Resveratrol-Mediated Protection via Hypoxia-Inducible Factor
Received: 30-Jun-2022 / Manuscript No. SCIENCE-22-68102 / Editor assigned: 04-Jul-2022 / PreQC No. SCIENCE-22-68102 / Reviewed: 18-Jul-2022 / QC No. SCIENCE-22-68102 / Revised: 29-Aug-2022 / Manuscript No. SCIENCE-22-68102 / Published Date: 05-Sep-2022 DOI: 10.4172/SCIENCE.1000127
Abstract
Hypoxia Inducible Factor (HIF) is a dimeric protein complex that involves the body's response to hypoxia or low oxygen concentrations. The HIF pathway is induced due to insufficiency in oxygen supply into cells and tissues. It has been proposed that HIF signalling pathway contributes to the development of pathological conditions, particularly cancer. Therefore, there have been attempts to target HIF signalling pathway to inhibit the development of pathological conditions. Plant derived chemicals have shown a promising profile due to their common side effects and significant pharmacological effects. Resveratrol is a naturally occurring polyphenol exclusively found in plants such as grapes and peanuts. Resveratrol has a variety of biological and therapeutic activities, including antioxidant, anti-inflammatory, hepatoprotective, cardioprotective, anti-tumor and anti-diabetic. In the present review, we focus on the modulatory effect of resveratrol on the HIF pathway and its relationship with its protective and anti-tumor effects.
Keywords: Cancer therapy, Herbal medicine, Hypoxia inducible factor, Resveratrol
Introduction
A number of criteria are considered for selecting a drug in the treatment of a disorder. It seems that the side effects and great therapeutic effects are more important than others. On the other hand, the cost is another determining factor. Over the past decades, scientists have focused on developing novel drugs, particularly anti-tumor drugs. However, it has been shown that the new synthetic drugs are associated with high side effects and they also suffer from high cost. Researchers have paid attention to nature, as a valuable source of naturally occurring compounds having favorable biological and therapeutic effects. Regardless of pharmacological effects, naturalderived compounds have minimal side effects. These are the most important factors which have resulted in attention to plant derived chemicals. Resveratrol (Res) is one of the members of polyphenols exclusively found in plants such as Eucalyptus, lilium, black mulberry, peanut and all kinds of grapes. The discovery of Res returns back to 1940 when this naturally occurring compound was isolated from the root of the Veratrum grandiflorum. It was extensively applied in traditional Chinese and Japanese medicine in 1960. Res are synthesized in response to stress conditions such as mechanical damage, ultraviolet light, and fungal contamination. Drinking red wine is a common habit among French people, it has been demonstrated that they are resistance to Cardiovascular Disorders (CVDs) than others. After extensive research, it was found that this resistance to CVDs is due to the presence of Res in wine [1].
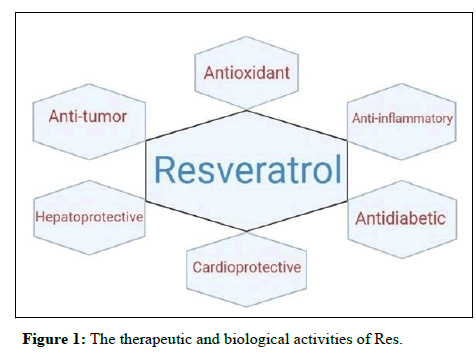
The attention towards Res is due to its various pharmacological effects such as antioxidant, anti-inflammatory, antitumor, hepatoprotective, cardioprotective and anti-diabetic (Figure 1). Markus and colleagues have shown that Res consumption is associated with a decreased incidence rate of CVD. Research has indicated that Res is able to reduce lipid peroxidation and provide the dilation of blood vessels, leading to a decreased incidence rate of CVDs. The inhibitory effect of Res on high blood pressure is a result of its antioxidant activity. Res have a potential candidate for decreasing the volume of adipose tissue. It has been suggested that providing a regimen containing Res is beneficial for inhibiting metabolic syndrome. A Res in combination with Mesenchymal Stem Cells (MSCs) diminishes the adverse effects of diabetes. Based on the antioxidant activity of Res, nuclear factor erythroid 2 related factors 2 (Nrf2) and its downstream mediators such as Heme Oxygenase-1 (HO-1) can be involved by Res consumption.
