Review of Thyroid Cancer Diagnosis
Received: 01-May-2023 / Manuscript No. jcd-23-92189 / Editor assigned: 04-May-2023 / PreQC No. jcd-23-92189 / Reviewed: 18-May-2023 / QC No. jcd-23-92189 / Revised: 22-May-2023 / Manuscript No. jcd-23-92189 / Published Date: 29-May-2023 DOI: 10.4172/2476-2253.1000183
Abstract
One of the prevalent, life-threatening disorders that have been on the rise in recent years is thyroid nodule. A frequent diagnostic technique for locating and identifying thyroid nodules is ultrasound imaging. Yet, it takes time and presents difficulties for the professionals to evaluate all of the slide photos. Automated, dependable, and objective methods are required for accurately evaluating ultrasound pictures. Recent developments in deep learning have completely changed several facets of image analysis and computer-aided diagnostic (CAD) programmes that deal with the issue of identifying thyroid nodules. We reviewed the literature on the potential, constraints, and present applications of deep learning in thyroid cancer imaging and discussed the study’s goals.
Keywords
Thyroid cancer; Diagnosis; Ultrasound; Medullary thyroid carcinoma
Introduction
The last three decades have seen an increase in thyroid cancer cases. The American Cancer Society’s most recent estimate for thyroid cancer for 2022 is roughly 43,800 new cases and 2,230 deaths. The thyroid gland is located at the front base of the throat, and thyroid cancer is a solid tumour that typically manifests as a nodule or mass there. When rogue cells proliferate too quickly for the immune system to manage, thyroid cancer results. Cancer typically originates from gene mutations or alterations to the genes that regulate how cells function. As a result, cells multiply uncontrollably and invade nearby tissues. There are several different varieties of thyroid cancer, but two types are by far the most prevalent types and account for 95% of thyroid malignancies. Follicular and papillary thyroid carcinoma is two examples of this [1-6].
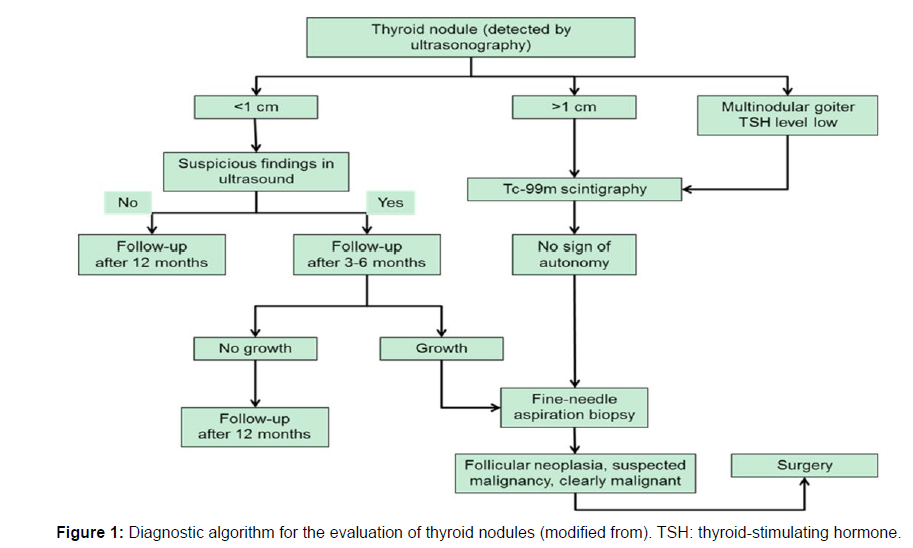
Early detection of malignant thyroid nodules can lead to efficient therapy and minimal harm if the nodules are treated before the cancerous cells in the thyroid gland spread. A procedure for the early diagnosis of malignant thyroid nodules is called thyroid cancer screening. Two primary techniques are used to identify thyroid cancer: (1) neck palpation during a physical examination, and (2) ultrasonography, which can identify both palpable and nonpalpable nodules, particularly those with a diameter of less than 1 cm. The main diagnostic method for determining the characteristics of thyroid nodules is ultrasonography. These traits are used to categorise nodules into benign and malignant types shown in [7] (Figure 1).
