Research Article Open Access
Retrospective-Prospective Study on Efficacy & Safety of Entecavir in Chronic Hepatitis B West Asian Patients with Genotype D
Akrouf K1, Gad A1,2*, Ibraheem E3, Kassem M3, Abdul Monem FM3 and El Kholy N31Amiri Hospital, Kuwait, Thanian al Ghanem Gastrointestinal Centre, Kuwait
2Suez Canal University School of Medicine, Internal Medicine Department, Egypt
3Al Azhar University, Tropical Medicine Department, Egypt
- *Corresponding Author:
- Gad A
Internal Medicine Department, Suez Canal University School of Medicine
Al-Nouras City, Blok 29, Group 2, Ismailia, Egypt
Tel: +96565935445, +201097226108
Fax: +96522469628
E-mail: amalahgad@gmail.com
Received date: February 10, 2017; Accepted date: March 02, 2017; Published date: March 06, 2017
Citation: Akrouf K, Gad A, Ibraheem E, Kassem M, Abdul MFM, et al. (2017) Retrospective-Prospective Study on Efficacy & Safety of Entecavir in Chronic Hepatitis B West Asian Patients with Genotype D. J Clin Infect Dis Pract 2:117. doi:10.4172/2476-213X.1000117
Copyright: © 2017 Akrouf K, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Clinical Infectious Diseases & Practice
Abstract
Aim: To assess the long term efficacy and safety of Entecavir in the treatment of CHB in Asian-Arabic patients with genotype D, for both nucleoside-naïve as well as experienced, comparing HBeAg positive and negative group.
Methods: This study included 70 CHB consecutive patients who were maintained on Entecavir for at least 36 months retrospectively and followed up prospectively every 3 months for a total of 18 months, at 2 centers in Kuwait (October 2012 and April 2014).
Results: It showed that 23 (32.8%) were HBeAg +ve. All were found to be HBV genotype D, 47.1% naive, 22 (31.4%) females, with a mean age of 42.9 ± (13), 14 (20%) cirrhotic and 1(1.4%) decompensated. There were no significant differences in the pre-treatment HBV-DNA level among HBeAg+ve and HBeAg-ve group, with a mean± SD log 10 of 7.9 ± 5.4 and 7.4 ± 5.0 respectively (P=0.237). There was a significant viral load reduction in both groups after 6 months of treatment with Entecavir. The reduction was more pronounced in the HBeAg–ve compared to HBeAg +ve group. In HBeAg +ve, 19 patients (82.61%) had complete HBV-DNA suppression after a median period of 7 month, while in HBeAg –ve group; all (100%) had complete HBV-DNA suppression after a median period of 5 month duration (P<0.05). None showed primary non response to Entecavir in both groups. In the HBeAg-ve group; one (2.13%) had HBsAg loss at 45 month compared to non in HBeAg+ve group (P<0.001), while in the HBeAg+ve group; 5 (21.74%) showed HBeAg clearance after a median of 16 month during Entecavir treatment. Multivariate analysis identified HBeAg negative status, pre-treatment as the only independent factor affecting complete viral suppression on Entecavir treatment. The drug showed 100% safety, as there were no serious adverse events throughout 54 month of follow up. None showed hepatic decompensation, HCC or need for liver transplantation during follow up. Also, there was no reported death throughout study period in both groups.
Conclusions: In real life data; long term Entecavir treatment for up to 54 months in West Asian CHB patients, with genotype D suppressed HBV-DNA to an undetectable level in 100% of HBeAg-ve, compared to 82% in HBeAg +ve group. Entecavir is considered an effective and safe choice on long term use for treatment CHB patients.
Keywords
HBV; Antigen; Antibody; Sero-conversion; Side effects
Introduction
Hepatitis B virus (HBV) is a partially double spiral type DNA virus. Globally, approximately over 400 million individuals are infected with hepatitis B. HBV is known as one of the most important carcinogens. Every year, over one million individuals die due to HBV-related causes [1].
It is estimated that 5% to 15% of the population are chronic carriers of hepatitis B in developing countries, whereas in North America and Western Europe only 1% of the population is chronically infected. Chronic HBV infection is highly prevalent in sub-Saharan Africa, Southeast Asia, the Eastern Mediterranean region, the Amazon basin and the Caribbean [2].
Perinatal transmission is believed to be the most important mode in regions of developing world with high and intermediate HBV prevalence rates. HBV remains a major nosocomial pathogen in many hospitals. Transmission may occur due to unsafe injections, blood transfusions and lack of awareness of infection control. Sexual contact also accounts for some HBV transmission [3]. The spectrum of disease and natural history of chronic HBV infection is diverse and variable, ranging from a low viremic inactive carrier state to progressive chronic hepatitis, which may evolve to cirrhosis and HCC [4].
