Review of Petroleum: Source, Applications, Production, and Environmental Impact
Received: 03-Jun-2022 / Manuscript No. OGR-22-70028 / Editor assigned: 06-Jun-2022 / PreQC No. OGR-22-70028(PQ) / Reviewed: 21-Jun-2022 / QC No. OGR-22-70028 / Revised: 24-Jun-2022 / Manuscript No. OGR-22-70028(R) / Accepted Date: 29-Jun-2022 / Published Date: 30-Jun-2022 DOI: 10.4172/2472-0518.1000248
Abstract
The review aims to update and improve the body of knowledge regarding the definition, applications, origin, formation, exploration, processing, and products of petroleum, as well as the major petroleum-producing nations, octane rating, petrochemical industry, and petroleum and environment. It combined old and new reports using the table research technique. Many millions of years ago, organic substances found underground or as a result of chemical reactions in the atmosphere likely gave rise to petroleum or rock oil. During refinement, fractional distillation separates the mixture of gaseous, liquid, and solid hydrocarbons into several products. Petroleum has been a source of energy since the Middle Ages and has recently dominated the synthesis of chemical molecules. Using and exploring petroleum provide certain environmental challenges.
Introduction
“Petroleum” is short for “rock oil” or “earth oil” (EIA, 2005).
Crude oil and petroleum have been known to mankind since the beginning of civilization. It was primarily used as lamp fuel in ancient Persia and Burma. At Baba Gurgur in Iraq, burning natural gas (emanating from subterranean petroleum reservoirs) created a “perpetual fire”.
Uses of petroleum
Petroleum has two major applications. As fuel is the first. Crude oil burning opened the door for the extraction of several energy from petroleum. In particular, petroleum has been the most practical fuel for internal combustion engines and has been used as a source of energy for heating, lighting, and propulsion. With the development of the automobile and a wide range of other internal combustion engine uses, the significance of this utilisation has rapidly expanded.
The creation of organic compounds is petroleum’s second usage. By 1965, petroleum was used to create roughly 80% of all organic compounds used worldwide. This percentage increased to 98% in 1980 and to 99% in 2000. Petrochemicals, often known as petroleum chemicals, are numerous different types of chemicals. The two most significant in terms of commerce are kerosine and gasoline. An oily, gelatinous substance called petroleum jelly is used as a foundation for ointments, a lubricant, and a protective covering.
The origin of petroleum
Nelson (1954) discussed two reasonable schools of thinking concerning how petroleum is formed and formed. The “organic matter theory,” an older notion, contends that petroleum was created through the decay of plankton and other dead sea animals. Almost every plant and animal life contains compounds (like lipids) that are very similar to hydrocarbons and even small amounts of certain hydrocarbons. The discovery that fairly recent marine layers (10,000–15,000 years old) include asphaltic and hydrocarbon materials lends support to the notion. In addition, some of the smaller components of crude oil have molecular structures that are similar to chemicals found in living things. Again, if petroleum’s genesis is geologically relatively recent, it is difficult to imagine where else the carbon content of petroleum might have come from besides organic material.
Petroleum is crude oil that naturally occurs in sedimentary rocks, which is another argument in favour of this viewpoint. Small pools of it had formed as a result of seepage from below. In line with this assertion, EIA (2005) claims that petroleum was created from the remains of plants and animals that coexisted with dinosaurs millions of years ago in a marine (water) environment. Tiny marine plants and animals perished and were buried on the ocean floor about 300-400 million years ago. They were eventually covered by silt and sand layers. The remains were progressively buried deeper and covered with mud layers between 50 and 100 million years ago. The remnants were transformed into crude oil and gas by the intense heat and pressure of these layers. Today, reaching the geological formations containing crude oil and gas resources requires drilling through layers of silt, sand, and rock.
The idea faces a hurdle in explaining the size of petroleum deposits. The marine organisms would have to be plentiful over a period of several million years for such a significant amount of petroleum to accumulate. Accounting for the accumulating oil in huge resources is another difficulty. The scattered crude oil droplets must have travelled far in order for this to occur. However, it is questionable whether a mechanism within the circumstances and timeframe supported this [1-4].