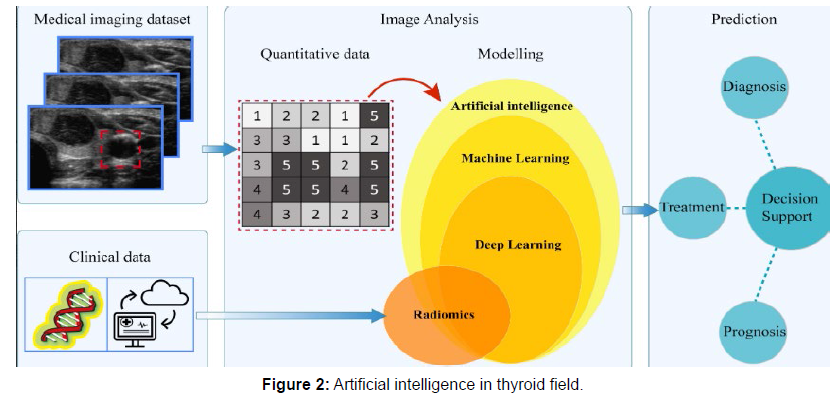
The use of computer-aided diagnosis (CAD) as a new method for automatically diagnosing thyroid nodules has increased during the past few decades. Artificial intelligence (AI) implementation in CAD tools makes them more intelligent and improves the accuracy and consistency of the interpretation of ultrasound findings, hence reducing the need for needless biopsies. The basic methodologies of AI-based CAD systems that have a significant impact on the medical industry are machine learning and deep learning [8]. These techniques rely on the expertise of specialists to select the key qualities from a list of predetermined specified criteria gathered from the area of interest. Several researches have employed thyroid ultrasound picture characteristics like margin, shape, echogenicity, calcifications, and composition to create CAD systems. These systems’ effectiveness has previously been mentioned. Prior study has revealed how the classic machine learning and deep learning methods, such as the support vector machines and convolutional neural network (CNN), have revolutionised the thyroid nodule diagnosis. The limitations of using CAD tools in doctors’ daily work have been greatly reduced because to the advancement of machine learning and artificial intelligence shown in [9] (Figure 2).
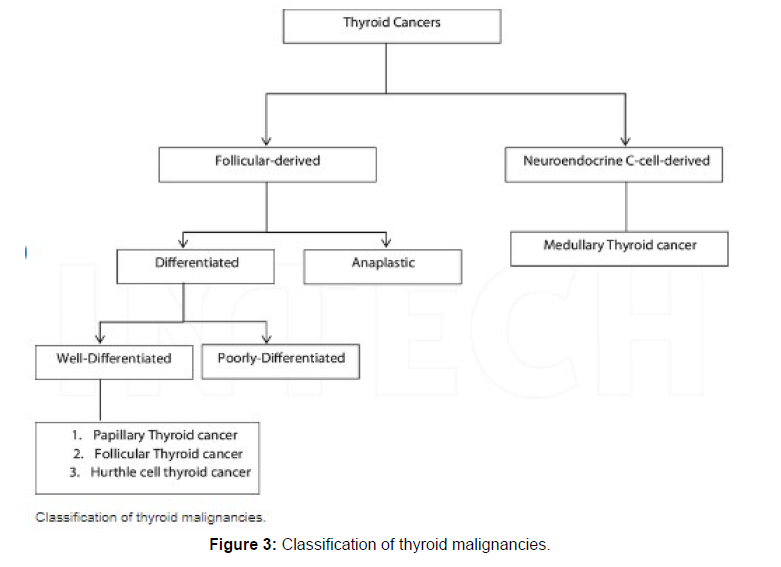
In-depth analysis of deep learning techniques for thyroid cancer diagnosis is provided in this research. Given that the majority of the articles were released after 2018, which shows that the deep learning algorithm performed well for classifying thyroid nodules, it has attracted a lot of attention in recent years [10]. In the section that follows, Section 2, an overview of the deep learning techniques that have previously been used for thyroid nodule classification is provided. Deep learning techniques like CNNs, generative adversarial networks (GANs), auto condors, long short-term memory (LSTM), deep belief networks (DBN), and recurrent neural networks (RNNs) were all thoroughly explained, and studies that used these techniques for classifying thyroid cancer were also introduced shown in [11] (Figure 3).
Since their introduction as a class of generative model, generative adversarial networks (GANs) have drawn a lot of interest from researchers studying artificial intelligence. Two-player zero-sum games are the source of inspiration for GANs. These models make an estimate of the dataset’s prospective distribution before creating new samples based on the distribution. Because to their excellent capacity to handle a range of issue types, including image processing, computer vision, audio processing, and language processing GANs techniques have been widely used in numerous industries [12], A generator and a discriminator learn simultaneously in a GAN, as is typical. The generator’s job is to capture the probability distribution of the provided datasets before using that distribution to create fresh data samples. The discriminator, which is typically a binary classifier, is in charge of telling actual data from bogus data. A deep neural network structure can be used for both the generator and the discriminator. With the aim of achieving Nash equilibrium, GAN uses minimax game optimization, and its straightforward design allows it to capture the distribution of input datasets [13].