Carriers of HBV are at increased risk of developing cirrhosis, hepatic decompensation and hepatocellular carcinoma (HCC). Although most carriers will not develop hepatic complications from chronic hepatitis B, 15% to 40% will develop serious squeal during their lifetime [5].
HBV-related end stage liver disease or HCC are responsible for over one million deaths per year and currently represent 5-10% of cases of liver transplantation [6].
Primary objective in hepatitis B treatment is to improve clinical and histological progression and to provide virus eradication. For many years, interferon, Lamivudine, Adefovir, Telbivudine, Entecavir and tenofovir still used in treatment of chronic hepatitis B. Entecavir and tenofovir are potent antiviral drugs. The treatment with these drugs leads to normalization in liver enzymes, improvement in liver histology, HBsAg and HBeAg loss and undetectable HBV-DNA levels [7,8]. Elevation of the decreased HBV-DNA during treatment is attributed to drug resistance or noncompliance [9].
Entecavir, a new guanosine nucleoside analogue with specific activity against HBV-DNA polymerase, represents a third agent within the nucleoside/nucleotide HBV polymerase inhibitor class. It has distinct advantages over Lamivudine and Adefovir Dipivoxil: it has a three-step mechanism of action, is the most potent inhibitor of HBVDNA polymerase, is not associated with any major adverse effects and has a limited potential for resistance. In clinical trials, Entecavir was superior to Lamivudine in all primary endpoints in both nucleosidenaive and Lamivudine-refractory hepatitis B e-antigen (HBeAg)- positive and HBeAg-negative patients [10].
Entecavir should be considered a first or second-line treatment option for the management of HBeAg-positive or HbeAg-negative nucleoside -naive or Lamivudine-refractory CHB patients [10].
This study aimed to assess the efficacy and safety of Entecavir in the treatment of chronic viral hepatitis B (CHB) and to check therapeutic end points for Entecavir and its predictors in Kuwait.
Material and Methods
This is a retrospective cohort-longitudinal study to assess the efficacy and safety of Entecavir in the treatment of CHB Asian-Arabic patients (in Kuwait) for 54 months who were nucleosides-naïve and experienced patients, comparing HBeAg positive and HBeAg-ve subgroup. A total of 70 patients were consecutively confronted according to selection criteria (mentioned below) at Gastroenterology centers of Amiri hospital and Al Adan hospital in Kuwait between October 2012 and April 2014.
*Inclusion criteria
• The following patients were included:
• Consecutively confronted adult (>18-year-old)
• Kuwaity CHB patients
• Currently on Entecavir therapy
*Exclusion criteria
• The following patients were excluded:
• IgM anti-HBc positive HCV-Ab positive
• positive serology for HDV
• HCC patients
• post-transplanted patients
Entecavir dosing was decided according to APASL 2012 with dose modification in some patients according to their renal function. In those with normal renal function; naïve patients received 0.5 mg while experienced ones received 1 mg daily dose [10,11]. hepatocellular carcinoma was diagnosed based on histopathology, Tri-phasic CT findings and/or S. AFP level.
End point assessment
Virological response was defined as undetectable HBV-DNA using branched chain DNA assay in IU/ml with HBV-DNA<20 IU/ml [11].
2) Biochemical response: was defined as ALT/AST normalization.
3) Sonographic response: was defined as improvement echogenicity, nodularity, hepatic size or signs of portal hypertension on treatment (size of portal, splenic veins and ascites).
A full history were taken from all patients including sociodemographic data, other co-morbid disease (s), current drug history, previous HBV treatment, drug adherence to treatment. Possible side effects of the Entecavir were checked every 3-6 months. A thorough clinical examination was performed to all patients to detect signs of liver cirrhosis and hepatic decompensation or evidence of other organ system affection.
Biochemical assessment was carried out every 3-6 months; platelet count, (Normal range 150-410 109 /L), INR, (Normal range up to 1.2), Fasting Blood Sugar (Normal range 3.9-6.1 mmol./L), ALT, (range 10-60 IU/L), AST (range 10-42 IU/L), total S. bilirubin (range 3-25 umol./L), S. albumin (range 35-48 g/L), BUN (range 2.5-7.1 mmol./L), S. creatinine (range 62-115 umol/L). Reference for FBS, LFT and RFT is quoted from manufacture documentation in the leaflets and user manual guides the machine used is Beckman Coulter instruments DXC 800).
Virological assessment was carried out every 3-6 months for HBsAg, HBsAb, HBeAg, HBeAb and HBV-DNA (using quantitative PCR. complete viral suppression was considered at 20 IU/ml, according to Kit Roche diagnostic range). Alpha fetoprotein (AFP) was also assessed every 3-6 months (normal range 2-10 ng/ml), quoted from manufacture documentation in the leaflets and user manual guides according to the international accepted range. Conventional abdominal ultra-sonography was done using 2 apparatus of Aquson Antas/ Siemens by convex curved probes equivalent to 4.1 MHz for assessment liver cirrhosis. Cirrhosis was diagnosed if coarse texture liver or shrunken liver with either splenomegaly or portal vein diameter more than 14 mm.