According to the more modern school of thought known as the “atmospheric chemical reaction theory,” petroleum is much older than the Earth’s habitability. The earth is over 5,000 million years old, and when it was a hot, molten mass primarily made of hydrogen (H) and carbon dioxide (CO2), chemical reactions between them produced a wide variety of hydrocarbons that rose to the atmosphere as vapour clouds similar to those that are thought to currently surround the planet Venus:
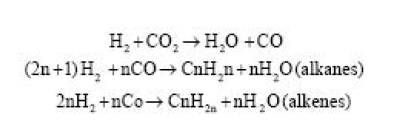
Condensation caused a “petroleum-rain” that lasted for 2,000 million years while the earth continued to cool. The oil and its sediments were gathered in numerous valleys and basins. The water-rain that the oil floats on arrived much later.
Both hypotheses struggle to explain the tremendous amount of the earth’s crude oil deposits.
Exploration of petroleum and important petroleum producing countries
Crude oil can flow up the well pipe under reservoir pressure or with mechanical aid from a pump or applied pressure, according to Arene and Kitwood (1979). Since Drake drilled crude oil in Pennsylvania, United States of America (USA), the petroleum business has continuously expanded, and numerous petroleum reserves have been found and explored throughout the world [5].
EIA (2005) states that scientists and engineers investigate a selected area using earth rock samples. If the site appears promising after the measurements, drilling is started. To house the equipment and pipelines entering the well, a building known as a “derrick” is constructed above the hole. A constant supply of oil will be brought to the surface after the drilled well is complete.
The top five producers of crude oil globally are Saudi Arabia, Russia, the United States, Iran, and China. Offshore in the Gulf of Mexico, more than one-fourth of the crude oil produced in the US is produced. Texas, Alaska, California, Louisiana, and New Mexico are the leading states for producing crude oil (NRC, 2003).
The volume of crude oil produced annually in the United States has been decreasing. On the other hand, as demand for items derived from crude oil has increased, it has become necessary to import additional oil from other nations that are major producers of petroleum. As a result, nearly 58% of the crude oil and petroleum products utilised in the US are imported.
According to OPEC (2011), five nations—the Islamic Republic of Iran, Iraq, Kuwait, Saudi Arabia, and Venezuela—signed an agreement in Baghdad, Iraq, in September 1960, establishing the Organization of the Petroleum Exporting Countries (OPEC). They were supposed to be the Organization’s founding members.
Later nations that joined these ones include Qatar (1961), the Socialist People’s Libyan Arab Jamahiriya (1962), the United Arab Emirates (1967), Algeria (1969), Nigeria (1971), Ecuador (1973), and Angola (2007).
Ecuador’s participation was suspended from December 1992 to October 2007. In 1995, Gabon resigned from the organisation. Indonesia’s participation was terminated as of January 2009. The Islamic Republic of Iran, Iraq, Kuwait, Saudi Arabia, Venezuela, Qatar, the Socialist People’s Libyan Arab Jamahiriya, the United Arab Emirates, Algeria, Nigeria, Ecuador, and Angola are the organization’s current 12 Member Countries.
The OPEC Statute makes a distinction between Founder Members and Full Members, or nations whose membership petitions have been approved by the Conference. “Any country with a substantial net export of crude petroleum, which has fundamentally similar interests to those of Member Countries, may become a Full Member of the Organization, if accepted by a majority of three-fourths of Full Members, including the concurring votes of all Founder Members,” the charter states. The Statute also mentions Associate Members, which are nations that do not meet the requirements for full membership but are nonetheless accepted under any unique restrictions that the Conference may impose.
The nature and composition of petroleum
A foul-smelling, yellow to black liquid known as crude oil is typically found in underground spaces called reservoirs (EIA, 2005). Sweeney (1950), Sachanen (1945), Hobson (1973), Bankole and Ogunkoya (1978), and McCain (1970) According to Shreve and Brink (1977) and Arene and Kitwood (1979), crude oil is mostly composed of a blend of gaseous, liquid, and solid hydrocarbons. Sulfur-containing substances such as thiol (mercaptans) such as ethanethiol (C2H5SH) and cyclic sulphides such as thiophen derivatives are present in some crude oils (e.g., tetrahydrothiophen and benzothiophen). Organic nitrogen compounds are also present in others. Typically, complicated ring structures containing nitrogen are seen, like the carbazole:
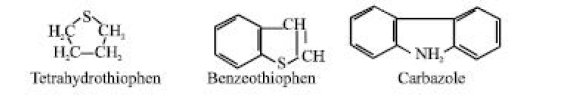
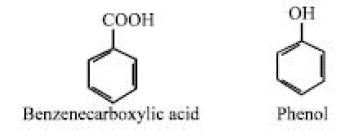
Again, a limited number of organic acids, whose molecules have the carbonyl group (-COOH), can be found in some crude oils (e.g., benzenecarboxylic acid). Additionally, certain crude oils have small amounts of phenol-type compounds, which are benzene rings that have hydroxyl groups (-OH) connected to them directly, such as phenol.
