Review Article Open Access
Re-thinking Vaccinology: “Act Universally, Think NK Cells”?
Geert Vanden Bossche*
Head of Vaccine Development Office, German Center for Infection Research, University Clinic Cologne, Belgium
- *Corresponding Author:
- Geert Vanden Bossche
Head of Vaccine Development Office
German Center for Infection Research
University Clinic Cologne
Joseph- Stelzmann-Str. 9b, D-50931 Cologne, Belgium
E-mail: geert.vandenbossche@live.be
Received date: August 05, 2017; Accepted date: August 23, 2017; Published date: September 7, 2017
Citation: Vanden Bossche G (2017) Re-thinking Vaccinology: “Act Universally, Think NK Cells”?. J Mol Immunol 2:112.
Copyright: © 2017 Vanden Bossche G. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Molecular Immunology
Abstract
Traditional and modern vaccines that are currently licensed for commercial use have proven safe and effective in fighting several infectious diseases; they are unquestionably among the most efficient tools for promoting individual, public and global health. However, the vaccine field is still facing important shortcomings in that the use of vaccines has not been successful yet in preventing many other, especially chronic, infectious diseases and that a therapeutic effect remains beyond reach of contemporary vaccines. As vaccine-mediated immune protection is widely acknowledged to result from the combined effect of specific target antigens and nonspecific immune stimulating agents, it is likely that limitations of conventional vaccinal antigens and adjuvants are responsible for these shortcomings.
This article aims at highlighting weaknesses in traditional vaccinology and calls for novel approaches to immune intervention to address those flaws and overcome the single-most important challenge in vaccinology, namely immune escape.
Keywords
Vaccinology; NK cells; Infectious diseases; Immune protection
Limitations of Conventional Target Antigens Used in Traditional/Contemporary Vaccines
To eliminate safety risks related to infectivity inactivated pathogens and, more suitably, well-characterised subunit or recombinant pathogen-derived antigens have increasingly been used as immunogens in ‘modern’ vaccines. Recombinant protein antigens can either be synthesised in vitro or in vivo (the latter as in case of nucleic acid-based vaccines, for example). The selection of vaccinal antigens/ epitopes is usually based on their capacity to induce functional immune responses that correlate with protection upon natural infection. This is to say that the vast majority of modern vaccines are designed at recapitulating naturally induced immune responses that correlate with protection against infection or disease. Consequently, target antigen (Ag) ‘discovery’ tends to concentrate on specific, naturally immunodominant epitopes that are expressed on the outer surface of free-circulating pathogens (hereafter called ‘B cell [Bc] target epitopes’) or on the surface of infected or diseased host cells within the context of MHC class I (hereafter called ‘T cell [Tc] target epitopes’). Both types of epitopes may be subject to antigenic change whereas their specific recognition is directly (e.g., in case of Tc target epitopes) and/or indirectly (e.g., due to T help-dependence of antibodies or effector T cells) restricted by the MHC background of the host. The more stringently the immune recognition of cognate T help (Th) epitopes is MHC class II-restricted, the higher the specificity with which a target epitope is recognised. It is reasonable to assume that the higher the level of specificity of target epitope recognition, the higher the likelihood for a pathogen to escape the host immune response.