Discussion
Due to its safety, effectiveness, non-invasiveness, and accessibility, ultrasound imaging has emerged as one of the most widely used modalities for assessing thyroid nodules. Yet, interpreting ultrasound images is a difficult undertaking that might vary depending on the radiologists’ prior medical expertise and observational abilities. As a result, there is a critical need for automated, trustworthy [14], and objective technology for the interpretation of ultrasound pictures. Recent advances in deep learning have changed a number of machine learning fields, including computer vision and image processing. Artificial intelligence-based CAD systems are developing quickly, but none of them have been widely adopted, and there are still problems with them. There is a huge need for AI-based CAD systems with superior designs and practicalities that deliver consistent nodule management solutions in practise [15].
In this article, we evaluated recent studies that used deep learningbased algorithms to examine photos of thyroid nodules from medical records. The literature showed that while CAD systems have sensitivity levels comparable to those of skilled radiologists, they fall short in terms of specificity and accuracy. As a result [16], combining the specificity and accuracy of radiologists with the sensitivity of CAD systems and using these systems as assistants for operators with less experience at primary care centres is probably a viable alternative to think about. As a result, it’s essential to use deep learning techniques and create models that have a high level of accuracy, specificity, and sensitivity. Future studies should examine how effective these strategies and tactics are. Also, as pre-processing techniques for pictures can considerably affect how well deep learning models work, they must be improved [17].
The management of data constraints, the development of reliable and accessible datasets, and the creation of uniform evaluation metrics are further issues that need to be addressed in future study. Additionally, all deep learning algorithms, including B-mode, Doppler, contrast-enhanced ultrasonography [18], and SWE, should be utilised on multimodal images to provide a complete view of the lesions. The multimodal pictures of thyroid nodules can be registered, trained, and evaluated to increase the accuracy of thyroid nodule diagnosis. Furthermore, it is challenging to evaluate the results of the offered strategies due to the absence of common measures for performance evaluation. According to a recent article, CNNs have been used the most frequently of all deep learning approaches to diagnose thyroid cancer. High sensitivity, specificity, and accuracy were found in the results. Other deep learning techniques haven’t seen as much use, and there aren’t enough papers to allow for a fair comparison of them. GANs are the second-most used deep learning technique for thyroid nodule diagnosis [19]. The high rates of sensitivity, specificity, and accuracy suggest that by using this method on multimodal pictures, models with improved performance may be discovered. Further research is needed to determine the accuracy rate for the other well-known deep learning techniques like RNN, DBN, and LSTM because they have not been applied widely [20].
Conclusion
As previously established, thyroid cancer starts when the cells multiply quickly and spread uncontrollably into the tissues around them. Consequently, in addition to lowering the number of fatalities, early diagnosis of malignant nodules is crucial for optimal disease management. Throughout the past several decades, the development of AI-based CAD systems for processing thyroid pictures has been remarkably rapid. If these technologies are adequately validated, thyroid nodule treatment will be improved. This article provides a thorough analysis of deep learning applications for evaluating thyroid nodules. The overall finding of this study showed that high specificity, sensitivity, and accuracy deep learning techniques with the most recent advancements will significantly assist the classification and analysis of thyroid tumours.
Today, it can be stated that further research is necessary to produce systems with high accuracy when compared to studies that employed deep learning algorithms for various cancer diagnosis, such as breast cancer and brain cancer. Many deep learning techniques still need to be applied to ultrasound photos in order to evaluate their performance, despite the empirical advantages and accomplishments of earlier deep learning algorithms and methodologies in the assessment of ultrasound thyroid images. There are currently insufficient public datasets available for thyroid cancer imaging. Thus, challenges that need to be addressed in future studies include the adoption of uniform assessment measures and the provision of trustworthy and accessible information.
It can be stated that CNN is by far the most widely used deep learning method for thyroid cancer diagnosis based on the findings of specificity, sensitivity, accuracy, and rate of previously presented methodologies. The method that has been most frequently used for classifying thyroid nodules is the VGG16 method. In other studies, GANs, RNNs, and LSTM techniques have also been used. However, further research is needed because the number of publications that have been published is insufficient. Also, it is essential to create better preprocessing techniques to enhance the functionality of deep learning models.