Duration of treatment
For the CHB Entecavir long-term cohort, duration of treatment was defined as the total duration in months from the first dose Entecavir to the last date of dosing follow up.
Assessment of entecavir efficacy
Efficacy assessments included the following endpoints: HBV-DNA ≤ 20 IU/ml, HbsAg/Ab Seroconversion and HbeAg/Ab Seroconversion [11].
Drug safety assessment
Safety of Entecavir was assessed throughout the study period (every 3-6 months) while on treatment which included ongoing hepatic events, adverse events (headache, fatigue, dizziness, nausea, vomiting diarrhea, insomnia and hematuria), treatment discontinuation due to adverse events, LFT worsening on treatment (e.g. hyperbilirubinemia or hepatic decompansation) or death while on-treatment.
Statistical analyses
All data analyses are descriptive. Tabulations by treatment groups are presented for each of the efficacy and safety variables. Continuous variables are summarized using the mean and the median values. Binary variables are summarized by counts and percentages. Efficacy endpoints were assessed among patients. Data were fed to the computer using Statistical package for Social Science (SPSS version). Simple descriptive statistics such as frequency and percentage distribution for categorical variables and mean with the standard deviation for quantitative variables were used. The median was also calculated for all scores and none normally distributed variables. For comparative purposes Chi-square and Fisher exact test (when the expected number of a cell is less than 5 in a two by two table) tests were used for categorical variables, student-t test and Mann Whitney (for discrete variables or those not normally distributed) tests for quantitative variables. For comparison between groups, analysis was initially carried out based on a series of univariate analysis comparison. In order to control simultaneously for possible confounding effect of the variables, multiple, logistic regression was used for the final analysis. In the univariate analysis, the association between exposure and outcome was expressed in terms of odds ratio (OR) together with their 95% confidence interval (95% CI). Appropriate inferential statistics was done with level of significance of (0.05). Quality control of data was assured through double check by supervisors to assure accuracy and reliability of data.
Assay methodology
Serum HBV-DNA was quantified by a central laboratory (AL sahibmain capital virology lab) using PCR assay by Roche Molecular Systems, Inc.-Branchburg (lower limit of detection 20 IU/mL). HBV serology (HBsAg, HBsAb, HBeAg, and HBeAb) were assessed using, enzyme immunoassay (Abbott Diagnostics, Germany). HBV-DNA Genotype involved PCR amplification of the HBV reverse transcriptase domain, followed by nucleotide sequence analysis. (Roche diagnostic Kits).
Ethical considerations
All administrative approvals were taken before the conduction of the study. All the examined patients was informed about the aim of the study and informing them that all data were treated in confidential manner; written/oral consent was taken from every examined patient.
Results
The present study included 70 Asian-Arabic adult patients with chronic hepatitis B infection referred to gastroenterology department, Thunayan Al-Ghanem center in Amiri hospital and gastroenterology department in Al-Adan hospital in Kuwait. Results showed that 23 (32.8) were HBeAg +ve. All were found to be of genotype D, 47.1% naive, 22 (31.4%) females, with a mean age of 42.9 ± (13), 14 (20%) showed evidence of cirrhosis and one patient was (1.4%) decompensated.
Background profile
There was no significant difference among HBeAg positive and negative sub-groups in their background data, except for the frequency of co-morbidities (Table 1). As in HBeAg +ve group; 20 (87%) had no co-morbid condition compared to 26 (55.3%) of HBeAg –ve group (P=0.009). There was a statistically significant difference in their previous NUC experience (16, 69.6% vs. 21, 44.7%, p<0.05) respectively (Table 2). There was no significant difference in the pretreatment ALT, AST, S. bilirubin, S. albumin levels, INR or platelets count among the two groups (Table 3). In addition, no significant difference in the pre-treatment ultrasonographic findings was found among the two groups Also, there was no significant differences in the pre-treatment HBV-DNA level (Table 4) with a mean ± SD log 10 of 7.9 ± 5.4 and 7.4 ± 5.0 respectively (P=0.237).
| HBeA+ve N=23 | HBeAg-ve N=47 | Total) (n=70) | p. Value | ||
|---|---|---|---|---|---|
| *Age in yrs.: Mean (± SD) | 37(13.42) | 48.81(12.1) | 42.9(13) |    0.001 | |
| Range | (20-77) | (23-83) | (20-83) | ||
| Female n(%) | 6(26.1) | 16(34.0) | 22(31.4) | Â Â Â 0.501 | |
| Co-morbidity: n(%) | 0.009 | ||||
| Non | 20 (87) | 26 (55.3) | 46 (65.7) | ||
| DM | 2 (8.7) | 3 (6.4) | 5 (7.1) | ||
| HTN | 0 | 3 (6.4) | 3 (4.3) | ||
| Multiple medical problems | 1 (4.3) | 15 (31.9) | 16 (22.9) | ||
Table 1: Comparison of background data among the studied group.