Prophylactic anti-viral or anti-bacterial vaccines, for instance, primarily rely on the induction of functional but strain-specific antibody (Ab) responses to Bc epitopes. To broaden the immune response, multiple Bc and/or Tc target epitopes can be combined in one and the same vaccine (so-called ‘multivalent’ vaccines). However, such combinatorial approaches often result in manufacturing complexity and issues of immunodominance hierarchy of immune responses to the different valences used. Although capable of preventing extracellular spreading microbes from reaching their tissuespecific target cells or organ-specific target tissues and thus, preventing them from causing systemic infection or disease, antibodies (Abs) cannot usually eliminate infected or diseased host cells. Although cytotoxic Abs (e.g., antibody-dependent cell-mediated cytotoxicity [ADCC] Abs) do have the potential to kill infected or diseased host cells, their maturation and persistence is likely to require persistent antigenic stimulation or repeated boosting [1,2]. The benefit, therefore, of contemporary Ab-based vaccines (so-called ‘Bc vaccines’) is largely limited to prophylactic immune intervention in diseases caused by extracellularly spreading microbial toxins or by infectious pathogens that are decorated with protective antigens and disseminate into the bloodstream to reach their target tissue or tissue-specific target cells. Immune subversive antigens (e.g., allergens or tumorigenic antigens) as well as infectious pathogens that are decorated at their surface with self-mimicking components (e.g., certain parasites) or propagate via cell-to-cell transmission or lymphatic trafficking of infected, mucosalresident dendritic cells (e.g., certain viruses ) largely remain beyond reach of contemporary vaccines. Mucosal Abs (i.e., IgA) as, for example, induced by live attenuated vaccines, or high titres of systemic Abs (i.e., IgG) may enable protection against local infection with certain infectious pathogens by neutralising these pathogens at the portal of entry. Live attenuated vaccines may, however, be hazardous in that they can occasionally cause debilitating disease when vaccine virus reverts or recombines to form virulent virus, as observed in some rare cases of immunisation with OPV [3,4]. In addition, Abs directed at Bc epitopes exposed on the surface of pathogens are often strain-/ serotype-specific (see above). Hence, minor changes in pathogen-encoded Bc target epitopes, for example due to spatial rearrangements (i.e., in case of conformational epitopes) or a spontaneous genetic mutation or recombination, may already suffice for the pathogen to escape a previously naturally induced or vaccine-mediated Ab response. Ab-based vaccines may, therefore, fail to induce protection against distinct, although phylogenetically related, pathogen strains/ serotypes. In addition, immune recognition of cognate T help (Th) epitopes may be poor or lacking in a subset of vaccine recipients (socalled ‘non-responders’) due to MHC polymorphism, thus resulting in the absence of functionally protective Ab responses. Due to the abovementioned limitations, pathogens may succeed in escaping the host immune response and preserve their fitness. Although immune escape can to some extent be mitigated by enhanced T help through coformulation of antigen with adjuvant, vaccine adjuvantation does not come without risk of side effects (see below). Last, to ensure adequate Ab isotype switching and high, long-lasting titres of protective Abs against surface-exposed proteins or other protein-conjugated antigens (e.g., polysaccharides), traditional prophylactic, non-live vaccines usually require one or more booster doses. To enable targeting of epitopes that are expressed on infected or diseased cells, new vaccine candidates frequently also comprise conserved linear peptide epitopes that are presented on MHC class I molecules (hereafter called ‘Tc target epitopes’). However, immune recognition of these epitopes is contingent on their specificity to the polymorphic MHC haplotype background of the host. This already implies that conserved Tc target epitopes will only be protective in individuals who are already genetically predisposed to Tc-mediated pathogen control by virtue of expression of MHC alleles matching the specificity of these epitopes (so-called ‘protective’ MHC alleles) [5]. In order to broaden the spectrum of effector Tc responses, multiple Tc target epitopes can be incorporated into vaccines. Multi-epitope vaccines can, for example, be produced by using sophisticated nucleic acid technology or recombinant viral or bacterial vectors. However, similarly to the situation described above for Bc target epitopes, the induction of fullfledged immune responses toward multivalent pathogen-encoded Tc epitopes requires booster injections (or ‘prime-boost’ regimens) whereas immune escape issues remain due to immune dominance hierarchy (i.e., resulting in suboptimal immune responses to certain Tc epitopes) or because of MHC-restriction of helper or effector T cells or spontaneous or vaccine-mediated mutation of immunodominant Tc epitopes (a phenomenon called ‘immune pressure’). Hence, multiepitope Tc vaccine candidates have not been effective in inducing broad and long-lasting immune protection in target populations with a heterogeneous MHC background.
Notwithstanding the nature of their target epitopes, traditional nonlive vaccines require adjuvantation and inclusion of MHCII-binding antigenic determinants (hereafter called ‘Tc helper epitopes’) to induce cognate T helper cells that can assist priming of effector B cells or MHCI- restricted T cells. Traditional pro-inflammatory adjuvants/ immune potentiators may, however, cause local reactogenicity and raise safety concerns. Hence, regulatory hurdles to the use of adjuvants in vaccines, especially if their use is unprecedented (e.g., other than Alum), may be substantial. Traditional Th2 adjuvants (e.g., Alum, oilin- water emulsions) are most commonly used to enhance humoral Ab responses whereas Th1 adjuvants (e.g., Pathogen Associated Molecular Patterns [PAMPs]) are regularly used to enhance T cell-mediated effector responses. However, alike Tc target epitopes, Tc helper epitopes are subject to MHC-restriction. To overcome absence of natural Th2 epitopes or mitigate limitations of cognate type 2 T help that are due to immunogenetic restriction, Bc target epitopes can be conjugated to a conserved promiscuously MHCII-binding Th peptide or to a protein comprising one or more promiscuous Th peptides (e.g., tetanus toxoid, diphtheria toxoid or a non-toxic mutant of diphtheria toxin [e.g., CRM197]) as in case of multivalent conjugate vaccines (e.g., glycoconjugate vaccines). Likewise, limitations due to immunogenetic restriction of cognate type 1 T help can be mitigated by co-localisation of Tc target epitopes and promiscuous Th peptide(s) to the same protein/polypeptide Ag. Alternatively, co-localisation of Tc target epitopes to multiple cognate MHCII-restricted Th peptides (as in case of multivalent polypeptide or nucleic acid-based Tc vaccines) combined with concomitant provision of type 1 immune stimulatory signals (e.g., via co-formulation with immune potentiating nucleic acid sequences or other PAMPs) may equally lead to enhanced breadth of Tc target epitope recognition by MHC class I-restricted effector T cells. Cognate T help facilitated by Th1 or Th2 adjuvants tends to broaden immune recognition of MHCI-restricted Tc epitopes or specific Bc epitopes, respectively. Promiscuously MHCII-binding Th peptides are ideally suited to broaden T help across a broad spectrum of MHCII haplotypes. Based on observations from natural infection, it cannot be ruled out, however, that the combination of promiscuous helper peptides and adjuvants is at risk of causing immune pathology, for example by promoting priming of autoreactive T cells (see also below under ‘Limitations and risks associated with the use of adjuvants in vaccines’).
Limitations and Risks Associated with the Use of Adjuvants in Vaccines
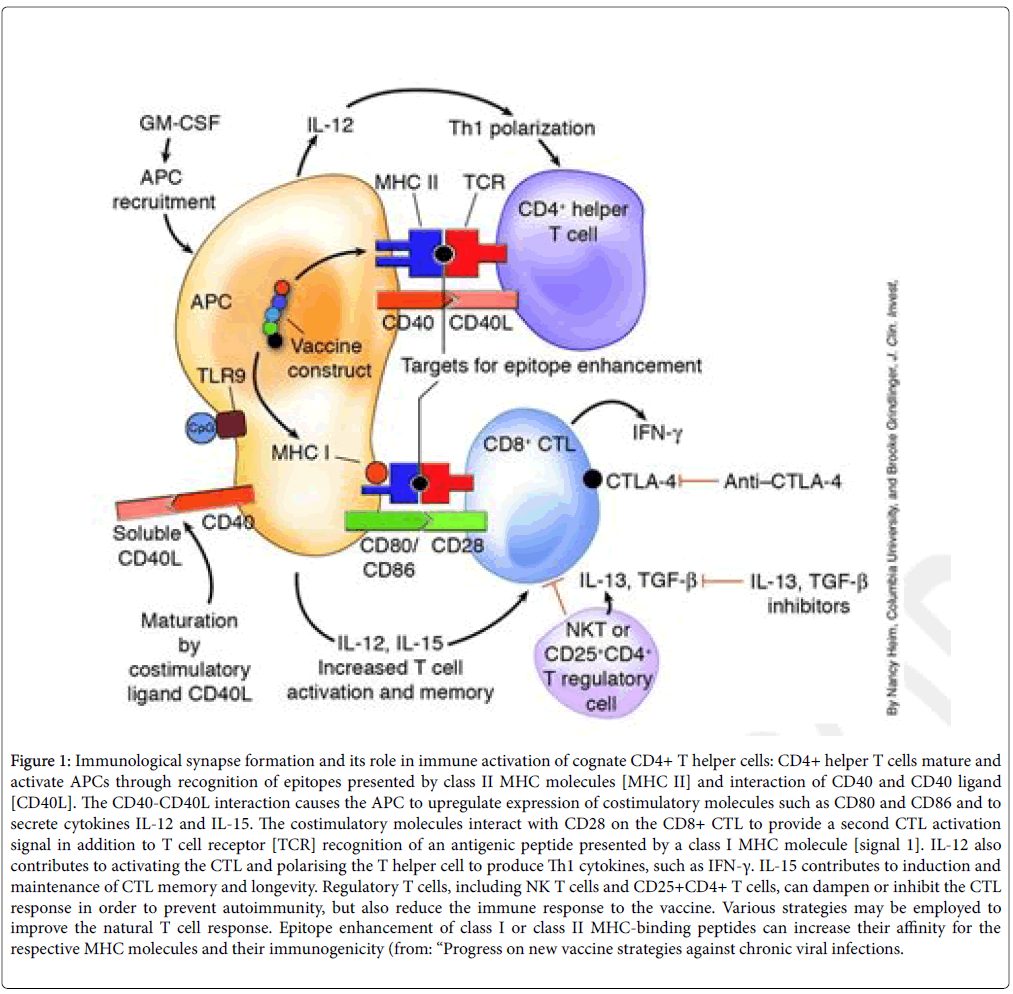
To mitigate limitations due to the high level of specificity and/ or immunogenetic restriction of Ag recognition, traditional vaccinology has been largely relying on the use of several different types of adjuvants. Adjuvants primarily enhance the immune response through upregulation of presentation of Ag-MHC complexes on the surface of Ag-presenting cells (APCs), thereby licensing MHCII-restricted T helper cells to assist priming of effector B cells or MHCI-restricted effector T cells Figure 1.
Figure 1: Immunological synapse formation and its role in immune activation of cognate CD4+ T helper cells: CD4+ helper T cells mature and activate APCs through recognition of epitopes presented by class II MHC molecules [MHC II] and interaction of CD40 and CD40 ligand [CD40L]. The CD40-CD40L interaction causes the APC to upregulate expression of costimulatory molecules such as CD80 and CD86 and to secrete cytokines IL-12 and IL-15. The costimulatory molecules interact with CD28 on the CD8+ CTL to provide a second CTL activation signal in addition to T cell receptor [TCR] recognition of an antigenic peptide presented by a class I MHC molecule [signal 1]. IL-12 also contributes to activating the CTL and polarising the T helper cell to produce Th1 cytokines, such as IFN-γ. IL-15 contributes to induction and maintenance of CTL memory and longevity. Regulatory T cells, including NK T cells and CD25+CD4+ T cells, can dampen or inhibit the CTL response in order to prevent autoimmunity, but also reduce the immune response to the vaccine. Various strategies may be employed to improve the natural T cell response. Epitope enhancement of class I or class II MHC-binding peptides can increase their affinity for the respective MHC molecules and their immunogenicity (from: “Progress on new vaccine strategies against chronic viral infections.
Limitations of Th2-Adjuvanted Vaccines
Conventional purified (e.g., recombinant/ subunit) pathogenderived Ags comprising one or more Bc target epitopes have been combined with type 2 T help-activating adjuvants (e.g., Alum, oil-inwater emulsion etc.) to enhance their immunogenicity. Th2 polarisation of vaccine-induced immune responses results from adjuvant-mediated triggering of Th2 cytokine secretion (e.g., IL-4, IL-5, IL-6). Th2 adjuvant-mediated upregulation of Th epitopes enhances immune recognition of Bc target epitopes that are associated with these Th epitopes (via a so-called ‘cognate’ mechanism of immune recognition). This increases the breadth of the Ab response in a way that promotes immune recognition of a more diversified spectrum of Bc epitopes across a set of heterologous pathogen strains (a phenomenon called ‘epitope spreading’). It is, however, reasonable to assume that epitope spreading is able to induce changes in the hierarchy of immune responses to pathogen-specific vaccinal epitopes. This may result in diminished recognition of so-called ‘protective’ Bc epitopes (i.e., Bc epitopes that are of vital importance to the pathogen), thus promoting immune escape of the target pathogen. On the other hand, nonAg-specific type 1 stimulation of CD4+ bystander T cells may occasionally lead to stimulation of noncognate B cells, thereby posing a risk of inducing allergic humoral immune responses towards pathogen- or even nonpathogen-related vaccine components [6-8].
Formulation of conformational Bc epitopes on Alum or their coformulation with other particulate adjuvants (e.g., emulsions, liposomes) may also lead to spatial rearrangements and, therefore, elicit Abs that do not match the natural antigenic conformation of these epitopes. This not only allows the pathogen to escape vaccineinduced immune responses but could possibly even raise safety concerns related to an increased likelihood of vaccine-mediated exacerbation of disease upon natural, post-immunisation exposure to certain viral pathogens [9-11].
Limitations of Th1-Adjuvanted Vaccines
To enhance cell-mediated immune responses towards target pathogens, contemporary vaccine approaches increasingly combine conventional pathogen-derived epitopes with type 1 T help-activating adjuvants (‘Th1 adjuvants’). Th1 adjuvants mostly mimic natural innate immune modulators (i.e., PAMPs comprising TLR agonists such as lipopolysaccharide, lipoproteins, lipopeptides, flagellin, doublestranded RNA, unmethylated CpG). Depending on their formulation and use in mutually synergising combinations, PAMPs may also induce Th17 immune signalling [12]. In case of protein-based vaccines, physical or chemical binding of the adjuvant with the vaccinal protein antigen is critical to ensure optimal biological activity. The ‘success’,therefore, of traditional Th1 adjuvants typically requires sophisticated conjugation technology or formulation with a macromolecular or particulate carrier (e.g., emulsions, liposomes, VLPs) capable of binding both, the vaccinal protein (or protein-conjugated) antigen and the adjuvant. Alternatively, Th1-assisted immune responses towards cell-bound target antigens can also be induced upon delivering these antigens as part of a recombinant genetic construct (e.g., by way of viral vectors, DNA-based vaccines, recombinant alpha-virus replicon particles or reassorted viruses) or as nucleic acids (e.g., in form of DNA, RNA or messenger RNA [mRNA]).
Th1 adjuvant-mediated upregulation of Th epitopes mitigates the impact of MHC class II restriction on immune recognition of cognate MHC class I-restricted target epitopes that are co-localised to these Th epitopes. This increases the breadth of the Tc effector response in a way that promotes immune recognition of a more diversified spectrum of Tc epitopes across vaccine recipients with a heterogenous MHC background (so-called ‘epitope spreading’). However, the resulting changes in the hierarchy of immune responses to vaccinal Tc epitopes may lead to poor or deficient recognition of ‘protective’ MHCIrestricted pathogen-specific Tc epitopes, thus enabling the target pathogen to escape vaccine-mediated cellular immune responses. On the other hand, nonAg-specific type 1 stimulation of bystander T cells could occasionally lead to stimulation of noncognate effector cells, thereby posing a risk of inducing autoreactive immune responses towards certain tissue-specific self-antigens [13-16].
Given the occasional observation of autoimmune responses in association with natural infection (e.g., certain viral infections), it is also conceivable that co-formulation of strong adjuvants (e.g., Th1 or Th17 adjuvants) with specific pathogen-derived promiscuously MHCII-binding Th peptides (PPPs), or proteins comprising such peptides, promotes Tc-mediated recognition of self-peptides that share homology in amino acid composition with said specific PPPs. Such autoreactive responses are likely to be triggered by ‘degenerate specificity of TCR-mediated recognition’, also called ‘TCR degeneracy of immune recognition’ [17-20]. The author postulates that this phenomenon could also occur as a result from adjuvant-mediated enhancement of PPP presentation on APC surface-expressed MHCII molecules.
