Retrovirus Pseudomembranous Atrophic Enteritis caused by Suicidal Emperipolesis; A Light and Ultrastructural Study
Received: 22-Sep-2015 / Accepted Date: 11-Nov-2015 / Published Date: 13-Nov-2015 DOI: 10.4172/2161-0681.1000257
Abstract
Benign retroviral infections are common but are usually not recognized. This histologic and ultrastructural study identifies such an infection that became clinically evident in the small bowel following a partial colectomy for multiple micro invasive adenomas. Suspected is a low-grade retroviral infection of myeloid progenitor cells activated by inflammatory cytokines released by the operative procedure. Multiple infected neutrophils migrated to the small bowel adjacent to the operative anastomosis and through emperipolesis entered the luminal facing enterocytes. Budding of the retrovirus beginning at the neutrophil cell membrane within enterocytes assembled an immature retrovirus virus lacking a central core because translational and transcriptional viral events were not possible in the organelle poor and nuclear damaged neutrophils. Apoptosis of the infected neutrophil released toxic products and water produced in the mitochondria that distended the vacuoles encircling the neutrophils. There disruption spilled these contents directly into enterocyte cytoplasm producing collateral organelle injury that killed and detached surface enterocytes. This effaced the intestinal villi to produce atrophic and pseudomembranous enteritis with ulcers and bowel perforations.
Conclusion: The incomplete assembly of retrovirus in neutrophils rendered the virus non-infective but suicidal emperipolesis in neutrophils assembling retrovirus grouped in enterocytes created the collateral damage that caused the enteritis. This article is the first to report this suicidal variant of emperipolesis and its causative retroviral enteritis.
Keywords: Apoptosis neutrophil; Emperipolesis suicidal; Pseudomembranous enteritis; Retrovirus
323806Introduction
Three horizontally spread-person to person-retroviral infections in humans are associated with recognized diseases: Human T-cell Leukemia-1 (HTLV-1) with adult T-cell leukemia and the neurologic disorder tropical spastic paraparesis; Human T- cell Leukemia-2 (HTLV-2) with hairy cell leukemia and other hematopoietic malignancies and human immunodeficiency virus (HIV) with acquired immunodeficiency syndrome (AIDS) [1,2]. However, retroviruses can also cause relatively or even completely benign human infections that maybe unrecognized and lifelong [2]. The literature about these infections in humans is sparse. The retrovirus in these circumstances may not be cytopathic, the infection may be minimal since only small amounts of cellular metabolism are committed toward virus replication, or the virus may be suppressed by a vigorous immunologic response [2]. This article will describe another pathway of how a retrovirus infection may remain clinically innocuous and show how, under the aegis of emperipolesis and certain co-existing conditions, it became clinically relevant.
Materials and Methods
Multiple sections of the resected bowel were selected and placed into 12 blocks that were cut and stained with hematoxylin-eosin. The Leder stain, used to identify polymorphonuclear leukocytes (PMN’s), was applied to selected slides. Areas containing the enteritis previously fixed in formalin were prepared for transmission electron microscopy utilizing a previously reported methodology [3].