| HBeAg +ve N=23 |
HBeAg-ve N=47 | Total | Â p. value | ||||
|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | ||
| Naïve | 7 | 30.4 | 26 | 55.3 | 33 | 47.1 | |
| Experienced | 16 | 69.6 | 21 | 44.7 | 37 | 52.9 | <0.05 |
| *This table shows that there was statistically significance difference with regards to previous NUC exposure in the studied groups. | |||||||
Table 2: Demonstration of the frequency of Naïve versus experienced CHB patients.
| Group ( 1 ) HBeAg+ve N = 23 | Group ( 2 ) HBeAg-ve N = 47 | p.Value | |||
|---|---|---|---|---|---|
| Mean | ± SD | Mean | ± SD | ||
| ALT (IU/L) | 51.13 | 22.82 | 47.98 | 99.37 | 0.837 |
| AST (IU/L) | 38.87 | 19.63 | 44.68 | 83.86 | 0.654 |
| T.BiL (Umol/L) | 21.08 | 16.48 | 17.44 | 13.58 | 0.365 |
| S.Alb ( g/L) | Â 37.91 | Â 4.33 | 37.63 | 10.51 | 0.875 |
| PLT (109/L) | 237.04 | 67.25 | 225.34 | 66.62 | 0.496 |
| INR | 1.09 | 0.15 | 1.17 | 0.57 | 0.384 |
| AFP (ng / L ) | 2.16 | 1.53 | 2.90 | 2.24 | 0.158 |
| *This Table shows that there was no statistically difference as regards Liver function test, PLT, and AFP in the studied groups. | |||||
Table 3: Comparison of liver function, mean PLT and AFP in the studied groups pre-treatment.
| Group(1) N=23 | Group(2) N=47 | p. value | |||
|---|---|---|---|---|---|
| Mean | ± SD | Mean | ± SD | ||
| Mean HBV DNA Log 10(IU/ml) | 7.9 ± | 206,084,000.1 | 28,774,877.94 | 110,100,715.5 | 0.237 |
| Genotype (D) | N | % | N | % | |
| 23 | 100 | 47 | 100 | ||
| *This table shows that there was no statistically difference as regards HBV-DNA level in the studied groups and all patients were Genotype | |||||
Table 4: HBV-DNA level and geno-type in the studied groups before start of treatment.
Biochemical response
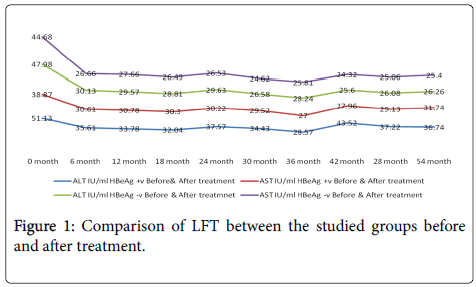
There was a statistically significant improvement in the mean ALT, PLT and S.albumen in HBeAg +ve group throughout the study period (54 months). While, there was no significant changes in LFT or PLT count in the HBeAg -ve group (Figure 1).
There was a statistically significant improvement in the mean ALT & AST in the HBeAg +ve compared to the HBeAg -ve group throughout the study period (54 months).
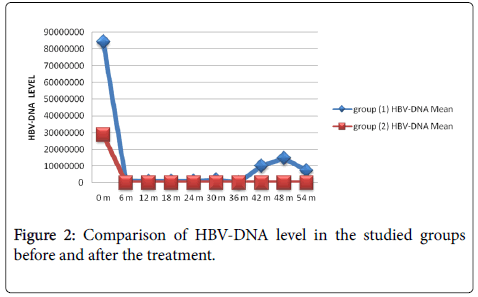
Virological response: There was a statistically significant reduction in HBV viral load (Table 5) in the HBeAg –ve compared to the HBeAg +ve group throughout the follow up period (Figure 2).
| Marker | HBeAg +ve N =23 |  HBeAg âÂ?Â?ve N= 47 | p. Value | ||
|---|---|---|---|---|---|
| N | % | N | % | ||
| *HBsAb seroconvert ion | 0 | 0 | 1 | 2.13 | <0.001 |
| **HBeAb seroconvert ion | 5 | 21.74 | - | - | |
| Complete viral suppression | 19 | 82.6 | 47 | 100 | <0.001 |
| *One patient from group (2) was seroconverted from HBsAg to HBsAb after 48 months of being on Entecavir. **Five patients from group (1) were seroconverted from HBeAg to HBeAb ; 1 after 12 months , 3 after 15 months & 1 after 24 months from start of treatment with Entecavir. |
|||||
Table 5: HB viral response in the studied groups after treatment.