Petroleum is a highly complex mixture, according to Shreve and Brink (1977), Nelson (1958), Rossini (1947), Sweeney (1950), and Arene and Kitwood (1979), with the main components being compounds (gases, liquids, and solids) of hydrocarbons (11–15% hydrogen and 83–87% carbon), which are categorised as alkanes, cycloalkanes, and aromatics. Crude oil is a sand, water, and viscous mobile liquid. It might be colourless or green-black in appearance. The density of crude oils ranges from 0.78 to 1.00 gcm-3.
90% of crude oils are alkanes. They are solely carbon and hydrogen compounds. Pentane is one example of an alkane with a “straight” chain:
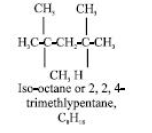
Others, like 2,2,4-trimethylpentane (iso-octane), have branched chains:
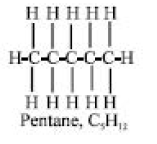
Straight-chain alkanes are preferable in kerosine and diesel fuels but undesirable in gasoline. Additionally, the creation of detergents and the bacteriological generation of protein both prefer straight-chain alkanes (Eneh, 2000a).
Cycloalkanes, such as cyclopentane and cyclohexane, are hydrocarbons having a ring structure but only one bond:

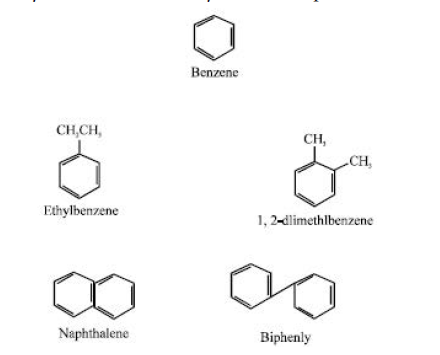
According to Arene and Kitwood (1979), aromatic compounds include molecules with the benzene ring structure, such as benzene, ethylbenzene, 1,2-dimethylbenzene, naphthalene, and biphenyl.
Petroleum processing and products
Refining is the process of turning crude oil into useful products, according to Sharma, Bankole and Ogunkoya, Hobson, McCain, Kimberlin, Rossini and Mair, Shreve and Brink, Arene and Kitwood, Eneh, USDE, and Saepudin et al. [6-8]. After being extracted from the ground, crude oil is transported to a refinery via pipeline, ship, or barge, where its components are divided into usable petroleum products.
Fractional distillation was used to separate kerosine and gasoline from petroleum. Numerous methods for transforming the principal distillation products into useful compounds have been developed in response to the increased demand for products that are more effective than those made from crude oil.
Sand and other solid materials are filtered out of the crude oil, which is then left to stand in a reservoir so that a layer of denser water can separate it from the oil layer above. The latter is then sent to the refinery by pipeline, where continuous distillation or fractionation divides the crude oil into a number of “cuts” or fractions with various boiling points.
Products from the refining of petroleum have boiling points that range from 20 to more than 400°C. The number of carbon atoms in the compound and the type of the compound (gas or liquid) and application of the product are related to the boiling range. For instance, C5–C10 compounds primarily consist of liquids and have a boiling range of 20–160°C.
The ratios of each fraction obtained depend on the kind of crude oil used as well as the distillation procedure. For instance, the yields of the three most valuable fractions from Nigeria’s refinery are around gasoline (10%), kerosine (20%), and gas (30%). Nigerian crude oil is in demand because it has a lower sulphur level (0.14%) than other crudes. For instance, Persian Gulf crude oil has a sulphur content of roughly 7%. Petroleum was found in Nigeria in 1956 in Oloibiri village, which is now Bayelsa State, and commercial production began in 1957. Since then, it has been continuously increasing [9-12].
Barrels are used to measure crude oil (bbls). More than 44 gallons of petroleum products can be produced from a 42-US gallon barrel of crude oil. When crude oil is refined, approximately 20 gallons of finished motor gasoline (19.6%), 7 gallons of diesel (10%), 4% of jet fuel, 1% of heavy fuel oil, 1% of liquefied petroleum gas, and other petroleum products are produced from one barrel of crude oil (7.6%). About 5% of the gain is attributable to processing. The majority of petroleum products are employed in the energy sector. For instance, a large number of Americans fuel their cars and heat their homes using propane. Ink, crayons, bubble gum, dishwashing detergent, deodorant, eyeglasses, records, tyres, ammonia, and heat valves are some further petroleum-based goods (USDE, 1999).