Results
Case presentation
The patient was an obese 68- year old white female in apparent good health who in participating in a screening program was found to have quaiac positive stools. Colonoscopy examination revealed 2 colonic polyps that were biopsied and identified as tubulovillus adenomas both showing foci of adenocarcinoma superficially invading into the submucosa. Twenty days later the patient underwent a partial colectomy that on pathologic examination revealed no residual carcinoma at the prior polypectomy sites, eight additional adenomas and no metastases in accompanying colonic lymph nodes. The bowel was anastomosed and the patient had an uneventful immediate postoperative course with spontaneous recovery of gastrointestinal function. However, about 2 weeks later she developed cramping abdominal pain and severe watery blood-flecked diarrhea. A chest Xray showed air under the diaphragm indicating possible intestinal perforation. An exploratory laparotomy was performed and several ileal perforations were identified. Approximately 120 cm of the ileum was resected and an ileostomy and a mucus fistula were created. Surgical pathological diagnosis was acute pseudomembranous enteritis with multiple perforations and acute peritonitis. A search for the etiology of the perforating enteritis was attempted including workup for Clostridium difficile, ova and parasites, Campylobacter and Escherichia coli overgrowth. These were all negative. Because preliminary electron microscopic studies showed viral particles, Enzyme-linked immunosorbent assay (ELISA) for rotavirus was performed. It was positive. The patient continued to have uncontrollable high output- up to 12 liters daily from her ileostomy requiring a side-to-end anastomosis of the ileum to the colon. Her white blood count was 6,700 with 11 segmental leukocytes, 76 bands and 5 metamyelocytes. Cultures of peritoneal fluid were positive for multiple enteric organisms. An enterocutaneous fistula formed that became infected and increased bowel drainage continued. She developed decreased renal function, hypotension and sepsis. Multiple blood cultures were positive for Morganella morganii. She became severely acidotic, oliguric, hypotensive and died from her sepsis. Autopsy revealed a perforation of the blind ileum that was preoccupied by granulation tissue and peritonitis. The mucosa of the gastrointestinal tract away from the site of perforation appeared grossly and microscopically unremarkable. A short bowel measuring only 90 cm was believed to be responsible for her persistent abdominal fluid loss. Serology for HIV was negative and the Cytomegalovirus titer was 1:8 and those for Ebstein-Barr virus was 1:160 both suggestive of a prior infection.
Morphologic Findings
Gross description
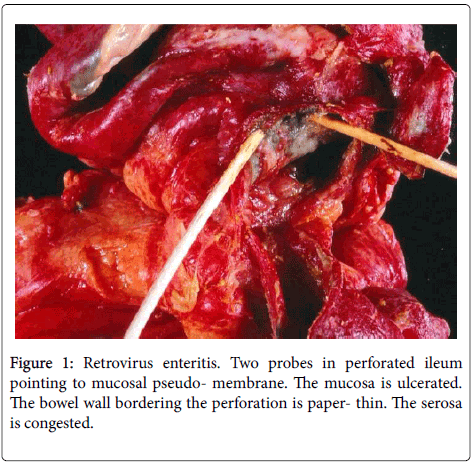