There was a statistically significant reduction in HBV viral load in the HBeAg –ve compared to the HBeAg +ve group throughout the follow up period.
In HBeAg +ve, 19 patients (82.61%) had complete HBV-DNA suppression after a median period of 7 month. The other 4 (17.39%) had showed secondary non-response after a median period of 24 months, while in HBeAg –ve group; all 47 (100%) had complete HBVDNA suppression after a median period of 5 months.
Serological response
All HBeAg +ve, patients (100%) remained HBsAg positive. However, 5 of them (21.74%) HBeAg /Ab seroconverted after a median period of 13 month. While in HBeAg –ve patients, one patient (2.13%) had HBsAg/Ab seroconversion after 45 months of treatment with Entecavir. this patient was previously treated with pegylated interferon then Lamivudine with Adefovir, but was non-responder. On Entecavir therapy; he showed complete viral suppression after 3 months only.
Sonographic response
In HBeAg+ve group; one (4.35%) showed improvement of US detected pre-treatment (regression in hepatic size) three (13.05%) showed regression of pre-treatment heterogeneous texture to homogenous pattern post treatment, while 6 patients (26.10%) showed no improvement. At the Meanwhile, 17 patients (73.91%) maintained normal hepatic texture pre and post treatment till the end of follow up period (54 months).
In HBeAg-ve group, 2 (4.24%) showed improvement of US detected pre-treatment hepatomegaly to normal sized liver post-treatment, while 4 (8.48%) showed improvement in texture. On the other hand, 25 (53.19) maintained normal hepatic texture pre and post treatment till the end of follow up period (54 months).
Factors associated with undetectable HBV-DNA after 54 months follow up:
As shown in univariate analysis (Table 6); four factors affected viral load suppression (Age, P=0.0014, ALT, P=0.016, AST, P=0.006, HBeAg–ve, P=0.00), while in multivariate analysis; pretreatment hepatitis Antigen status (HBeAg –ve) was the only independent factor affecting viral load suppression (OR=16.9, 95% CI 20.287 (0.0-∞), P=0.000).
| Variables | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| OR(95%CI) | P. Value | OR(95%CI) | p. Value | |
| Age | 6.044 (4.31-15.6) | 0.014 | 0.286 | |
| Gender | 0.081 (0.54-3.2) | 0.837 | 0.837 | |
| Drug History | 0.014 (0.65-3.69) | 0.906 | 0.480 | |
| Past Medical History | 0.162(1.35- 4.97) | 0.689 | 0.469 | |
| ALT | 5.751(0.07-40.91) | 0.016 | 0.529 | |
| AST | 7.428(0.58-36.87) | 0.006 | 0.178 | |
| T. BiL | 2.792 (0.76-9.93) | 0.126 | 0.126 | |
| S. AlB | 4.53(602 -34.72) | 0.132 | 0.134 | |
| AFP | 2.123 (0.27-15.97) | 0.699 | 0.699 | |
| U/S Finding | 3.372(0.69-13.58) | 0.066 | 0.217 | |
| Seroconversion | 0.398(0.176-1.46) | 0.528 | 0.512 | |
| HBeAg negative | 16.5 (0.0--8) | 0.000 | 20.287( 0.0---8) | 0.000 |
| *In Univariate analysis 4 factors affected HBV viral load suppression, while in multivariate analysis the only independent factor was HBeAg âÂ?Â?ve. | ||||
Table 6: Univariate and multivariate analysis of the studied groups.
Safety and Adverse effects
On-treatment symptoms
In HBeAg +ve group; 21(91%) were asymptomatic, one patient had fatigue (4.3%), and one another patient (4.3%) had fatigue, abdominal pain, jaundice and distention before treatment that did not show evidence of improvement on Entecavir therapy.
In HBeAg -ve group; 44 (93.6%) were asymptomatic, one patient (2.1%) had fatigue, another patient (2.1%) had abdominal pain, and also one patient (2.1%) had both jaundice and fatigue before treatment that did not improve on Entecavir therapy. As overall; 65 patients (92.9%) from both groups were asymptomatic throughout treatment follow up period (54 month) with no adverse hepatic events, treatment discontinuations or hepatic decompensation detected throughout follow up period. No deaths reported on-treatment for a total of 54 month follow up (Table 7).
| Group ( 1 ) HBeAg +ve N = 23 |
Group ( 2 ) HBeAg-ve N = 47 |
Total | p. Value | ||||
|---|---|---|---|---|---|---|---|
| N | % | N | % | ||||
| Asymptomatic | 21 | 91 | 44 | 93.6 | 65 | 92.9 | 1.00 |
| Fatigue | 1 | 4.3 | 1 | 2.1 | 2 | 2.9 | 1.00 |
| Abdominal pain | 0 | 0 | 1 | 2.1 | 1 | 1.4 | 1.00 |
| Jaundice + fatigue | 0 | 0 | 1 | 2.1 | 1 | 1.4 | 1.00 |
| *All symptoms | 1 | 4.3 | 0 | 0 | 1 | 1.4 | 1.00 |
| * Having all symptoms of fatigue +abdominal pain +jaundice and distention *this table shows that there were no statistically significance differences in the symptoms between the studied groups. | |||||||
Table 7: Frequency of occurrence of different symptoms during Entecavir therapy.
