Research Article Open Access
Retrospective Analysis of Chemotherapy-Induced Nausea and Vomiting (CINV) in Colorectal Cancer Patients Treated with Antiemetics
Takanori Goi1*, Toshiyuki Nakazawa1, Youhei Kimura1, Katsuji Sawai1, Mitsuhiro Morikawa1, Kanji Katayama1, Akiko Momota2, Hiroko Kubo2, Kyouhei Watanabe3, Mikio Masada3 and Akio Yamaguchi11First Department of Surgery, University of Fukui, 23-3, Eiheiji-cho, Yoshida-gun, Fukui, Japan
2Ambulatory Therapy Center, University of Fukui, 23-3, Eiheiji-cho, Yoshida-gun, Fukui, Japan
3Department of Pharmacy, University of Fukui, 23-3, Eiheiji-cho, Yoshida-gun, Fukui, Japan
- *Corresponding Author:
- Takanori Goi
First Department of Surgery, University of Fukui
23-3, Eiheiji-cho, Yoshida-gun, Fukui, Japan
Tel: 81-776-61-3111
Fax: 81-776-61-8113
E-mail: tgoi@u-fukui.ac.jp
Received date: May 11, 2012; Accepted date: August 28, 2012; Published date: August 30, 2012
Citation: Goi T, Nakazawa T, Kimura Y, Sawai K, Morikawa M, et al. (2012) Retrospective Analysis of Chemotherapy-Induced Nausea and Vomiting (CINV) in Colorectal Cancer Patients Treated with Antiemetics. J Palliative Care Med S1:006. doi: 10.4172/2165-7386.S1-006
Copyright: © 2012 Goi T, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and
Visit for more related articles at Journal of Palliative Care & Medicine
Abstract
Purpose: The aim of this retrospective study was to clarify the effect of the antiemetics for chemotherapy-induced nausea and vomiting associated with FOLFOX chemotherapy.
Methods: Fifty patients were given FOLFOX as chemotherapy for colorectal cancer, and granisetron were used as first-line antiemetics. The severity of CINV was evaluated using (1) questioning, (2) Common Terminology Criteria for Adverse Events version 4.0, and (3) Multinational Association of supportive care in cancer method for patient self�assessment. When a patient indicated that another antiemetic was desired, granisetron was switched to palonosetron.
Results: Forty two patients did not express a desire for another antiemetic, but eight patients expressed a desire for it. They were evaluated as Grade 2 according to the CTCAE 4.0. The MAT method identified a score of 6 points or more. Granisetron was switched to palonosetron as a second-line antiemetic. The severity of CINV decreased to Grade 1 or less, while the MAT method score decreased to 0 points in 3 patients and ≤ 4 points in 5 patients. None of the 8 patients expressed a desire for another antiemetic.
Conclusion: Granisetron/palonosetron can be thought to have improved the patients’ QOL, relieved their anxiety, and contributed to continuation of the chemotherapy.
Keywords
Colorectal cancer; Chemotherapy; Chemotherapyinduced nausea and vomiting; Antiemetic; Palonosetron; Granisetron
Abbreviations
QOL: Quality of Life; CTCAE: Common Terminology Criteria for Adverse Events; CINV: Chemotherapy- Induced Nausea and Vomiting
Introduction
The last 10 years have seen striking advances in chemotherapy for unresectable, advanced, recurrent colorectal cancer. In the early 2000s, the Median Survival Time (MST) was about 14-17 months [1,2], whereas survival has been steadily extended since then, recently reaching approximately 30 months [3,4]. However, conversely, chemotherapy-related adverse reactions due to chemotherapy have become an issue, and it is not unusual for such reactions to decrease patients’ Quality of life (QOL). Nausea and vomiting rank high on the list of such chemotherapy-related adverse reactions that especially impact on the daily life of patients and cause anxiety [5,6]. Granisetron is a first-generation 5-HT3 receptor antagonist and commonly used as a first-line antiemetic. Palonosetron is a second-generation 5-HT3 receptor antagonist that has recently (April, 2010) gone on the market in Japan. Compared with the first-generation antiemetic, granisetron, palonosetron is characterized by stronger affinity for the 5‑HT3 receptor and a plasma half-life that is 40 hours longer [7]. For these reasons, palonosetron is said to show both acute(up to 24 hours postchemotherapy) and delayed (after 24 hours postchemotherapy) antiemetic activity. However, there have not yet been any reports of studies that investigated the efficacy of palonosetron, the secondgeneration 5‑HT3 receptor antagonist, in colorectal cancer patients who did not respond sufficiently to the first-generation antiemetic, granisetron. The present retrospective study aimed to clarify the efficiency of the antiemetics.
Materials and Methods
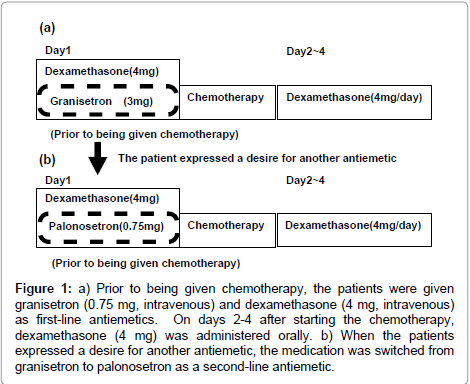
Prior to being given FOLFOX as chemotherapy for unresectable, advanced, recurrent colorectal cancer, 50 patients were given granisetron (0.75 mg, intravenous) and dexamethasone (4 mg, intravenous) as first-line antiemetics to suppress Chemotherapy- Induced Nausea and Vomiting (CINV). On days 2-4 after starting the chemotherapy, dexamethasone (4 mg) was administered orally (Figure 1). Following the chemotherapy, the following were done: (1) the patient was questioned (i.e., asked whether another antiemetic was desired), (2) the severity of nausea and/or vomiting was evaluated using CTCAE version 4.0 (CTCAE 4.0), and (3) the Multinational Association of supportive care in cancer (MAT) method developed by Multinational Association of Supportive Care in Cancer (MASCC) was used for patient self assessment and recording of the severity of nausea and vomiting [8].
Figure 1: a) Prior to being given chemotherapy, the patients were given granisetron (0.75 mg, intravenous) and dexamethasone (4 mg, intravenous) as first-line antiemetics. On days 2-4 after starting the chemotherapy, dexamethasone (4 mg) was administered orally. b) When the patients expressed a desire for another antiemetic, the medication was switched from granisetron to palonosetron as a second-line antiemetic.
Results
Forty-two patients did not express a desire for another antiemetic. The CTCAE 4.0 classification of nausea/vomiting was Grade 1 or less. The MAT method showed that nausea/vomiting was a score of 3 points or less. Eight patients expressed a desire for another antiemetic (Table 1). Using the CTCAE 4.0, nausea was rated as Grade 2 in all 8 patients, while vomiting was rated as Grade 2 in 3 patients, Grade 1 in 4 patients, and Grade 0 in 1 patient. In addition, using the MAT method, the same patients each showed a score of 6 points or higher.
| Granisetron | Palonosetron | ||||
|---|---|---|---|---|---|
| Age | Sex (grade)* | Nausea/vomit score | **MAT (grade)* | Nausea/vomit score | **MAT |
| 57 | M | (G2)/(G1) | 7 | (G0)/(G0) | 1 |
| 61 | M | (G2)/(G1) | 6 | (G1)/(G0) | 0 |
| 49 | M | (G2)/(G0) | 8 | (G1)/(G0) | 0 |
| 51 | F | (G2)/(G2) | 8 | (G1)/(G0) | 3 |
| 56 | M | (G2)/(G2) | 10 | (G1)/(G0) | 4 |
| 54 | F | (G2)/(G1) | 8 | (G1)/(G0) | 4 |
| 69 | M | (G2)/(G1) | 9 | (G0)/(G0) | 0 |
| 52 | F | (G2)/(G2) | 7 | (G1)/(G0) | 1 |
**Maximum: acute and delayed CIMV
Table 1: The results of eight patients expressed a desire for another antiemetic (Forty-two patients did not expresse a desire for another antiemetic).
Subsequently, the medication was switched from granisetron to palonosetron as a second-line antiemetic. The CTCAE 4.0 classification of nausea/vomiting decreased to Grade 1 or less in all 8 patients, while the MAT method showed that nausea/vomiting was completely suppressed to a score of 0 points in 3 patients and to a score of 4 points or less in the remaining 5 patients. None of the 8 patients expressed a desire for another antiemetic. There were no serious antiemeticrelated adverse effects that were considered to have been caused by palonosetron (Table 2).
| Grade 1-2 | Grade 3-4 | |
|---|---|---|
| Constipation | 0 (0%) | 0 (0%) |
| Headache | 0 (0%) | 0 (0%) |
| Increased AST concentration |
0 (0%) | 0 (0%) |
| Prolonged ECG QTc |
0 (0%) | 0 (0%) |
| Increased ALT concentration |
0 (0%) | 0 (0%) |
| Angiopathy | 0 (0%) | 0 (0%) |
| Protein urine present |
0 (0%) | 0 (0%) |
| Increased blood bilirubin concentration | 0 (0%) | 0 (0%) |
| Increased ganma-GTP concentration |
0 (0%) | 0 (0%) |
| Constipation | 0 (0%) | 0 (0%) |
Table 2: Toxicity (CTCAE v4.0).
Discussion
The efficacy rates of the mainstay FOLFOX chemotherapy regimen, which consist of combinations of 5-fluorouracil, Oxaliplatin, and leucovorin, in the treatment of unresectable, advanced, recurrent colorectal cancer are generally said to be in the range of about 50-60% [9,10]. Moreover, in recent years, molecularly targeted drugs such as bevacizumab, cetuximab, and panitumumab have been added to the therapeutic arsenal, and the survival rate has been prolonged [3,4,11-13]. However, chemotherapy-related adverse reactions have become an issue, and, in particular, it is said that 70-80% of patients undergoing CINV [14]. Moreover, the patients themselves rank CINV as top issues causing misgivings regarding their cancer chemotherapy [5,6]. In addition, CINV can not only exert bad effects, such as anorexia and malnutrition, but it can also lead to a marked decrease in the patient’s QOL and interfere with continuation of the cancer chemotherapy.
In consideration of that situation, the National Comprehensive Cancer Network (NCCN) and American Society for Clinical Oncology (ASCO) have prepared guidelines for antiemetic therapy. In these guidelines, the FOLFOX regimens for unresectable, advanced, recurrent colorectal cancer are classified as Moderate Emetic Risk (MER) in the emesis risk classification. In Japan, many institutions administer granisetron and dexamethasone as first-line antiemetics. However, it is said that these agents are unable to control CINV in some patients. Nevertheless, to date, there have been few reports of studies aimed at identifying effective antiemetics for colorectal cancer patients. The objective of the present study was to generate data in regard to this important aspect of patient care.
Our findings indicated that 84% of patients did not express a desire for another antiemetic, but 16% of patients expressed a desire for it. Control of CINV was poor in 16% of colorectal cancer patients undergoing chemotherapy and that a back‑up strategy was needed for management of CINV in such cases. Palonosetron, the secondgeneration 5‑HT3 receptor antagonist that was used in this study, is characterized by stronger affinity for the 5‑HT3 receptor and a plasma half-life that is 40 hours longer in comparison with granisetron, which is a first-generation antiemetic [7]. Prior to this, Saito et al. performed a comparative study of palonosetron and granisetron as first-line antiemetics for acute and delayed CINV caused by highemetic- risk chemotherapy in breast cancer patients. They reported that palonosetron was significantly more effective than granisetron in suppressing CINV [15].
The present study focused on 50 patients who received the FOLFOX regimen, which are classified as MEC in the emesis risk classification, to treat unresectable, advanced, recurrent colorectal cancer. CINV for 8 patients was not effectively controlled by granisetron and dexamethasone as first-line antiemetics. However, when palonosetron was given as a second-line antiemetic, replacing granisetron, it was found to safely control CINV in all patients.
Granisetron/palonosetron can be thought to have improved the patients’ QOL, relieved their anxiety, and contributed to continuation of the chemotherapy.
References
- Saltz LB, Cox JV, Blanke C, Rosen LS, Fehrenbacher L, et al. (2000) Irinotecan plus fluorouracil and leucovorin for metastatic colorectal cancer. Irinotecan Study Group. N Engl J Med 343: 905-914.
- Douillard JY, Cunningham D, Roth AD, Navarro M, James RD, et al. (2000) Irinotecan combined with fluorouracil compared with fluorouracil alone as first-line treatment for metastatic colorectal cancer: a multicentre randomised trial. Lancet 355: 1041-1047.
- Hochster HS, Hart LL, Ramanathan RK, Childs BH, Hainsworth JD, et al.(2008) Safety and efficacy of oxaliplatin and fluoropyrimidine regimens with or without bevacizumab as first-line treatment of metastatic colorectal cancer: results of the TREE Study. J Clin Oncol 26: 3523-3529.
- Grothey A, Sugrue MM, Purdie DM, Dong W, Sargent D, et al.(2008) Bevacizumab beyond first progression is associated with prolonged overall survival in metastatic colorectal cancer: results from a large observational cohort study (BRiTE). J Clin Oncol 26: 5326-5334.
- de Boer-Dennert M, de Wit R, Schmitz PI, Djontono J, v Beurden V, et al. (1997) Patient perceptions of the side-effects of chemotherapy: the influence of 5HT3 antagonists. Br J Cancer 76: 1055-1061.
- Lindley C, McCune JS, Thomason TE, Lauder D, Sauls A, et al. (1999) Perception of chemotherapy side effects: cancer versus non-cancer patients. Cancer Pract 7: 59-65.
- Aapro MS (2007) Palonosetron as an anti-emetic and anti-nausea agent in oncology. Ther Clin Risk Manage 3: 1009-1020.
- Molassiotis A, Coventry PA, Stricker CT, Clements C, Eaby B, et al. (2007) Validation and psychometric assessment of a short clinical scale to measure chemotherapy-induced nausea and vomiting: the MASCC antiemesis tool. J Pain Symptom Manage 34: 148-159.
- Goldberg RM, Sargent DJ, Morton RF, Fuchs CS, Ramanathan RK, et al. (2004) A randomized controlled trial of fluorouracil plus leucovorin, irinotecan, and oxaliplatin combinations in patients with previously untreated metastatic colorectal cancer. J Clin Oncol 22: 23-30.
- Tournigand C, Cervantes A, Figer A, Lledo G, Flesch M, et al. (2006) OPTIMOX1: a randomized study of FOLFOX4 or FOLFOX7 with oxaliplatin in a stop-and-Go fashion in advanced colorectal cancer--a GERCOR study. J Clin Oncol 24: 394-400.
- Saltz LB, Clarke S, Díaz-Rubio E, Scheithauer W, Figer A, et al. (2008) Bevacizumab in combination with oxaliplatin-based chemotherapy as first-line therapy in metastatic colorectal cancer: a randomized phase III study. J Clin Oncol 26: 2013-2019.
- Douillard JY, Siena S, Cassidy J, Tabernero J, Burkes R, et al. (2010) Randomized, phase III trial of panitumumab with infusional fluorouracil, leucovorin, and oxaliplatin (FOLFOX4) versus FOLFOX4 alone as first-line treatment in patients with previously untreated metastatic colorectal cancer: the PRIME study. J Clin Oncol 28: 4697-4705.
- Van Cutsem E, Köhne CH, Hitre E, Zaluski J, Chang Chien CR, et al. (2009) Cetuximab and chemotherapy as initial treatment for metastatic colorectal cancer. N Engl J Med 360: 1408-1417.
- NCCN Clinical Practice Guidelines in Oncology (2010) Antiemesis 2.
- Saito M, Aogi K, Sekine I, Yoshizawa H, Yanagita Y, et al. (2009) Palonosetron plus dexamethasone versus granisetron plus dexamethasone for prevention of nausea and vomiting during chemotherapy: a double-blind, double-dummy, randomised, comparative phase III trial. Lancet Oncol 10: 115-124.
Relevant Topics
- Caregiver Support Programs
- End of Life Care
- End-of-Life Communication
- Ethics in Palliative
- Euthanasia
- Family Caregiver
- Geriatric Care
- Holistic Care
- Home Care
- Hospice Care
- Hospice Palliative Care
- Old Age Care
- Palliative Care
- Palliative Care and Euthanasia
- Palliative Care Drugs
- Palliative Care in Oncology
- Palliative Care Medications
- Palliative Care Nursing
- Palliative Medicare
- Palliative Neurology
- Palliative Oncology
- Palliative Psychology
- Palliative Sedation
- Palliative Surgery
- Palliative Treatment
- Pediatric Palliative Care
- Volunteer Palliative Care
Recommended Journals
- Journal of Cardiac and Pulmonary Rehabilitation
- Journal of Community & Public Health Nursing
- Journal of Community & Public Health Nursing
- Journal of Health Care and Prevention
- Journal of Health Care and Prevention
- Journal of Paediatric Medicine & Surgery
- Journal of Paediatric Medicine & Surgery
- Journal of Pain & Relief
- Palliative Care & Medicine
- Journal of Pain & Relief
- Journal of Pediatric Neurological Disorders
- Neonatal and Pediatric Medicine
- Neonatal and Pediatric Medicine
- Neuroscience and Psychiatry: Open Access
- OMICS Journal of Radiology
- The Psychiatrist: Clinical and Therapeutic Journal
Article Tools
Article Usage
- Total views: 7276
- [From(publication date):
specialissue-2012 - Apr 02, 2025] - Breakdown by view type
- HTML page views : 2717
- PDF downloads : 4559