The resected segment of small bowel was 120 cm long and the distal end included the previous anastomotic site. It was dusky red brown for most its length and exhibited several perforations ranging in size and shape from 1 cm slits to 4 cm irregular lesions. The mucosa showed multiple varying sized ulcers the largest bordering the perforations. The ulcerated bowel wall adjacent to many perforations was paperthin. A whitish-grey pseudomembrane irregularly bedecked the ulcers and the non-ulcerated congested and focally edematous mucosa (Figure 1).
Microscopic description
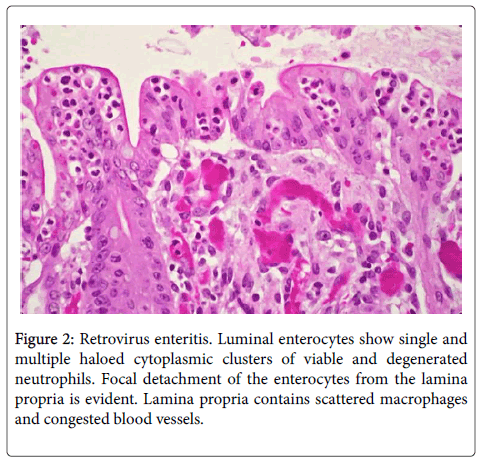
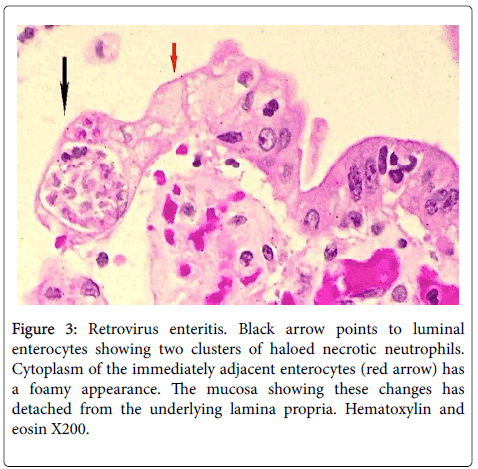
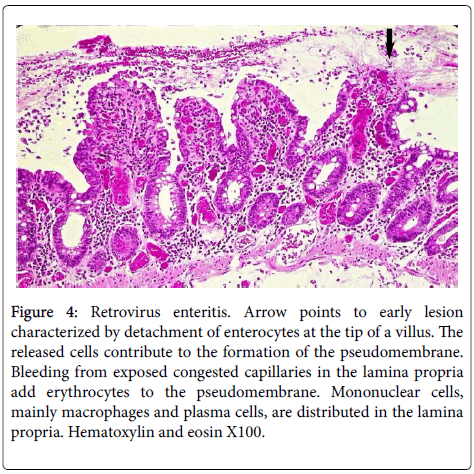
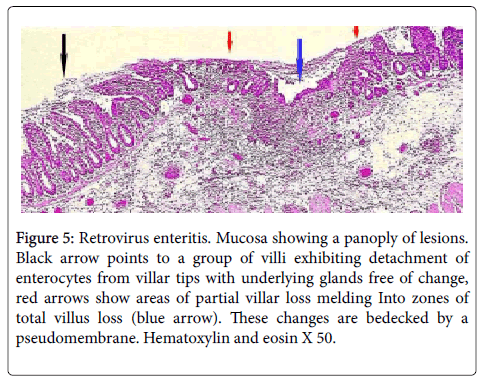
Single or multiple polymorphonuclear leukocytes (PMN’s)-Leder positive cells-surrounded by clear halos were principally distributed within the cytoplasm of luminal facing enterocytes (Figure 2) although a few were also apparent in the enterocytes lining the glands. Most neutrophils showed degenerative alterations-cytoplasmic eosinophilia and fragmentation and nuclear pyknosis, karyorrhexis and karyolysis (Figures 2 and 3). Scattered engulfing and surrounding enterocytes exhibited a foamy cytoplasm (Figure 3). In areas, the superficial enterocytes exhibiting these changes uncoupled from the underlying lamina propria (Figures 2 and 3). The detachment of enterocytes caused villus decapitation, shortening, widening and eventual elimination (Figures 4 and 5). Injured and necrotic PMN’s and enterocytes expelled into the intestinal lumen combined with fibrin and red cells to form a pseudomembrane (Figures 1,4 and 5). Further depopulation of enterocytes in colonized segments of small bowel resulted in glandular injury and loss and led to perforating ulcerations with surrounding peritonitis. Increased numbers of mononuclear cells principally macrophages and plasma cells (Figures 2-4) were evident in the lamina propria in zones of enterocyte loss.
Figure 3: Retrovirus enteritis. Black arrow points to luminal enterocytes showing two clusters of haloed necrotic neutrophils. Cytoplasm of the immediately adjacent enterocytes (red arrow) has a foamy appearance. The mucosa showing these changes has detached from the underlying lamina propria. Hematoxylin and eosin X200.
Figure 4: Retrovirus enteritis. Arrow points to early lesion characterized by detachment of enterocytes at the tip of a villus. The released cells contribute to the formation of the pseudomembrane. Bleeding from exposed congested capillaries in the lamina propria add erythrocytes to the pseudomembrane. Mononuclear cells, mainly macrophages and plasma cells, are distributed in the lamina propria. Hematoxylin and eosin X100.
Figure 5: Retrovirus enteritis. Mucosa showing a panoply of lesions. Black arrow points to a group of villi exhibiting detachment of enterocytes from villar tips with underlying glands free of change, red arrows show areas of partial villar loss melding Into zones of total villus loss (blue arrow). These changes are bedecked by a pseudomembrane. Hematoxylin and eosin X 50.
Ultrastructural Findings
Viral assembly
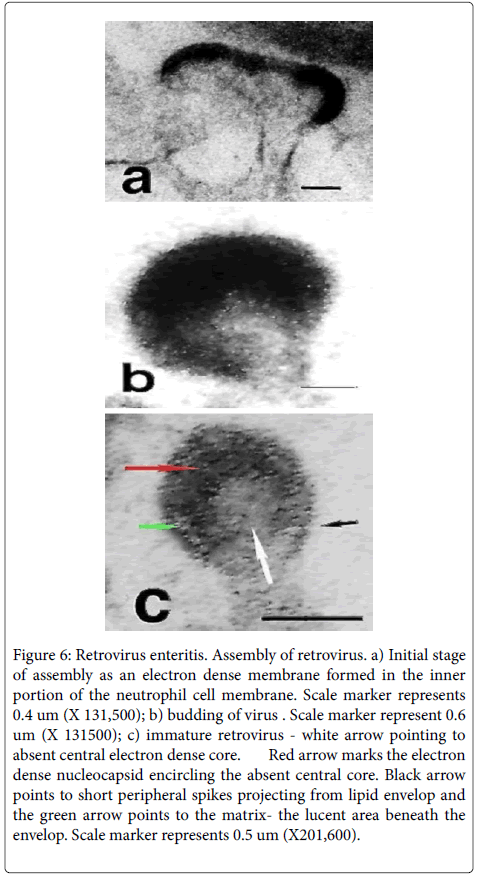
Discrete electron dense linear patches, around 39nm in size, intimately associated with the cytoplasmic face of the cell membrane of PMN’s (Figure 6) characterized the initial phase of viral assembly. The outward bowing of this patch by a budding process created a domeshaped virus particle with short peripheral spikes often situated atop a peninsula of externally projected cytoplasm (Figure 6). With further assembly a slightly ovoid viral particle formed- about 94 nm- that was encircled by an electron dense capsule (Figure 6)-the lipid envelopfrom which fine short electron dense spikes projected-surface glycoprotein (black arrow). Internal to the envelope was a lucent zone, the matrix (green arrow), which was internally bounded by an irregularly thickened electron-dense membrane, the nucleocapsid (red arrow). The latter encircled a central electron-lucent round zone representing the immature core (white arrow). Released viruses with electron dense cores were not identified in the 32 ultra-structural photomicrographs taken of this case. Viral assembly was principally observed in the luminal pseudomembrane in intact degenerate or fragmented PMN’s having preserved cell membranes.
Figure 6: Retrovirus enteritis. Assembly of retrovirus. a) Initial stage of assembly as an electron dense membrane formed in the inner portion of the neutrophil cell membrane. Scale marker represents 0.4 um (X 131,500); b) budding of virus . Scale marker represent 0.6 um (X 131500); c) immature retrovirus - white arrow pointing to absent central electron dense core. Red arrow marks the electron dense nucleocapsid encircling the absent central core. Black arrow points to short peripheral spikes projecting from lipid envelop and the green arrow points to the matrix- the lucent area beneath the envelop. Scale marker represents 0.5 um (X201,600).
Polymorphonuclear leukocytes within enterocytes
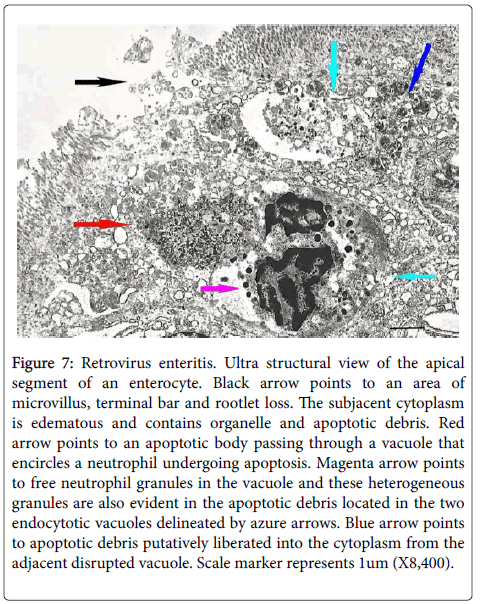
Many of the ultrastructural alterations distribute below are evident in Figure 7. PMN’s distributed within the cytoplasm of enterocytes exhibited degradative changes characterized by chromatin condensation and vacuolization and loss of nuclear matrix and condensation and disintegration of cytoplasmic organelles into varying sized electron dense fragmented masses (cyan arrows) many containing heterogeneous cytoplasmic granules. The cell membrane of these damaged cells often was partially or totally lost. The varying sized cellular debris was situated in dilated irregular rounded vacuoles with clear content some containing isolated neutrophil granules (magenta arrow). Disruption of these vacuoles caused spillage of the neutrophil cellular debris into the cytoplasm of the enterocytes (blue and red arrows). The enterocyte cytoplasm bordering these vacuoles often was edematous and contained damaged organelles (black arrow) and disrupted enterocyte junctions. Patchy loss of microvilli with it terminal web and underlying rootlets also occurred (black arrow). Damaged neutrophils or its apoptotic fragments were expelled into the intestinal lumen through these defects. The above degenerative neutrophil changes were principally located in the mid and apical cytoplasmic zones of the enterocytes while more intact neutrophils were localized to the basal cytoplasm of enterocytes.
Figure 7: Retrovirus enteritis. Ultra structural view of the apical segment of an enterocyte. Black arrow points to an area of microvillus, terminal bar and rootlet loss. The subjacent cytoplasm is edematous and contains organelle and apoptotic debris. Red arrow points to an apoptotic body passing through a vacuole that encircles a neutrophil undergoing apoptosis. Magenta arrow points to free neutrophil granules in the vacuole and these heterogeneous granules are also evident in the apoptotic debris located in the two endocytotic vacuoles delineated by azure arrows. Blue arrow points to apoptotic debris putatively liberated into the cytoplasm from the adjacent disrupted vacuole. Scale marker represents 1um (X8,400).
Pseudomembrane
The pseudomembrane was composed of neutrophil debris exhibiting varying degrees of degradative changes, detached enterocyte fragments containing neutrophil apoptotic debris, fibrin and occasional red cells. Mature retrovirus was not recognized and rotavirus was not detected.
Discussion
Two major problems arose in the morphologic interpretation of this enteritis. The first concerned the identification of the virus, its infectivity and its role in the pathogenesis of the enteritis. The ultrastructure of the offending virus described in this case was that of a retrovirus most likely a gamma retrovirus (C-type) since the viral assemble occurred on the neutrophil plasma membrane, the surface projections were barely visible and precursor viral particles were not evident in cytoplasm of the infected PMN’s [1,2,4]. The outward budding of sthe nascent viral particles from the plasma membrane assembled a retrovirus with an electron-lucent inner core indicating that the virus was immature. The intact inner core of the mature retrovirus consists of the RNA genome, nucleocapsid protein, reverse transcriptase, protease and integrase [1,2,4]. Viral protease is responsible for viral maturation [2.4]. Immature retroviruses are noninfective since they are incapable of being uncoated and initiating reverse transcription. Given the absence of rough endoplasmic reticulum and polyribosomes in PMN’s [5] it is suspected that these end-stage cells with very short life- spans are unable to complete the translational events needed for viral maturation. Additionally apoptotic induced degenerative nuclear changes possibly aborted the transcription of viral RNA by the provirus formed by the transformation of the viral RNA to DNA and its incorporation into host genome. The absence of inner core contents expressed by its electron-lucent appearance called attention to these nuclear and cytoplasmic liabilities.