Discussion
Current CHB treatment recommendations advocate sustained suppression of HBV-DNA as the primary goal of antiviral therapy. A key requirement for maintaining long-term HBV-DNA suppression is the avoidance of resistance to the antiviral [11].
Patients with persistently elevated viral load are at the greatest risk of developing liver disease progression and adverse outcomes. It has also been shown that even patients with low-level HBV-DNA viremia 104 to 105 copies/mL) are at risk of fibrosis, cirrhosis, and HCC [12].
In 2010, interferon (IFN)-based therapies (conventional IFN and pegylated-IFN-alpha-2a or pegylated-IFN-alpha-2b [pegIFN-α2a or pegIFN-α2b, respectively]) and the nucleos(t)ide; Lamivudine, Adefovir, Telbivudine, and Entecavir are currently recommended for the treatment of patients with hepatitis B e-antigen (HBeAg)-positive CHB [13].
For patients with HBeAg-negative CHB and those with cirrhosis; nucleos(t)ide analogs with high potency and low resistance rates, such as Entecavir, are preferred. The Asian-Pacific consensus statement on the management of CHB recommends that conventional IFN or pegIFN-α2a, Lamivudine, Adefovir, Entecavir, Telbivudine, and the nucleotide analogue, Tenofovir, can all be considered for initial therapy in patients without liver decompensation [14].
According to data released by the market research firm, IMS Health, Entecavir currently accounts for 26% of the market share for HBV treatment in the People’s Republic of China, compared with 39% for Adefovir. Lamivudine and Telbivudine, account for 22% and 8% respectively of the market share [15].
It is demonstrated that long-term Entecavir therapy in HBeAg +v achieved and maintained HBV-DNA suppression. At fifth year, 94% of patients in the Entecavir long-term cohort had HBV-DNA 300 copies/ml. The importance of maintaining prolonged HBV-DNA suppression to avoid or minimize the long-term complications of CHBV has been recognized in several long-term studies of disease progression and outcome [16].
In our study, we were able to demonstrate Entecavir efficacy and safety in a real world study of a cohort of Asian Arabic patients (Kuwaiti) with genotype D. For our knowledge, this is the first long term retrospective-prospective study from west Asia in this regards. However, a limited study from Saudi Arabia showed conflicting results compared to ours. It was a retrospective study only, with a shorter follow up period (48 wks.) and smaller population, (43 patients) which makes it difficult to compare results [17].
The biochemical response to treatment in our data was rather promising; as there was significant decrease in mean ALT after 6 months of starting Entecavir from (51.13-35.61 IU/L, P=0.034) in the HB eAg +ve compared to (47.98-30.13 IU/L) in the HB eAg -ve group respectively. All the studied patients showed normal ALT at the end of follow up period (54 month) with a mean of 36.74 and 26.26 IU/L in both groups respectively. Such a finding coincides with other previous studies that showed a significant reduction of the mean ALT on treatment with Entecavir [12,17-19].
Similarly, Luo et al. from china showed, in a retrospective study of 230 nucleos(t)ide naïve CHB patients; an ALT normalization throughout the study period (5 yr.) from 73.9% at first year to 100% at 5th year of treatment [18].
Our results showed 82.61% complete HBV-DNA suppression in HBeAg+ve, compared to 100% in HBeAg-ve patients. While, in Al- Ashqar et al., from Saudi-Arabia [17], only 20% of the HBeAg-+ve patients achieved undetectable HBV-DNA at 48 weeks compared to 60.7% in the HBeAg-ve ones [17]. The shorter follow up duration in their study compared to ours in addition to the small number of studied patients could stand behind such a difference in results.
While our results coincide with data from Far east and Middle of Asia [18-22]. Chang et al. from Taiwan, showed in their 5 year follow up study of 146 Entecavir treated patients that 94% (88/94) had HBVDNA <300 copies/mL and 80% (78/98) had normalized ALT levels [19].
In addition, Mann et al. reported comparable results after 3 years follow up of Europian CHB patients regarding efficacy and safety of entecavir [23]. In North America, Morris and his colleagues from Toronto-Canada, found in his study of (HBeAg)-positive, Lamivudine refractory patients that switching to Entecavir was superior to continued Lamivudine at week 48 it regards to histological improvement, viral load reduction, and ALT normalization [24]. Results from South America are also comparable to our data regarding efficacy and safety of entecavir [25,26]. However, a similar but smaller study from Morocco, North Africa showed only 85% viral response in 48 weeks follow up of 41 patients on Entecavir therapy [27].
We presented 21.74% HBeAg sero-conversion, and only 2.13% HBsAg sero-conversion. These findings coincide with Ting and his colleagues who 23% achieved HBeAg sero-conversion and 1.4% HBsAg sero-conversion during the study [19]. Also similar results were shown by Cheng-Yuan Peng et al., from Taiwan [21]. In addition, they found that complete virological response at 6 months is a favorable predictive of HBeAg loss at 2 years of Entecavir therapy. Therefore, measurement of serum HBV-DNA level at 6 months of Entecavir therapy is optimal to predict HBeAg loss at 2 years of therapy in HBeAg-positive CHB patients [21]. Data from Japan, by Atsushi and his colleagues assured the antiviral potency and viral resistance rate after 4 years of continuous Entecavir treatment in patients with CHB infection. They reported 96% chance of undetectable HBV-DNA with similar rates of HBeAg sero-conversion and HBsAg loss [22].
Multivariate analysis in our study showed that HBeAg status was the only independent factor affecting the suppression of viral load on Entecavir treatment (OR=16.9, 95%). As we showed that the response rate was better in HBeAg -ve patients when compared to HBeAg +ve patients. At the meanwhile, in univariate analysis, It was shown that 4 factors were affecting viral load suppression; Age, ALT, AST and HBeAg–ve status. Similarly, in a Korean national study on 1009 HBV naïve patients: multivariate analysis showed that the pre-treatment (HBeAg) negative status (p<0.001) and lower HBV-DNA (p<0.001) were the predictors of virological response [20].
Our results reported that there were no serious adverse events of Entecavir usage, which corresponds with that of Man and his colleagues; who showed that there were no serious adverse events on Entecavir treatment of naïve CHB patients, followed for a total of 3 years. Also it was associated with >90% chance of HBV-DNA clearance [27]. In a real life study, Jie et al. also reported safety of Entecavir with lack of serious adverse events however; they described few cases of cutaneous adverse events [18].
The most frequent adverse events previously reported for Entecavir were increased ALT, upper respiratory tract infection, headache, abdominal pain, cough, pyrexia, fatigue, and diarrhea.
Ting-Tsung and his colleagues also agreed with our finding regarding safety and tolerability of the drug. None of patients discontinued therapy due to adverse events. One patient experienced an ALT flare and one case of HCC [19]. Jimi and his colleagues reported a 65-year-old woman with facial granulomatous eruption. The patient is known to be HBV carrier for 35 years. Since her serum AST and ALT levels started to be elevated persistently for 3 months; she had been started on Entecavir at a dose of 0.5 mg daily. Two months after initiating the antiviral therapy, she presented with multiple pruritic erythematous papules and telangiectasia on the forehead, both pre-orbital areas and the cheeks [18].
Limitations
The retrospective part of our study with a relatively small number of studied patients could be limitations in such a study, but being a real life data of West Asian, with HBV genotype D has its weight, adding to our daily practical knowledge and experience, and this is a lacking point in the RCT. As in the later; although valid data can be obtained, however it can't be generalized in our daily life practice.
Conclusion
As a real life data; long term Entecavir treatment for up to 54 months in West Asian CHB patients, with genotype D suppressed HBV-DNA to an undetectable level in 100% of the HBeAg -ve, compared to 82% in the HBeAg +ve group. Entecavir is considered an effective and safe choice on long term use for treatment CHB patients.
References
- Kwon H, Lok AS (2011) Hepatitis B therapy. Nat Rev Gastroenterol Hepatol 8: 275-284.
- Abbas Z, Siddiqui AR (2011) Management of hepatitis B in developing countries. World J Hepatol 3: 292-299.
- Lynch P, Pittet D, Borg MA, Mehtar S (2007) Infection control in countries with limited resources. J Hosp Infect 65 Suppl 2: 148-150.
- Ganem D, Prince AM (2004) Hepatitis B virus infection--natural history and clinical consequences. N Engl J Med 350: 1118-1129.
- Bosch FX, Ribes J, Cléries R, DÃaz M (2005) Epidemiology of hepatocellular carcinoma.Clin Liver Dis 9: 191-211.
- Hoofnagle JH, Doo E, Liang TJ, Fleischer R, Lok AS (2007) Management of hepatitis B: summary of a clinical research workshop. Hepatology 45: 1056-1075.
- EASL Clinical Practice Guidelines (2009) Management of chronic hepatitis B. J Hepatol 50: 227-242.
- Kim SS, Cheong JY, Cho SW (2011) Current Nucleos(t)ide Analogue Therapy for Chronic Hepatitis B. Gut Liver 5: 278-287.
- Lok AS, McMahon BJ (2007) Chronic hepatitis B. Hepatology 45: 507-539.
- Rivkin A (2007) Entecavir: a new nucleoside analogue for the treatment of chronic hepatitis B. Drugs Today (Barc) 43: 201-220.
- Lok ASF, McMahon BJ, Brown RS, Wong JB, Ahmed AT, et al. (2016) Antiviral therapy for chronic hepatitis B viral infection in adults: A systematic review and meta-analysis. Hepatology 63: 284-306.
- Yuen MF, Yuan HJ, Wong DK, Yuen JC, Wong WM, et al. (2005) Prognostic determinants for chronic hepatitis B in Asians: therapeutic implications. Gut 54: 1610-1614.
- Chinese Society of Hepatology and Chinese Society of Infectious Diseases, Chinese Medical Association (2011) The guideline of prevention and treatment for chronic hepatitis B (2010 version). Zhonghua Gan Zang Bing Za Zhi 19: 13-24.
- Liaw YF, Kao JH, Piratvisuth T, Chan HLY, Chien RN, et al. (2012) Asian-Pacific consensus statement on the management of chronic hepatitis B: a 2012 update. Hepatol Int 6: 263-283.
- CHPA (2012) IMS China Hospital Market Overview.
- Iloeje UH, Yang HI, Su J, Jen CL, You SL, et al. (2006) Predicting cirrhosis risk based on the level of circulating hepatitis B viral load. Gastroenterology 130: 678-686.
- Al-Ashqar HI, Al-Quaiz M, Dahab ST, Peedikayil MC (2013) Entecavir for the treatment of real-life chronic hepatitis B patients: a study from Saudi Arabia. Ann Saudi Med 33: 119-23.
- Yoon J, Park D, Kim C (2013) A granulomatous drug eruption induced by entecavir. Ann Dermatol 25: 493-495.
- Chang TT, Lai CL, Kew Yoon S, Lee SS, Coelho HS, et al. (2010) Entecavir treatment for up to 5 years in patients with hepatitis B e antigen-positive chronic hepatitis B. Hepatology 51: 422-430.
- Cho JY, Sohn W, Sinn DH, Gwak GY, Paik YH, et al. (2016) Long-term real-world entecavir therapy in treatment-naïve hepatitis B patients: base-line hepatitis B virus DNA and hepatitis B surface antigen levels predict virologic response. Korean J Intern Med.
- Peng CY, Hsieh TC, Hsieh TY, Tseng KC, Lin CL, et al. (2013) HBV-DNA level at 6 months of Entecavir treatment predicts HBeAg loss in HBeAg-positive chronic hepatitis B patients. J Formos Med Assoc 275: 235-250.
- Ono A, Suzuki F, Kawamura Y, Sezaki H, Hosaka T, et al. (2012) Long-term continuous entecavir therapy in nucleos(t)ide-naïve chronic hepatitis B patients. J Hepatol 57: 508-514.
- Yuen MF, Seto WK, Fung J, Wong DK, Yuen JC, et al. (2011) Three Years of Continuous Entecavir Therapy in Treatment-Naïve Chronic Hepatitis B Patients: viral Suppression, Viral Resistance, and Clinical Safety. Am J of gastroenterol 106: 1264-1271.
- Sherman M, Yurdaydin C, Simsek H, Silva M, Liaw YF, et al. (2008) Entecavir Therapy for Lamivudine-Refractory Chronic Hepatitis B: Improved Virologic, Biochemical, and Serology Outcomes Through 96 Weeks. Hepatol 48: 99-108.
- Ridruejo E, Marciano S, Galdame O, Reggiardo MV, Muñoz AE, et al. (2014) Efficacy and safety of long term entecavir in chronic hepatitis B treatment naïve patients in clinical practice. Ann Hepatol 13: 327-36.
- Pereira CV, Tovo CV, Grossmann TK, Mirenda H, Dal-Pupo BB, et al. (2016) Efficacy of entecavir and tenofovir in chronic hepatitis B under treatment in the public health system in southern Brazil. Mem Inst Oswaldo Cruz 111: 252-257.
- Chakkor A, Rouibaa, F, Elaboudi S, Aourarh A (2016) An evaluation of entecavir treatment among nucleos(t)ide-naïve Moroccan patients with chronic hepatitis B. BMJ Open Gastro 3: e000081.
Relevant Topics
- Antibiotics and Resistance
- Antifungal
- Antiviral therapy
- Bacteremia
- Bacterial diseases
- Broad Spectrum of Antibiotics
- Clinical Infectious Diseases
- Diagnosis of Pathogenic microorganisms
- Emerging infections
- Natural Antibiotics
- Opportunistic Pathogens
- Parasitic Diseases
- Pertussis Vaccines
- Prevention of infection
- Septicemia
- Viral Infections
- Viremia
Recommended Journals
Article Tools
Article Usage
- Total views: 5152
- [From(publication date):
February-2017 - Apr 03, 2025] - Breakdown by view type
- HTML page views : 4318
- PDF downloads : 834