As a result, primary distillation of crude oil produces a tiny amount of gasoline compared to demand while producing significantly more of the higher-boiling elements than necessary. The process used to break down huge hydrocarbon molecules into smaller ones is known as cracking. Temperatures of 300–700°C and pressures of up to 30 atms were utilised in the previous thermal cracking process. According to Arene and Kitwood (1979), catalytic cracking, which uses a catalyst (such as silicon IV oxide or aluminium III oxide), about 500°C temperature, and approximately atmospheric pressure, has essentially supplanted it.
Catalytic cracking produces particular products and is simpler to manage. Alkane is first cracked into a tiny alkane molecule and an alkene molecule (with a carbon-carbon double bond, C = C):
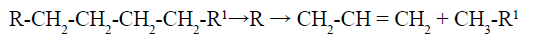
The resultant alkene is extremely reactive and can be isomerized (changing its structure). Additionally, polymerization is an option (joining together to form larger unit). Both procedures produce a variety of gases in addition to hydrocarbons fit for use in gasoline, kerosine, and diesel (butane and butene, 45%; propane and propene, 3%; ethane and ethene, 15% ; methane 10%; and a little hydrogen). These are crucial as petrochemical industry raw ingredients. The overall gas yield typically ranges between 15 and 20% of the charge [13-15].
By adding hydrogen to the catalytic cracking process to promote the synthesis of lesser alkanes under high pressures (150–200 atmospheres) and moderate temperatures (400–450°C), hydrocracking increases the yield of the gasoline. The catalysts could be palladium and nickel cations or aluminosilicate compounds. In contrast to ordinary cracking, which also produces alkenes, hydrocracking primarily produces alkanes. Altering the hydrocarbons’ molecular structure in the lower fractions obtained from primary distillation or from cracking is a process known as reforming. Branched-chain hydrocarbons in the C6–C10 range, which are required for high-grade motor gasoline, are the yields. The “feed” typically comprises of substances that are boiling up to 200°C. The best catalyst consists of highly pure aluminium (III) oxide on platinum (approximately 0.5% by weight). Three common types of reforming reactions are as follows:
When an aromatic (benzene-type) chemical is created from a cycloalkane, for example:
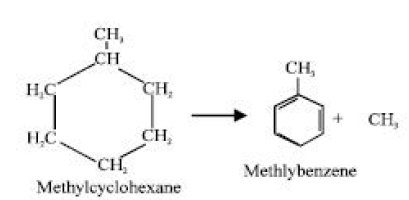
Dehydrocyclization is the process of forming a chemical with a ring structure out of one with a straight chain and losing hydrogen afterward. For example:
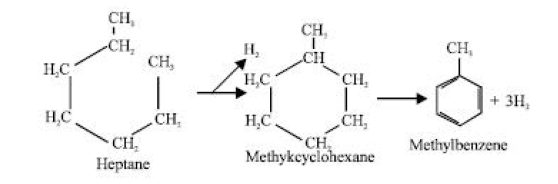
Without a net loss of any ingredient, isomerization or rearrangement, for example
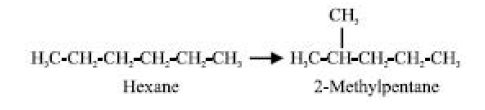
Octane number
Poor quality fuel has a propensity to “knock” or detonate unevenly and prematurely, especially in a high-compression engine, according to Sharma and Arene and Kitwood. A useful fuel is 2,2,4-trimethylpentane, often known as iso-octane, which burns smoothly and does not create knocking. Consequently, it is given the arbitrary octane number of 100 (on a zero-to-100 scale). Heptane, on the other hand, is a terrible fuel since it is prone to knocking in particular. Therefore, it is given the octane number of 0. The amount of “iso-octane” combined with heptane determines the octane number or rating of gasoline, which represents the fuel’s knocking tendencies. It serves as a gauge for how well an internal combustion engine performs.
By adding branched-chain hydrocarbons or aromatic chemicals, the quality of the fuel can be increased (or the fuel’s octane number or octane rating can be increased). To enhance the performance of various fuels, ethanol and methanol are added. Tetraethyl-lead (IV), Pb(C2H5)4, is another additive that significantly raises a fuel’s octane number; 0.03% by weight can result in an increase of 15-20 units. However, due to the environmental pollution and lead poisoning that occur from the use of such fuels, this practise has been discouraged and forbidden in the majority of countries [16-19].