Conflicts of Interest
None
Acknowledgement
None
References
- Santos IP, Barroso EM, Schut TCB (2017) Raman spectroscopy for cancer detection and cancer surgery guidance: translation to the clinics. Analyst 142: 3025-3047.
- Jermyn M, Desroches J, Aubertin K (2016) a review of Raman spectroscopy advances with an emphasis on clinical translation challenges in oncology. Physics in Medicine and Biology 61: 370-400.
- Yue S, Cheng JX (2016) Deciphering single cell metabolism by coherent Raman scattering microscopy. Current Opinion in Chemical Biology 33: 46-57.
- Sun J, Zhang J, Lu J, Gao J, Ren X, et al. (2016) BRAF V600E and TERT Promoter Mutations in Papillary Thyroid Carcinoma in Chinese Patients. PLoS One 11: 153-319.
- Jin L, Sebo TJ, Nakamura N, Qian X, Oliveira A et al (2006) BRAF Mutation Analysis in Fine Needle Aspiration (FNA) Cytology of the Thyroid. Diagn Mol Pathol 15.
- Nikiforova MN, Kimura ET, Gandhi M, Biddinger PW, Knauf JA, et al. (2003) BRAF mutations in thyroid tumors are restricted to papillary carcinomas and anaplastic or poorly differentiated carcinomas arising from papillary carcinomas. J Clin Endocrinol Metab 88: 5399-5404.
- Travis WD (2014) Pathology and diagnosis of neuroendocrine tumors: lung neuroendocrine. Thorac Surg Clin 24: 257-66.
- Rudin CM, Brambilla E, Faivre-Finn C, Sage J (2021) Small-cell lung cancer. Nat Rev Dis Primers 17: 3.
- Nicholson SA, Beasley MB, Brambilla E, Hasleton PS, Colby TV, et al. (2002) Small cell lung carcinoma (SCLC): a clinicopathologic study of 100 cases with surgical specimens. Am J Surg Pathol 26: 1184-97.
- Liu Y, Wang H, Li Q, McGettigan MJ, Balagurunathan Y, et al. (2018) Radiologic Features of Small Pulmonary Nodules and Lung Cancer Risk in the National Lung Screening Trial. Radiology 286: 298-306.
- Nijssen A, Schut TCB, Heule F (2002) Discriminating basal cell carcinoma from its surrounding tissue by Raman spectroscopy. Journal of Investigative Dermatology 119: 64-69.
- Bodanese B, Silveira FL, Zangaro RA, Pacheco MTT, Pasqualucci CA, et al. (2012) Discrimination of basal cell carcinoma and melanoma from normal skin biopsies in vitro through Raman spectroscopy and principal component analysis. Photomedicine and Laser Surgery 30: 381-387.
- Frank CJ, Redd DCB, Gansler TS, McCreery RL (1994) Characterization of human breast biopsy specimens with near-IR Raman-spectroscopy. Analytical Chemistry 66: 319-326.
- Huang ZW, McWilliams A, Lui H, McLean DI, Lam S, et al. (2003) Near-infrared Raman spectroscopy for optical diagnosis of lung cancer. International Journal of Cancer 107: 1047-1052.
- Bergholt MS, Zheng W, Lin K (2015) Characterizing variability of in vivo Raman spectroscopic properties of different anatomical sites of normal colorectal tissue towards cancer diagnosis at colonoscopy. Analytical Chemistry 87: 960-966.
- Slipchenko M, Oglesbee RA, Zhang D, Wu W, Cheng JX, et al. (2012) Heterodyne detected nonlinear optical microscopy in a lock-in free manner. Journal of Bio photonics 5: 801-807.
- Chen W, Tsai P, Chen Y (2008) Functional nanoparticle-based proteomic strategies for characterization of pathogenic bacteria. Analytical Chemistry 80: 9612-9621.
- Sasaki T, Rodig SJ, Chirieac LR, Jänne PA (2010) The biology and treatment of EML4-ALK non-small cell lung cancer. Eur J Cancer 46: 1773-1780.
- Loo PS, Thomas SC, Nicolson MC, Fyfe MN, Kerr KM, et al. (2010) Subtyping of undifferentiated non-small cell carcinomas in bronchial biopsy specimens. J Thorac Oncol 5: 442-447.
- El-Deiry WS (1998) the p53 pathway and cancer therapy. J Cancer 11: 229-236.
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, CrossRef , Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar , Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Citation: Kaplan E (2023) Review of Thyroid Cancer Diagnosis. J Cancer Diagn 7: 183. DOI: 10.4172/2476-2253.1000183
Copyright: © 2023 Kaplan E. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.