Kong and colleagues demonstrated that Res can exert a beneficial effect in the treatment of Alzheimer’s Disease (AD) by stimulation of the Nrf2/HO-1 signaling pathway as well as improving antioxidant defense system. Res can be considered as an adjuvant for chemotherapy. The anti-tumor activity of Res is also attributed to its modulatory effect on the differentiation and self-renewal of cancer stem cells. Song and colleagues, have found that Res effectively inhibits the self-renewal capacity of glioma stem cells by decreasing B cell-specific Moloney murine leukemia virus integration site 1 (Bmi1) and SRY related HMG-box-2 (Sox2) markers. Research has shown that Res ameliorates bladder cancer cells. Res remarkably reduce the viability and proliferation of tumor cells and stimulate apoptotic cell death by down regulation of SRC, protein kinase B (Akt) and mammalian Target of Rapamycin (mTOR). Res stimulated S phase arrest by down regulating Polo Like Kinase-1 (PLK-1). Res have demonstrated a promising profile for protecting cells against radiation. Regardless of extensive research about the protective and anti-tumor activities of Res, there is a lack of precise mechanisms as well as the impact of this naturally occurring on the Hypoxia Inducible Factor (HIF). At the present review, we evaluate the effects of Res on the HIF and reveal how this compound affects HIF to exert its therapeutic effects [2-6].
Materials And Methods
HIF signalling pathway
Hypoxia Inducible Factor (HIF) is a kind of DNA binding protein being able to enhance or suppress a wide range of homeostasis genes. HIF is stimulated during hypoxic conditions. Moreover, except hypoxia, there are some other conditions which can control HIF expression such as growth factors, lack of tumor suppressing mechanism and increasing oncogene factors. Induction of HIF can protect the cells. HIF as a heterodimeric transcription factor has α- and β-subunit. The first subunit is oxygen-regulated but the second is oxygen independent. The α- and β-subunit have respectively three and two isoforms that are involved during hypoxia [7-10].
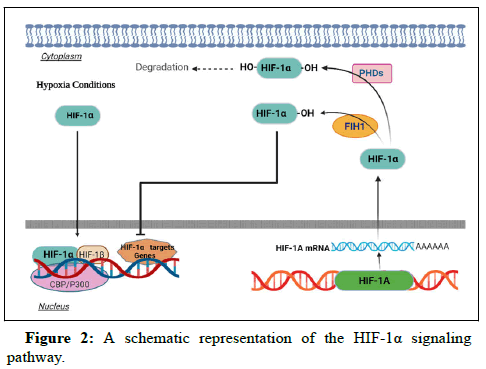
The RNA transcription of HIF-1α gene occurs in the nucleus by some proteins, known as HIF-1β Specificity protein (Sp) 1, P300. The HIF-1α protein is hydroxylated and then ubiquitinated but it can be destructed with the help of proteasomes during the oxygenated term. During hypoxic conditions, HIF-1α protein enters into the nucleus and binds to HIF-1β subunit to constitute a specific transcription complex. The complex regulates the target genes involved in hypoxia conditions. The degeneration of HIF-1α by the hydroxylation, linking of HIF-1α to other subunit and regulation of the HIF-1α gene are therapeutic methods following by the scientists (Figure 2). The energetic protein links to Hypoxia Response Elements (HREs) on the regions of determined genes, specially enhancer or promoter regions, leading to the activation of the transcriptional factors. The former research has shown that HIFα subunit interacts with HIFβ subunit and then links to specific regions of DNA. PER-ARNT-SIM (PAS) and basic Helix Loop Helix (bHLH) A and B domains help to the dimerization of mentioned subunits. The process of linking to DNA happens via the bHLH domains. The activity of HIF protein is regulated by Transcriptional Activation Domains (TADs) locating in the C-terminal part. Both of the HIF1α and HIF2α subunits possess two TADs. They are called the C-TAD and N-TAD. The hydroxylation of Factor Inhibiting Hif (FIH) activates C-TAD, head whereas N-TAD activity is not dependent on HIF. Therefore, HIFα is activated and inactivated by the FIH function. The HIF1α and HIF2α isoforms can cooperate together to regulate the genes by a fundamental overlap. At present, there is the contradictory information about the expression and roles of HIF3α isoform in hypoxic and non-hypoxic conditions. The activity of more than 60 genes is increased by the HIF signaling pathway. The most well-known gene among them is Vascular Endothelial Growth Factor A (VEGF-A). HIFs play significant roles to active the involved genes in homeostasis, erythropoiesis, vasodilation, metabolism and autophagy mechanisms. In spite of an increase in the gene expression in the mentioned mechanisms, HIFs are also able to suppress some other genes [11-15].
HIF-1α transcriptionally triggers target genes in reaction to hypoxia. Under normoxic conditions, PHDs and other prolyl hydroxylases lead to hydroxylation of HIF-1α. VHL proteins realize Hydroxylated HIF-1α to ubiquitinated and degrade. Moreover, FIH1 regulates the transcriptional activity of HIF-1α via hydroxylating an asparagine residue of HIF-1α in its C-terminal transactivation domain leading to the blockage of the interaction between CBP/p300 and HIF-1α. Hypoxia conditions reduce the hydroxylation reactions leading to HIF-1α accumulation, decrease transcriptional function, dimerization with HIF-1β, binding to target genes and regulation of the genes via CBP/p300 and the transcription initiation complex [16-20].
HIF signalling pathway in pathological conditions
Many researchers have confirmed the role of HIF signaling pathway in the development of pathological conditions, particularly cancer. It has been shown that HIF-2α can be considered as a potential target in breast cancer therapy. Therefore, it has been suggested that stimulation of HIF-2α is associated with invasion and Epithelial to Mesenchymal Transition (EMT) of tumor cells. HIF-1α is also important for inhibiting the malignancy of breast cancer cells. Decreasing the HIF-1α expression remarkably reduces the metastasis of murine breast cancer cells, demonstrating the pivotal role of HIF signaling in cancer therapy. Cancer is not the only pathological condition that HIF signaling pathway plays a remarkable role, but it has also been shown that the HIF pathway is vital in the progression of osteoarthritis. There have been attempts to elucidate the role of the HIF signaling pathway in OA therapy. Cho and colleagues indicated the ameliorative impact of apigenin on OA. The findings demonstrated that administration of apigenin significantly reduces the expression of HIF-2a and also its downstream mediators such as Matrix Metalloproteinase 3 (MMP3), MMP13, Interleukin-6 (IL-6) and Cyclooxygenase-2 (COX-2), leading to the inhibition of inflammation in chondrocytes. It has been demonstrated that enhanced expression of HIF-1a effectively elevates knee OA synovial fibrosis. Therefore, a variety of studies have been designed in order to target HIF signaling pathway in the pathological conditions. Several hypoxia mediated mechanisms such as apoptosis and angiogenesis are modulated by HIF which play a remarkable role in metastasis and tumor growth. As an important mediator of placenta development, intense expression of HIF-1α is associated with congenital disorders. The limited fetal growth and preeclampsia are induced by high expression of HIF-1α. It has been suggested that HIF-2 has harmful impacts on bone for decreasing born mass in some of the disorders such as osteoporosis, chronic diseases and aging. It has been supposed that the HIF signaling pathway has a considerable role in lipid metabolism and its impairment is observed in metabolism disorders.
Results And Discussion
Ameliorative effect of resveratrol mediated by HIF modulation
Anti-tumor activity: Cancer cells need a hypoxia condition to grow; therefore, targeting hypoxia is of interest. The exposure of Res to the PC3 prostate cancer cells showed the anti-tumor activity of Res. The findings have shown that Res effectively suppressed the growth and viability of tumor cells by inhibiting glucose fermentation as well as elevating respiration and prevention of hypoxia condition. The inhibitory impact of Res is partially mediated by decreasing the level of HIF-1. The prevention of the growth of tumor cells under stress conditions is a potential strategy in inhibiting the progress of tumor cells. It seems that Res is able to diminish the growth of tumor cells under chronic stress. Res remarkably stimulate apoptotic cell death in tumor cells and reduce their viability at a dose-dependent manner. Researchers have shown that these anti-tumor effects are mediated through the α-Adrenergic Receptor (ADRB-2)/HIF-1α axis. Res decreases the expression profile of ADRB2, resulting in reduced expression of HIF-1α. However, in order to exert anti-tumor activity, Res may upregulate the expression of HIF-1. Wang and colleagues investigated the effect of Res on the viability of prostate cancer cells. The results have revealed that Res significantly inhibits the migration and cell survival of tumor cells, whereas it stimulates apoptotic cell death via enhancing the expression of Bax and down regulation of Bcl-2. In order to evaluate the role of HIF-1α in the anti-tumor effects, HIF-1α was knockdown, leading to the decreased anti-tumor effects. Research has demonstrated that Res enhanced the expression of HIF-1α, resulting in an enhanced concentration of ROS and upregulation of p53.
Arthritis therapy: The tumor cells enhance angiogenesis to make more progress in their migration and malignancy. Therefore, targeting angiogenesis is of importance in the treatment of many disorders including cancer. It has been suggested that inhibition of angiogenesis can be considered as a promising strategy in Rheumatoid Arthritis (RA) therapy. The effect of Res was examined on the Sprague-Dawley rat arthritis model as well as an in vitro model of Interleukin-1β (IL-1β) mediated Rat Synovial Cells (RSC-364). The findings have revealed that Res remarkably attenuates RA by down-regulation of HIF-1α and subsequently, a decrease in HIF-1α mediated angiogenesis. One of the priorities in OA therapy is the prevention of cartilage degeneration. It has been shown that Res administration is advantageous in OA treatment so that Res remarkably diminishes cartilage degeneration and improves chondrocyte autophagy partly by affecting the expression profile of HIF-1α and HIF-2α. Researchers have shown that Res is respectively associated with up-regulation and down regulation of HIF-1α and HIF-2α.
Protection against stress: The ameliorative impact of Res on hypobaric hypoxia induced adverse effects on rat retina was evaluated. The results have shown that Res is able to remarkably decrease the harmful effects of stress on the retina by down regulation of hypoxia stress associated genes such as HIF-1.
Hepatoprotective activity: Oxidative stress can predispose into Alcoholic Fatty Liver Disease (AFLD). Research has shown that Res can ameliorate AFLD. The results have demonstrated that Res administration remarkably diminishes alanine Aminotransferase (ALT) and Aspartate aminotransferase (AST) levels. Res exposure was also associated with decreased fat deposition, Triglyceride (TG) content and liver injury. It seems that the ameliorative impacts are partially mediated by down-regulation of HIF-1α.
Neuroprotective activity: A variety of studies have indicated the neuroprotective activity of Res, however, at the present review, we focus on the neuroprotective effect of Res mediated by modulation of the HIF signaling pathway. Rosa and colleagues examined the protective effect of Res on glial cells. Lipopolysaccharide (LPS) induced stress and inflammation in the glial cells. Finding has shown that Res effectively inhibits LPS induced inflammation partly via down-regulating of HIF-1α.
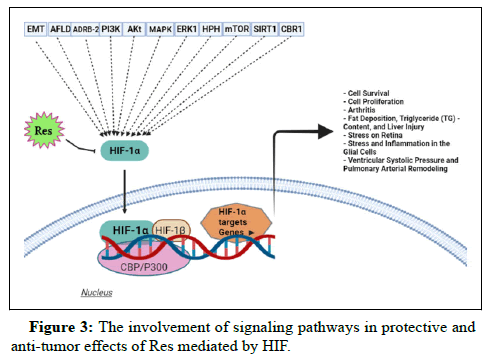
Lung-protective activity: The HIF signaling pathway can be effective for ameliorating the Hypoxic Pulmonary Hypertension (HPH). Administration of Res considerably ameliorated right ventricular systolic pressure and pulmonary arterial remodeling induced by hypoxia. Res also suppressed the infiltration of inflammatory cells. It is supposed that the anti-inflammatory impacts of Res are mediated by down regulation of HIF-1α through upstream mediators such as the MAPK/ERK1 and PI3K/Akt pathways (Figure 3).
HIF-1α: Hypoxia Inducible Factor-1α; EMT: Epithelial to Mesenchymal Transition; AFLD: Alcoholic Fatty Liver Disease; ADRB-2: β-adrenergic Receptor; PI3K: Phosphatidylinositide 3- Kinase; Akt: protein kinase-B; MAPK: Mitogen Activated Protein Kinase; ERK1: Extracellular Signal Regulated Kinase 1; HPH: Hypoxic Pulmonary Hypertension; mTOR: mammalian Target of Rapamycin; SIRT1: Sirtuin 1; CBR1: Carbonyl Reductase 1.
Conclusion
Targeting the HIF signaling pathway by Res is of importance for its acting as anti-tumor and protection. It has been supposed that the HIF signaling pathway impairment is associated with the development of pathological conditions, specifically cancer. Res are able to modulate the HIF signaling pathway to exert protective and anti-tumor effects. Res show anti-tumor activity through downregulating the expression of the HIF signaling pathway which leads to the decreased viability, proliferation, invasion and EMT. Furthermore, