Commentary Open Access
Retinal and Optic Disc Alterations in Alzheimer's Disease: the Eye as a Potential Central Nervous System Window
Maria P Bambo1,2, Elena Garcia-Martin1,2*, Jose M Larrosa1,2, Vicente Polo1,2, Fernando Gutiérrez-Ruiz1,2, Vilades2, Laura Gil-Arribas1,2 and Luis E Pablo1,2
1Ophthalmology Department, Miguel Servet University Hospital, Zaragoza, Spain
2Aragon Institute for Health Research (IIS Aragon), University of Zaragoza, Zaragoza, Spain
- Corresponding Author:
- Elena Garcia-Martin
C/ Padre Arrupe. Consultas Externas de Oftalmologia 50009-Zaragoza, Spain
Tel: 0034-976765558
E-mail: egmvivax@yahoo.com
Received date: March 09, 2016; Accepted date: March 14, 2016; Published date: March 21, 2016
Citation: Bambo MP, Garcia-Martin E, Larrosa JM, Polo V, Gutiérrez-Ruiz F, et al. (2016) Retinal and Optic Disc Alterations in Alzheimer’s Disease: the Eye as a Potential Central Nervous System Window. J Alzheimers Dis Parkinsonism 6:223. doi: 10.4172/2161-0460.1000223
Copyright: © 2016 Bambo MP, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Alzheimers Disease & Parkinsonism
Abstract
Pathologic changes in the retina and optic nerve are observed in patients with Alzheimer´s disease (AD), even in early stages of the dementia. In our clinical ophthalmology practice, we use optical coherence tomography (OCT), a noninvasive, rapid, objective, and reliable technology that enables for quantification of the retinal nerve fiber layer (RNFL), namely the retinal ganglion cell axons that eventually form the optic nerve. The opportunity to analyze a part of the central nervous system by such a simple exploration led to several studies demonstrating thinning of the RNFL and central retina in AD patients compared with healthy subjects. Here we present some of our investigations in AD patients using Spectral Domain-OCT. Our results suggest that axonal loss secondary to pathologic alterations in the brains of AD patients can be observed by OCT. We also analyzed the association between retinal and RNFL thicknesses and neurologic characteristics, disease duration and severity, and found that mean RNFL thickness was significantly correlated with disease duration, indicating that the progression of AD is associated with a progressive loss of ganglion cells.
Keywords
Alzheimer’s disease; Retinal nerve fiber layer; Retinal thickness; Optic disc; Optical coherence tomography
Introduction
Alzheimer disease´s (AD) patients usually complain about visual symptoms such as blurry vision or reading difficulties. These complaints have been reported for more than 40 years, and they were initially considered to be of strictly cortical origin. In the last 20 years, however, some studies have identified pathologic changes in the retina and optic nerve [1,2] that could appear during the preclinical stages in patients with AD or in subjects with mild cognitive impairment.
The retinal nerve fiber layer (RNFL) comprises axons originating from retinal ganglion cells that finally form the optic nerve, namely, the first neurons of the visual pathway. The RNFL can be measured using ocular imaging technologies such as optical coherence tomography (OCT), Schuman [3] which provides noninvasive, rapid, objective, and reliable measurements. Ophthalmologists now use OCT as part of daily clinical practice and numerous studies have reported the ability of OCT to detect RNFL thickness abnormalities and changes in the macula of patients with neurodegenerative diseases [4,5]. OCT reveals thinning of the RNFL [6,7] and central retina [8] in AD patients compared with healthy subjects. There are some similarities between AD and certain eye diseases associated with aging. In animal models of AD andagerelated macular degeneration, extracellular deposits of amyloid beta are a common pathologic feature [9]. The visual pathway provides occasion to use noninvasive explorations to study and follow-up biomarkers of axonal loss in neurodegenerative diseases such as AD. In fact, retrograde loss of nerve fibers in the retina and optic nerve could be an early biomarker of axonal loss in AD, even before hippocampal damage, which conducts to memory impairment [10].
Five years ago, our Ophthalmology Department initiated a research line focusing on AD patients. The retinal resolution of Time Domain-OCT (TD-OCT) is approximately 10 μm, whereas the retinal resolution of the newer Spectral Domain-OCT (SD-OCT) to 5 μm or less. Further, SD-OCT acquires images faster, registers the retinal location, and requires no pupil dilation. Faster acquisition is important for patients with cognitive impairment or patients who have difficulty maintaining visual fixation, such as those with AD. Based on our experience, SD-OCT can detect axonal defects in AD patients, and specifically the Nsite Axonal application (especially designed for evaluating neuro-ophthalmic patients) of the Spectralis OCT device (Heidelberg Engineering, Inc., Heidelberg, Germany) has the greatest sensitivity for detecting subclinical RNFL atrophy in AD [11]. The temporal quadrant of the RNFL is the sector most often affected in early neurodegenerative diseases [12], as the fibers of the temporal quadrant follow the papillomacular bundle (PMB).In a recent study of 70 AD patients and 70 healthy subjects, we demonstrated that PMB thickness was decreased in patients with AD, with the temporal sector being the most susceptible (PMB thickness was 51.58 μmin AD patients and 56.79 μm in healthy subjects, p=0.012) [11]. Further, mean and superior RNFL thickness were more affected in patients with severe cognitive impairment [11,13]. Fast acquisition is an important feature of SD-OCT devicesfor examining patients with cognitive impairment or fixation difficulties, such as individuals with AD, and may improve the reliability of the technique. Due to the fast acquisition of SD-OCT, we found both retina and RNFL thickness measurements showed good reliability in AD patients (OCT measurements coefficient of variation ranged between 2.02 and 7.30; OCT measurements intraclass correlation coefficient ranged between 0.812 and 0.985) [11]. SD-OCT, therefore, is a useful and accurate clinical tool to evaluate subjects with AD, and the test is very fast and comfortable for the patient, who sits with the head positioned in a chin rest while the SD-OCT acquires images in 2 s. This type of imaging can easily be incorporated into eye exams for AD patients. The advent of the newer Swept source (SS)-OCT provides additional advantages over SD-OCT, including reduced fringe washout, better sensitivity with imaging depth, and a longer imaging range. SS-OCT increases penetration into the choroid and optic nerve head with reduced sensitivity to ocular opacities [14].
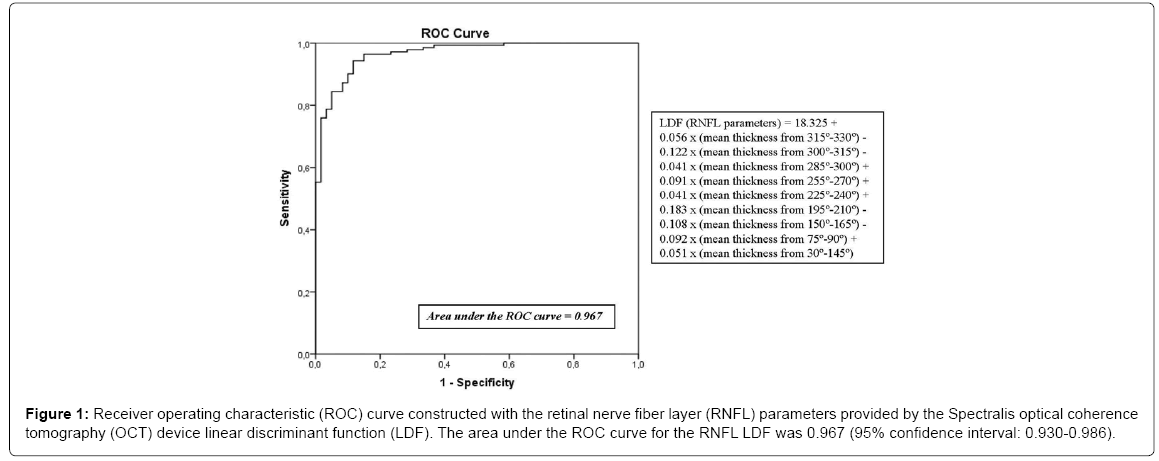
Currently, ophthalmologists don´t know what retinal or RNFL parameters provided by OCT could be better biomarker to diagnose AD. According to the area under the receiver operating characteristic (ROC) curve, RNFL mean thickness is the best diagnostic parameter assessed by OCT to identify various inner retinal or optic neuropathies, such as glaucoma, and it is the most sensitive parameter for identifying neurodegenerative diseases [15-17]. However, it’s probably that we achieve the optimal neurodegenerative disease detection by using different OCT parameters. In another study, we performed a logistic regression analysis to establish the relative importance of each independent variable using the forward Wald method, so the calculated linear discriminant function (LDF) has better diagnostic capability compared with individual OCT parameters. We obtained that the LDF built using RNFL measurements provided by Spectralis OCT procured the highest sensitivity at a high specificity compared to any single measurement obtained using OCT (Figure 1) [18].
Figure 1: Receiver operating characteristic (ROC) curve constructed with the retinal nerve fiber layer (RNFL) parameters provided by the Spectralis optical coherence tomography (OCT) device linear discriminant function (LDF). The area under the ROC curve for the RNFL LDF was 0.967 (95% confidence interval: 0.930-0.986).
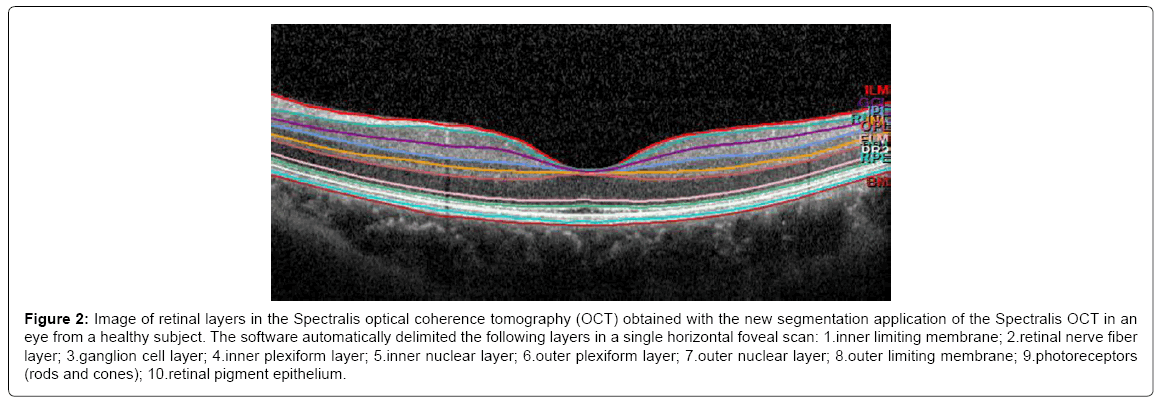
The latest commercialized OCT devices have excellent reproducibility and better resolution, making it possible to segment retinal layers (Figure 2). Ctori [19] recently observed that the retinal layers are differentially affected by AD: the disease causes a general decrease in RNFL foveal thickness, but only the inner retinal layers exhibit significant thinning compared with those of healthy subjects. Further, patients with longer AD duration (≥ 3 years) exhibited more thinning of the RNFL, ganglion cell, and inner plexiform layers (p < 0.05) [20]. This could be due to degeneration of the retinal ganglion cells and axons or to retrograde transsynaptic degeneration of the retinal ganglion cell layer and its axons in AD patients in association with posterior visual pathway lesions. Albrecht et al. [21] previous studies reported transsynaptic retinal ganglion cell degeneration in patients with homonymous hemianopsia due to retrogeniculate lesions. Jindahra et al. [22] and Reich et al. [23] reported an association between RNFL loss and retrogeniculate lesions, suggesting that measurements performed by OCT can reveal combined posterior and anterior abnormalities in the visual pathway.
Figure 2: Image of retinal layers in the Spectralis optical coherence tomography (OCT) obtained with the new segmentation application of the Spectralis OCT in an eye from a healthy subject. The software automatically delimited the following layers in a single horizontal foveal scan: 1.inner limiting membrane; 2.retinal nerve fiber layer; 3.ganglion cell layer; 4.inner plexiform layer; 5.inner nuclear layer; 6.outer plexiform layer; 7.outer nuclear layer; 8.outer limiting membrane; 9.photoreceptors (rods and cones); 10.retinal pigment epithelium.
Our results suggest that axonal loss secondary to pathologic alterations in the brains of AD patients can be observed by OCT. The new technologies can quantify and measure retinal ganglion cells, and changes detected by OCT in the RNFL may reflect similar pathologic alterations that occur elsewhere in the patient´s brain. OCT allows for observation of the axonal constituents of the visual pathway and direct visualization of the anterior part of the central nervous system through the eye [24]. We also analyzed the association between retinal and RNFL thicknesses and neurologic characteristics, disease duration, and severity, and found no significant correlation between the mean retinal and RNFL and the Mini Mental Examination (MMSE) score. Mean RNFL thickness (provided by the glaucoma application of the Spectralis OCT) was, however, significantly correlated with disease duration (r = 0.551, p = 0.043), indicating that progression of AD is associated with a progressive loss of ganglion cells. Mean RNFL mean and superior thicknesses were significantly reduced in patients with severe cognitive impairment (MMSE≤9) compared with those with mild cognitive impairment (MMSE between 19 and 24 points) [11].
These technologies still have some limitations: media opacity (like cataract), instrument variability, retinal pigment epithelium status, and centering and positioning of the images all affect the quality of the data obtained by these imaging devices. In patients in advanced stages of AD, good-quality scans are often not possible to acquire, so the number of severe AD patients included in these studies is limited. Some other ophthalmologic diseases and optic neuropathies can cause a reduction in RNFL and retinal thickness, and patients with these conditions were excluded in our investigations; however it is possible that some preclinical stages or normal-tension glaucoma could be included by error. These limitations must be taken into account when interpreting OCT data obtained from patients with AD.
Additional studies should be performed to evaluate the ability of OCT measurements to distinguish AD from other kinds of dementia, and the ability of the thickness of each layerto predict AD. This would be especially useful in the evaluation of AD patients in incipient phases or with a difficult or atypical diagnosis. OCT measurements are tools than can be used in combination with clinical explorations and other neurologic parameters, with the advantage that OCT is an inexpensive and non-invasive examination that only takes a few minutes. Future studies of AD using the new SS-OCT are needed, because the ability to rapidly acquire three-dimensional OCT data over a wide field of view, and zoom and image fine structures with high speed and high density promises to simplify ophthalmic examination protocols and to improve performance for ophthalmic instrumentation. The eye offers us a direct window to the central nervous system, and OCT allows for objective and reproducible evaluation.
Acknowledgements
Supported in part by the Fundación Mutua Madrileña grant FMMA 02/12.The authors would like to thank the Aragon Federation of Alzheimer´s Disease Patients and other Dementias (FARAL) for helping with patient inclusion.
References
- Dehabadi MH, Davis BM, Wong TK, Cordeiro MF (2014) Retinal manifestations of Alzheimer's disease. Neurodegener Dis Manag 4: 241-252.
- Tzekov R, Mullan M (2014) Vision function abnormalities in Alzheimer disease. Surv Ophthalmol 59: 414-433.
- Schuman JS, Pedut-Kloizman T, Hertzmark E, Hee MR, Wilkins JR, et al. (1996) Reproducibility of nerve fiber layer thickness measurements using optical coherence tomography. Ophthalmology 103: 1889-1898.
- Kallenbach K, Frederiksen J (2007) Optical coherence tomography in optic neuritis and multiple sclerosis: a review. Eur J Neurol 14: 841-849.
- Yu JG, Feng YF, Xiang Y, Huang JH, Savini G, et al. (2014) Retinal nerve fiber layer thickness changes in Parkinson disease: a meta-analysis. PLoS One 9: e85718.
- Lu Y, Li Z, Zhang X, Ming B, Jia J, et al.(2010) Retinal nerve fiber layer structure abnormalities in early Alzheimer's disease: evidence in optical coherence tomography. Neurosci Lett 480: 69-72.
- Coppola G, Di Renzo A, Ziccardi L, Martelli F, Fadda A, et al. (2015) Optical Coherence Tomography in Alzheimer's Disease: A Meta-Analysis. PLoS One 10: e0134750.
- Gharbiya M, Trebbastoni A, Parisi F, Manganiello S, Cruciani F, et al.(2014) Choroidal thinning as a new finding in Alzheimer's disease: evidence from enhanced depth imaging spectral domain optical coherence tomography. J Alzheimers Dis 40: 907-917.
- Park SW, Kim JH, Mook-Jung I, Kim KW, Park WJ, et al. (2014) Intracellular amyloid beta alters the tight junction of retinal pigment epithelium in 5XFAD mice. Neurobiol Aging 35: 2013-2020.
- Valenti DA (2011) Alzheimer's disease and glaucoma: imaging the biomarkers of neurodegenerative disease. Int J Alzheimers Dis 2010: 793931.
- Polo V, Garcia-Martin E1, Bambo MP1, Pinilla J1, Larrosa JM1, et al. (2014) Reliability and validity of Cirrus and Spectralis optical coherence tomography for detecting retinal atrophy in Alzheimer's disease. Eye (Lond) 28: 680-690.
- Garcia-Martin E, Pueyo V, Almarcegui C, Martin J, Ara JR, et al. (2011) Risk factors for progressive axonal degeneration of the retinal nerve fibre layer in multiple sclerosis patients. Br J Ophthalmol 95: 1577-1582.
- Bambo MP, Garcia-Martin E, Pinilla J, Herrero R, Satue M, et al. (2014) Detection of retinal nerve fiber layer degeneration in patients with Alzheimer's disease using optical coherence tomography: searching new biomarkers. Acta Ophthalmol 92: e581-2.
- Esmaeelpour M, Povazay B, Hermann B, Hofer B, Kajic V, et al. (2010) Three-dimensional 1060nm OCT: Choroidal thickness maps in normals and improved posterior segment visualization in cataract patients. Invest Ophthalmol Vis Sci 51: 5260-5266.
- Ratchford JN, Quigg ME, Conger A, Frohman T, Frohman E, et al. (2009) Optical coherence tomography helps differentiate neuromyelitis optica and MS optic neuropathies. Neurology 73: 302-308.
- Bennett JL, de Seze J, Lana-Peixoto M, Palace J, Waldman A, et al. (2015) Neuromyelitis optica and multiple sclerosis: Seeing differences through optical coherence tomography. Mult Scler 21: 678-688.
- Mailankody P, Battu R, Khanna A, Lenka A, Yadav R, et al. (2015) Optical coherence tomography as a tool to evaluate retinal changes in Parkinson's disease. Parkinsonism Relat Disord 21: 1164-1169.
- Larrosa JM, Garcia-Martin E, Bambo MP, Pinilla J, Polo V, et al. (2014) Potential new diagnostic tool for Alzheimer's disease using a linear discriminant function for Fourier domain optical coherence tomography. Invest Ophthalmol Vis Sci 55: 3043-3051.
- Ctori I, Huntjens B (2015) Repeatability of Foveal Measurements Using Spectralis Optical Coherence Tomography Segmentation Software. PLoS One 10: e0129005.
- Garcia-Martin E, Bambo MP, Marques ML, et al. (2016) Ganglion cell layer measurements correlate with disease severity in patients with Alzheimer's disease. Acta Ophthalmol .
- Albrecht P, Ringelstein M, Müller AK, Keser N, Dietlein T, et al. (2012) Degeneration of retinal layers in multiple sclerosis subtypes quantified by optical coherence tomography. Mult Scler 18: 1422-1429.
- Jindahra P, Petrie A, Plant GT (2009) Retrograde trans-synaptic retinal ganglion cell loss identified by optical coherence tomography. Brain 132: 628-634.
- Reich DS, Smith SA, Gordon-Lipkin EM, Ozturk A, Caffo BS, et al. (2009) Damage to the optic radiation in multiple sclerosis is associated with retinal injury and visual disability. Arch Neurol 66: 998-1006.
- Siger M, Dziegielewski K, Jasek L, Bieniek M, Nicpan A, et al. (2008) Optical coherence tomography in multiple sclerosis: thickness of the retinal nerve fiber layer as a potential measure of axonal loss and brain atrophy. J Neurol 255:1555-1560.
Relevant Topics
- Advanced Parkinson Treatment
- Advances in Alzheimers Therapy
- Alzheimers Medicine
- Alzheimers Products & Market Analysis
- Alzheimers Symptoms
- Degenerative Disorders
- Diagnostic Alzheimer
- Parkinson
- Parkinsonism Diagnosis
- Parkinsonism Gene Therapy
- Parkinsonism Stages and Treatment
- Stem cell Treatment Parkinson
Recommended Journals
Article Tools
Article Usage
- Total views: 11130
- [From(publication date):
April-2016 - Apr 02, 2025] - Breakdown by view type
- HTML page views : 10267
- PDF downloads : 863