In conclusion, immune responses induced by traditional vaccines are specifically directed to a limited number of immunodominant epitopes and restricted by the MHC background of the vaccine recipient. As a result, pathogens may escape vaccine-induced humoral and/or cellular host immune responses, thereby preventing vaccines from providing the target vertebrate population with broad and longstanding protection against target pathogens. Despite their capacity to successfully prevent systemic infection or disease caused by one or more (the latter, for example, in case of multivalent vaccines) specific pathogens, current Ab-based vaccines, for example, do not usually provide significant cross-protective immunity and may even fail to protect a subset of vaccine recipients (so-called ‘non-responders’). On the other hand, T cell-based vaccines targeted at cell-bound pathogenic antigens primarily protect individuals that are already naturally predisposed to T cell-mediated pathogen control by virtue of their expression of protective MHC or TCR alleles. Hence, the protective effect of T cell-based vaccine candidates in controlling infection or disease strongly depends on the immunogenetic background of the vaccine recipient.
Even ‘modern’ sophisticated vaccine constructs, for example comprising multiple Bc and/or Tc target epitopes combined with (promiscuous) T helper peptides and/ or adjuvants, are basically still mimicking naturally induced immune responses. This already explains why vaccines have failed to induce long-lasting, broadly and universally protective immunity and may ultimately allow the pathogen to escape from the host immune system. Because adjuvantation of vaccines may lead to changes in immunodominance hierarchy between the selected vaccinal epitopes, adjuvants are at risk of enabling protective vaccinal epitopes to evade vaccine-induced immune responses. This would particularly apply to situations where multi-epitope constructs co-formulated with adjuvants are used for mass vaccination campaigns or routine immunisation programs.
NK Cell-Based Immunotherapy and Vaccines: The New Holy Grail in Modern Immune Intervention?
Based on all of the above, there is an obvious medical need for vaccinal antigens that are truly and universally protective in that they are capable of educating the host immune system to mount a type of protective immune response which the target pathogen is unable to escape from.
In this regard, NK cell-based immune interventions are causing a great deal of excitement and have strengthened the belief that NK cells can effectively contribute to fighting infectious diseases or controlling cancer. Natural killer cells (NK cells) are, indeed, widely renowned for their role in eliminating virus-infected as well as damaged and malignant (i.e., transformed) cells very efficiently [21]. They are characterised by a thin line of discriminative capacity of ‘self ’ as compared to ‘non-self ’ and by their innocuity towards healthy host cells. Their cytotoxic action is balanced by signalling through patternrecognition receptors on the NK cell surface. These receptors serve an NK activating (e.g., via monomorphic NKG2D and NKp46 in man and mice, respectively) or inhibitory role (i.e., via family of polymorphic KIRs and Ly49 receptors in man and mice, respectively). By virtue of their MHC class I-specific inhibitory pattern recognition receptors, NK cells are capable of detecting and killing cells that have lost cognate self MHC class I-educating ligands [22,23]. In addition, the expression on target cells of ligands recognised by activating NK receptors (e.g., NKG2D and NKp46) serves as another important checkpoint for NK cell activation and induction of their cytolytic activity and cytokine production [24,25].
Germline-encoded activating NK cell receptors (NCRs) recognise aberrant expression of self-specific ligands on autologous cells. They sense alterations in expression patterns of self-ligands expressed on transformed, stressed or infected host cells [13,26]. NK cells use a diversified array of these activating NK cell receptors to detect changes in their environment and respond to alterations caused by transformation, cellular stress or infection. NK cells can also respond to specific antigens by receptors that are seemingly required for Agspecific recognition (e.g., NKG2C, NKG2D). However, the interaction between such specific antigens and activating receptors on NK cells cannot explain antigen-specific features of the immune response. This already suggests that interactions between specific antigenic ligands and receptors on NK cells do not require these ligands to display a specific antigenic sequence but rather enable sensing of ‘incompatible’ ligands [13,27]. Such incompatibility might be due to aberrant or unfavourable binding of antigenic ligands to cognate self MHC class I molecules. The idea that NK cells could be endowed with an alternative ligand-sensing system could also explain their capacity to recognise a broad and highly diversified spectrum of antigen patterns. It has repeatedly been reported that NK cells can be primed and educated to acquire memory if they are activated by sensitising signals that are delivered in the absence of activation of inhibitory MHCI- specific receptors [28]. Co-activation resulting from the loss of inhibitory control is thought to lower the activation threshold of NK cells for responding to a diversified range of virus-, tumour- or stress-derived signals or ligands. The resulting enhancement of NK cell-mediated responsiveness towards these ligands is thought to rely on several different mechanisms [29]:
Confined compartmentalisation of activation receptors (e.g. NKp46) in signalling microclusters/ nanodomains at the immune synapse.
Enhanced expression on NK cells of adhesion molecules (e.g. lymphocyte function-associated antigen 1, also known as LFA-1) that enable stable conjugate formation.
Down-regulated expression of inhibitory receptors specific for self MHCI molecules.
Meanwhile, several studies provide compelling evidence of antigenspecific recall responses of memory NK cells but the molecular interactions between NK cell receptors and cognate pathogen-derived antigens that underlie these responses are still largely unknown [30,31]. Scientists increasingly acknowledge that an improved understanding of the mechanisms that enable durable antigen-specific immune recognition by a diversified spectrum of memory NK cells may unleash the immunological and clinical potential of NK cell-based immunotherapies and even pave the way to NK cell vaccines that could help defeat infectious, immune-mediated or tumour diseases. The idea that NK cells could be endowed with an alternative ligandsensing system is particularly intriguing and may require the scientific and medical community to re-think approaches to investigate or exploit NK cell triggering. Unlike recognition of pathogen-derived target antigens used in traditional vaccines, NK cell-mediated recognition of pathogen-derived antigens that are reminiscent of ‘self ’ (so-called ‘altered self ’ antigens) is not MHC-restricted in that these cells recognise antigens regardless of the immunogenetic background of the host, sometimes even across phylogenetically unrelated host vertebrate species (hence called ‘universal’ vaccines). There is a particular medical need to better explore and exploit immunisation strategies that harness NK cells to acquire adaptive immune features and enable improved vaccines or immunotherapeutic approaches capable of eliminating infected or pathologically altered or transformed host target cells. As NK cells naturally serve a selfprotective function, more research into pathogen-derived immune subversive antigens that bear the hallmarks of ‘self ’ is warranted. An improved understanding of the role of pathogenic self-mimicking antigens combined with better insights in the immune pathogenesis of infectious or immune-mediated diseases would enable a more rational design for modern immune interventions. Novel, NK cell-based vaccines would ideally be capable of teaching these innate immune cells to mount a full-fledged adaptive immune response towards immune subversive antigens that deviate from ‘self ’.
It is reasonable to assume that immune interventions that have the capacity to prime NK cells into long-lived, effector memory cells which universally recognise a broad spectrum of pathogens, even across phylogenetically unrelated strains or species, would greatly contribute to the prevention or control of multiple infectious, immune-mediated or oncogenic diseases in naturally susceptible vertebrate species. Such broadly and universally protective vaccines would be highly costeffective and obviate the need for developing personalised vaccines,which would be prohibitively expensive and difficult to implement in most parts of the world.
References
- Alpert MD (2012) ADCC develops over time during persistent infection with live-attenuated SIV and is associated with complete protection against SIV (mac) 251 challenge. PLoS Pathog 8: e1002890.
- Bonsignori M (2012) Antibody-dependent cellular cytotoxicity-mediating antibodies from an HIV-1 vaccine efficacy trial target multiple epitopes and preferentially use the VH1 gene family. J Virol 86: 11521-11532.
- Bhasin VK (2008) Problems with the oral polio vaccine. Nature Medicine 14: 9-12.
- Willyard C (2007) Polio eradication campaign copes with unusual outbreak. Nature Med 13: 1394.
- Hansen SG (2013) Cytomegalovirus vectors violate CD8+ T Cell epitope recognition paradigms. Science 340: 1-34.
- Boyman O (2010) Bystander activation of CD4+ T cells. Eur. J. Immunol 40: 936-939.
- Chung EH (2014) Vaccine allergies. Clin Exp Vaccine Res 3: 50-57.
- Fritsche PJ (2010) Vaccine hypersensitivity - update and overview. Swiss Med Wkly. 140: 238-246.
- Openshaw PJM, Tregoning JS (2005) Immune Responses and Disease Enhancement during Respiratory Syncytial Virus Infection. Clin Microbiol Rev 18: 541-555.
- Takada A, Kawaoka Y (2003) Antibody-dependent enhancement of viral infection: Molecular mechanisms and in vivo implications. Rev Med Virol 13: 387-398.
- Thomas S (2006) Antibody-dependent enhancement and vaccine development. Expert Rev Vaccines 5: 409-412.
- Davila EA, Kolls J (2010) Toll for Th17 cell expansion. J Leukocyte Biol 88: 5-7.
- Dardalhon V (2008) Role of Th1 and Th17 cells in organ-specific autoimmunity. J Autoimmun 31: 252–256.
- Gilbertson B (2004) Bystander Activation of CD8+ T Lymphocytes during Experimental Mycobacterial Infection. Infection and Immunity 72: 6884-6891.
- Palmer MT, Weaver CT (2010) Autoimmunity: Increasing suspects in the CD4+ T cell lineup. Nature Immunol 11: 36-40.
- Wooldridge L (2012) A single autoimmune T cell receptor recognizes more than a million different peptides. J Biol Chem 287: 1168-1177.
- Grogan JL (1999) Cross-reactivity of myelin basic protein-specific T cells with multiple microbial peptides: experimental autoimmune encephalomyelitis induction in TCR transgenic mice. J Immunol 163: 3764-3770.
- Roep BO (2010) Islet inflammation and CXCL10 in recent-onset type 1 diabetes. Cl Exp Immunol 159: 338-343.
- Tavares RG (2012) Enterovirus infections and type 1 diabetes mellitus: is there any relationship? J Venom Anim Toxins 18: 3-15.
- Wucherpfennig KW, Strominger JL (1995) Molecular mimicry in T cell-mediated autoimmunity: viral peptides activate human T cell clones specific for myelin basic protein. Cell 80: 695-705.
- Vivier E (2008) Functions of natural killer cells. Nature Immunology 9: 503-510.
- Cruz-Munoz ME, Veillette A (2010) Do NK cells always need a license to kill? Nature Immunol 11: 279–280.
- Jaeger BN, Vivier E (2012) When NK cells overcome their lack of education. J Clin Invest 122: 3053-3056.
- Kwon HJ, Kim N, Kim HS (2017) Molecular checkpoints controlling natural killer cell activation and their modulation for cancer immunotherapy. Expe Mol Medicine 49: e311.
- Long EO (2013) Controlling NK Cell Responses: Integration of Signals for Activation and Inhibition. Annu Rev Immunol 31: 227-258.
- Lanier LL (2008) Up on the tightrope: natural killer cell activation and inhibition. Nature Immunol 9: 495-502.
- Rydyznski CE, Waggoner SN (2015) Boosting vaccine efficacy the natural (killer) way. Trends in Immunol 36: 536-546.
- Bryceson YT (2006) Activation, co–activation, and co–stimulation of resting human NK cells. Immunol Rev 214: 73-91.
- He Y, Tian Z (2016) NK cell education via nonclassical MHC and non-MHC ligands. Cell Mol Immunol 13: 1-10.
- Byrd A (2007) Expression Analysis of the Ligands for the Natural Killer Cell Receptors NKp30 and NKp44. PLoS ONE 2: e1339.
- Joyce MG, Sun PD (2011) The Structural Basis of Ligand Recognition by Natural Killer Cell Receptors. J Biomed Biotech.
Relevant Topics
- Bacteriostatic antibiotics
- Cell signaling and activation
- Chemokines
- Class I MHC molecules
- Class II MHC molecule
- Colitis Antibiotics
- Immune response
- Immunochemistry
- Immunogenicity of biopharmaceuticals
- Immunogenomics
- Immunoglobulins
- Immunoglycomics
- Immunomodulatory xenobiotics
- Immunopharmacology
- Immunoproteomics
- Immunosenescence
- Immunotolerance
- Molecular Immunology
- Non classical MHC class I molecules
Recommended Journals
Article Tools
Article Usage
- Total views: 16746
- [From(publication date):
October-2017 - Jul 15, 2025] - Breakdown by view type
- HTML page views : 15601
- PDF downloads : 1145