Since mature viral retroviruses also were not evident in the bowel luminal contents, bordering PMN’s or enterocytes in the many electron micrographs studied, this infection was probably not acute. Suspected is a low grade or latent retroviral infection of myeloid progenitor cells [6] activated by inflammatory cytokines formed and released in the immediate postoperative period. The precursor viral proteins utilized in the assembly of the immature virus apparently were already manufactured in the biosynthetically active organelles of these cells. The operative events in this patient putatively attracted a multitude of infected neutrophils to the mucosa of the small intestine in the vicinity of the earlier operative site. The enteritis was caused by collateral damage created by the lysis of multiple neutrophil cell membranes and death by apoptosis, typical cytopathic effects produced by viral infections [1]. Water produced and released from the mitochondria of neutrophils by the stepwise reduction of O2 to water by reactive oxygen species (ROS) in apoptosis [7,8] distended the vacuole surrounding these cells producing the halo effect seen microscopically. The degraded PMN’s released proteolytic enzymes, toxic oxygen compounds and possible toxic viral products into the lumen of the encompassing vacuole. The liberation of all these products into the enterocyte cytoplasm elicited by the destruction and/or rupture of the encircling vacuolar membrane caused enteric cytoplasmic edema with organelle degradative changes that created the foamy histologic appearance in the involved enterocytes. The loss of the microvilli with its terminal web and underlying rootlets and the lysis of the underlying organelles permitted unimpeded passage of the damaged and fragmented PMN’s into the intestinal lumen and junctional injury between enterocytes and their basement membranes caused the detachment of the injured enterocytes. We suspect that these lesions were created and/or intensified because the intracytoplasmic location of the neutrophils assured intimate contact of their released toxic substances with the immediately adjacent cytoplasmic organelles. These events represent a paradigm of complex viral pathologies-an infection in an intermediate cell type that ultimately leads to disease in another cell type [2]. It is also represents a type of low grade retroviral infections incapable of producing viremia and continuous infectionstates aborted by its infection in rapidly maturing cells incapable of assembling mature virus and collateral damage that expelled the infected cells (provirus) into the intestinal lumen. Unfortunately, like many examples in pathology such as immune-complex diseases and the fibrosis of chronic pulmonary tuberculosis, the collateral response was more damaging than the primary infection initiating it.
The second problem dealt with the biologic instrumentality responsible for carrying PMN’s into the cytoplasm of the enterocytes. Were we witnessing phagocytosis or emperipolesis? Enterocytes are non-professional phagocytic cells capable of phagocytizing bacteria and macromolecular substances from the intestinal luminal contents [9]. This putatively occurs at the apical aspect of the enterocytes. Ultrastructural evaluation showed intact neutrophils in the cytoplasm of the basal aspect of the enterocytes; while PMN’s exhibiting degenerative changes were evident in the mid and subapical zones of these cells. These observations suggest that the PMN’s entered the enterocyte from adjacent capillaries through its basilar cell membrane to be terminated as they apically ascended. In phagocytosis the endocytosed cell comes to lie in a heterophagosome. It is destroyed and digested following fusion of primary lysosomes to the phagosome. In our case the endocytosed neutrophils were terminated by the viral infection releasing toxic substances that injured and destroyed the supposed phagocytic enterocyte-a reversed situation to that occurring in phagocytosis. Moreover, we are not aware of any reports of enterocytes phagocytizing PMN’s- the phagocytosis of professional phagocytes by non-professional phagocytic cells. Indeed the phagocytic ability of enterocytes is very limited. Effete PMN’s are phagocytized by macrophages. Macrophages were abundant in the lamina propria but PMN’s were absent at this site. The above considerations militates against the notion that phagocytosis is the mechanism inserting PMN’s into enterocyte cytoplasm. The remaining recognized method is emperipolesis. This is an active process whereby one cell, the visitor cell, penetrates and wanders through the cytoplasm of another, the host cell, but classically the visitor cell is not phagocytized and destroyed by lysosomal enzymes and typically this process has no physiologic consequence to either cell [10-12]. Indeed the absence or rarity of cell death of the visiting cell is a major criterion for differentiating emperpolesis from phagocytosis. The penetrated cells most often reported are histiocytes (histiocytic emperipolesis) and megakaryocytes (megakaryocytic emperipolesis). Lymphocytes engulfed by the former are found in Rosai-Dorfman disease [13] and PMN’s by the latter in myelodysplastic disorders [14]. Emperipolesis by PMN’s also occurs in certain neoplasms such as giant cell tumor of the lung and in lymphomas [15]. Emperipolesis has been reported in certain intestinal disorders such celiac disease and lymphocytic colitis but the visiting cells have been lymphocytes. The emperipolesis in this case differs from classic emperipolesis in the following ways. The enterocyte host cell is entered by a visiting neutrophil a finding to our knowledge not previously reported. Secondly both host and visiting cell die in contrast to classic emperipoleis and these deaths have serious pathologic consequences. Thirdly the penetrating PMN is infected with a virus. Virally infected cells in emperipolesis are reported in Rosai-Dorfman disease as Parvovirus B19 detected in visiting lymphocytes and in Rabbit hemorrhagic disease where possibly immature calicivirus occur in rabbit heterophils [16,17]. The biologic events inciting emperipolesis and its purpose(s) have not been definitively defined. In myelodysplastic diseases, emperipolesis is believed connected to the increased expression of P-selectin (cell adhesion molecule) that allowed neutrophil sequestration on the outer surface of megakaryocytes, thus promoting increased neutrophilsmegakaryocytes interactions [11]. Wang and Li postulate that emperipolesis acts as a medium or pathway to mediate natural killer cell-mediated tumor cell death [18]. However, the interplay of visiting cells within host cells in this article does not involve megakaryocytes, tumor cells, lymphocytes or natural killer cells. Rather its defining characteristic is a viral infection that targets the myeloid cell line to create a novel double suicidal variant of emperipolesis, involving both host and visiting cells. The term suicidal emperipolesis was first used to describe the destruction of autoreactive visiting CD8 T cells in intact hepatocytes in order to induce tolerance to antigens produced by liver infections and thereby prevent the development of autoimmune hepatitis [19].
Differential Diagnosis
The morphologic findings in this case are similar to those encountered in acute viral infections of the bowel characterized by villus blunting and broadening with degenerative changes and sloughing of surface enterocytes [20]. What distinguishes these viral infections, where intracellular lymphocytes are evident in the surface and glandular epithelium from our retrovirus infection is the suicidal emperipolesis of PMN’s that principally develops in the luminal facing enterocytes. Concurrent emperipolesis of the glandular enterocytes is sparse. The necrotic PMN’s are widely surrounded by halos and distributed in foamy appearing host enterocytes, a pseudomembrane forms with and finally ultrastructural examination identifies the causative immature retrovirus.
Conclusion
• Retrovirus exclusively assembled in PMN’s, as illustrated in this report, are immature. They cannot cause viremia and infect other cells thereby bequeathing a benign clinical course to this type of infection.
• However, such infections can became clinically recognizable by initiating collateral damage through aggregation at one site and the promotion of suicidal emperipolesis. Suicidal emperipolesis in isolated organ cells would not cause significant tissue damage and therefore would be asymptomatic.
• The definition of emperipolesis requires further expansion to include visiting and host cell death that can cause pathologic lesions.
• Specific viral infections involving hematopoietic cells can trigger emperipolesis. This process may be viewed in two ways. One as a host defense mechanism originated to neutralize the infection or contrarily, as a viral survival stratagem created to cause collateral injury for the purpose of prolonging or initiating new infections.
• Suicidal emperipolesis of retrovirus -infected neutrophils in the bowel should be included in the differential diagnosis of pseudomembranous enteritis.
Acknowledgement
The authors express their appreciation to Dr. David Walker for his help in identifying the causative agent as a retrovirus, to Dr. Gerald Campbell for his help in acquiring the clinical history of this patient and Jason Slavin for his invaluable photographic assistance. No funding agent played a role in the preparation or writing of this manuscript.
References
- Goff SP (2001) Retroviridae: The retroviruses and their replication in Field Virology, DM Knipe and PM Howley(4thEds.), Lippincott Williamsand Wilkins, Philadelphia pp 1871-1940.
- Cloyd MW (1996) Human Retroviruses in Medical Microbiology, Baron S., (4thEds.),Galveston (TX): University of Texas Medical Branch at Galveston.
- Slavin RE, Christie JD, Swedo J, Powell LC (1986) Locally aggressive granular cell tumor causing priapism of the crus of the clitoris. A light and ultrastructural study, with observations concerning the pathogenesis of fibrosis of the corpus cavernosum in priapism. Am J SurgPathol 10: 497-507.
- Cawley JC, Hayhoe FG (1973) Ultrastructure of Haemic cells. WB Saunders, London.
- Banerjee P, Samuelson E, Feuer G (2010) The Role of hematopoietic progenitor cells in retroviral pathogenesis; in Recent Advances in Human retroviruses: Principles of Replication and Pathogenesis, Advances in retroviral research. Lever AML, Jeang K-T, Berkhout B., Eds., World Scientific Publishing Co. Pte. Ltd Singapore.
- Brookes PS, Yoon Y, Robotham JL, Anders MW, Sheu SS (2004) Calcium, ATP, and ROS: a mitochondrial love-hate triangle. Am J Physiol Cell Physiol 287: C817-833.
- Green DR, Kroemer G (2004) The pathophysiology of mitochondrial cell death. Science 305: 626-629.
- Neal MD, Leaphart C, Levy R, Prince J, Billiar TR, et al. (2006) Enterocyte TLR4 mediates phagocytosis and translocation of bacteria across the intestinal barrier. J Immunol 176: 3070-3079.
- Humble JG, Jayne WH, Pulvertaft RJ (1956) Biological interaction between lymphocytes and other cells. Br J Haematol 2: 283-294.
- Rastogi V, Sharma R, Misra SR, Yadav L, Sharma V (2014) Emperipolesis - a review. J ClinDiagn Res 8: ZM01-02.
- Ghadially FN (1988) Endocytotic structures and cell processes in Ultrastructural pathology of the cell and matrix, (3rdEditn.), Butterworths, London.
- Iyer VK, Handa KK, Sharma MC (2009) Variable extent of Emperipolesis in the evolution of Rosai-Dorfman disease: Diagnostic and pathogenetic implications. J Cytol 26:111-116.
- Sable MN, Sehgal K, Gadage VS, Subramanian PG, Gujral S (2009) Megakaryocytic emperipolesis: a histological finding in myelodysplastic syndrome. Indian J PatholMicrobiol 52: 599-600.
- SaadOsmani Z, Susan H, Hedge P (2010) Emperipolesis in giant cell carcinoma of lung. Community Oncology 17:233-235.
- Mehraein Y, Wagner M, Remberger K, Füzesi L, Middel P, et al. (2006) Parvovirus B19 detected in Rosai-Dorfman disease in nodal and extranodal manifestations. J ClinPathol 59: 1320-1326.
- Alexandrov M, Peshev R, Lasarova S, Doumanova L, Tchorbanov A, Bostandjieva R. (2009) Heterophilemperipolesis in rabbit haemorrhagic disease. Bulgarian J Vet Med 12: 43-53.
- Wang X, Li W (1987) Mechanisms of natural killer cell-mediated tumor cell cytolysis at a single cell level. J Med Coll PLA 2:107-117.
- Benseler V, Warren A, Vo M, Holz LE, Tay SS, et al. (2011) Hepatocyte entry leads to degradation of autoreactive CD8 T cells. ProcNatlAcadSci U S A 108: 16735-16740.
- Lamps LW (2010) Surgical pathology of the gastrointestinal system: bacterial, fungal, viral and parasitic infections, Springer-Verlag, New York
Citation: Slavin RE, John Swedo BS, Julie Wen MS (2015) Retrovirus Pseudomembranous Atrophic Enteritis caused by Suicidal Emperipolesis; A Light and Ultrastructural Study . J Clin Exp Pathol 5:257. DOI: 10.4172/2161-0681.1000257
Copyright: © 2015, Slavin RE, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 14125
- [From(publication date): 12-2015 - Apr 04, 2025]
- Breakdown by view type
- HTML page views: 13204
- PDF downloads: 921