The Petrochemical Industry
The manufacture of chemicals from petroleum has expanded significantly since World War II, according to Bankole and Ogunkoya (1978), Tedder et al. (1979), Waddams (1973), Shreve and Brink (1977), and Kimberlin (1957).
Even inorganic compounds, such as carbon black from methane, have been created from petrochemicals. Some of the chemicals made from petrochemicals are listed.
Petrochemicals such as methane, ethyne, and synthesis gas are used to make other compounds. Methane can be converted into carbon black, ethyne, synthesis gas, halogenmethanes, and hydrogen cyanide. Ethanal and chloroethene chemicals are both made from ethyne. Additionally, methanol and ammonia are made from synthesis gas.
Petroleum and the Environment
Even though utilising petroleum products for heating our homes, cooking stoves, vehicles, trucks, and other combustion engine applications makes life easier, it can also lead to environmental issues including air and water pollution. Burning petroleum-based products releases carbon dioxide (CO2), a greenhouse gas associated with global warming, when they are used as fuel. The usage of petroleum products also releases pollutants into the air we breathe, including unburned hydrocarbons, nitrogen oxides, particulate matter, and carbon monoxide (CO). Since vehicles and trucks contribute significantly to air pollution, numerous environmental rules have been passed to alter the composition of gasoline and diesel fuel to reduce emissions. Compared to 1990, when diesel and gasoline were first introduced, these “reformulated fuels” burn substantially cleaner. So that they can be utilised with modern, less-polluting technology, gasoline and diesel fuel’s sulphur content will be drastically lowered during the next few years (USDE, 1999).
Oil drilling and exploration might disrupt habitats on land and in the ocean. Specifically, oil exploration activities had a negative impact on soil/land resources, aquatic life/fisheries, water resources, crops, livestock, and forests/vegetation, according to a study on the assessment of the impact of oil exploration activities on agriculture and natural resources in the Niger Delta Region of Nigeria. Most agricultural lands have been damaged by oil spills, which have also decreased the availability of fish and fish products, contaminated surface and ground water supplies, destroyed arable and tree crops, and killed farm animals in the area due to poisonous substances in the soil and polluted water. Some forest animal and plant species, including primates, fish, turtles, and birds, have also disappeared as a result of oil drilling activity. The end result of these effects is a sharp decline in farm productivity and income from animal farms [17].
The quantity and size of drilling “footprints,” also known as disturbed areas, have been decreased thanks to new technologies. Fewer wells must be drilled in order to find oil deposits thanks to satellites, global positioning systems, remote sensing equipment, 3-D seismic technologies, and 4-D seismic technologies. Once more, the use of horizontal and directional drilling enables the production of oil from considerably larger areas from a single well. Due to the invention of mobile drilling rigs and smaller “slimhole” drilling rigs, today’s production footprints are only approximately one-fourth the size of those from 30 years ago. Even though a well’s soil fertility has been lost, it must be blocked underground when the oil is gone. Old offshore drilling platforms are toppled as part of the “rig-to-reefs” initiative and placed on the ocean floor to serve as artificial reefs that draw fish and other marine life. A rig becomes covered in barnacles, coral, sponges, clams, and other aquatic life within six months to a year of being overturned (USDE, 1999).
Wildlife may be harmed if oil is dumped into rivers or oceans. Oil spills can occur when petroleum products are utilised on land, such as gasoline that occasionally falls onto the ground when people are filling their gas tanks, motor oil that is thrown away after an oil change, or fuel that escapes from a leaking storage tank. They can also occur when ships collide [18-20]. The spilled goods end up in the gutter during a rainstorm and eventually end up in rivers and the ocean. Fuel leaks from motorboats and jet skis are another occasion where oil enters the sea (USDE, 1999).
Petroleum products may potentially seep into the earth when a storage tank or pipeline leaks. Methyl Tertiary Butyl Ether (MTBE), one of the components of gasoline, has occasionally entered local water supplies in areas where it has leaked from storage tanks. A number of states have banned the use of MTBE in gasoline, and the refining industry is moving away from using it when blending reformulated gasoline since it gives water a terrible flavour and many people are concerned about drinking it. All subterranean storage tanks are expected to be replaced with tanks that have a double lining to prevent leaks (USDE, 1999).
Conclusion
The review made an effort to combine old and recent petroleum literature. It has clarified the meaning of petroleum from the Medieval Latin words petra and oleum; the uses of petroleum; the two plausible theories on the origin and formation of petroleum; petroleum exploration and the major oil-producing nations in the world, including the OPEC members; the nature and composition of petroleum; petroleum processing and products; and the octane number or rating, the petrochemical industry; and petroleum and the environment.
Acknowledgement
I would like to acknowledge Graduate School of Global Environmental Studies, Kyoto University, Kyoto, Japan for providing an opportunity to do research.
Conflict of Interest
No potential conflicts of interest relevant to this article were reported.
References
- Kim JJ , AAPCEH (2004) Ambient air pollution: Health hazards to children. Pediatrics 114: 1699-1707.
- Eneh OC (2011) Managing Nigeria's environment: The unresolved issues. J Environ Sci Technol 4: 250-263.
- Eneh OC (2011) Effects of water and sanitation crisis on infants and under-five children in Africa. J Environ Sci Technol 4: 103-111.
- Goss CH, Newsom SA, Schildcrout JS, Sheppard L, Kaufman JD (2004) Effect of ambient air pollution on pulmonary exacerbations and lung function in cystic fibrosis. Am J Respir Crit Care Med 169: 816-821.
- Kimberlin CN (1957) Chemistry in the manufacture of modern gasoline. J Chem Educ 34: 569-569.
- Kymisis M, Hadjistavrou K (2008) Short-term effects of air pollution levels on pulmonary function of young adults. Internet J Pulmonary Med 9: 136-148.
- Saepudin D, Sukarno P, Soewono E, Sidarto KA, Gunawan AY (2010) Oil production optimization in a cluster of gas lift wells system. J Appl Sci 10: 1705-1713.
- Sweeney WJ (1950) Petroleum and its products. J Chem Educ 27: 696.
- Tabieh MAS, Al-Horani A (2010) An economic analysis of water status in Jordan. J Appl Sci 10: 1695-1704.
- Ugwu DS (2009) Effects of oil exploration on agriculture and natural resources in the niger delta region of Nigeria. Sustain Human Dev Rev 1: 117-130.
- Ramos M, Dias APS, Puna JF, Gomes J, Bordado JC (2019) Biodiesel production processes and sustainable raw materials. Energies 12: 4408.
- Gebremariam SN, Marchetti JM (2018) Economics of biodiesel production. Energy Convers Manag 168: 74-84.
- Aghbashlo M, Peng W, Tabatabaei M, Kalogirou SA, Soltanian S, et al. (2021) Machine learning technology in biodiesel research: A review. Prog Energy Combust Sci 85: 100904.
- Singh D, Sharma D, Soni SL, Sharma S, Sharma PK, et al. (2020) A review on feedstocks, production processes, and yield for different generations of biodiesel. Fuel 262: 116553.
- Rizwanul FIM, Ong HC, Mahlia TMI, Mofijur M, Silitonga AS, et al. (2020) State of the art of catalysts for biodiesel production. Front. Energy Res 8: 101.
- Tabatabaei M, Aghbashlo M, Dehhaghi M, Panahi HKS, Mollahosseini A, et al. (2019) Reactor technologies for biodiesel production and processing: A review. Prog Energy Combust Sci 74: 239-303.
- Mahlia TMI, Syazmi ZAHS, Mofijur M, Abas AP, Bilad MR, et al. (2020) Patent landscape review on biodiesel production: Technology updates. Renew Sustain Energy Rev 118: 109526.
- Panwar NL, Kaushik SC, Kothari S (2011) Role of renewable energy sources in environmental protection: a review. Renew Sustain Energy Rev 15: 1513-1524.
- Mekhilef S, Siga S, Saidur R (2011) A review on palm oil biodiesel as a source of renewable fuels. Renew Sustain Energy Rev 15: 1937-1949.
- Sirisomboonchai S, Abuduwayiti M, Guan G, Samart C, Abliz S, et al. (2015) Biodiesel production from waste cooking oil using calcined scallop shell as catalyst. Energy Convers Manage 95: 242-247.
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Citation: Trencher G (2022) Review of Petroleum: Source, Applications, Production, and Environmental Impact. Oil Gas Res 8: 248. DOI: 10.4172/2472-0518.1000248
Copyright: © 2022 Trencher G. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 2426
- [From(publication date): 0-2022 - Apr 03, 2025]
- Breakdown by view type
- HTML page views: 2036
- PDF downloads: 390
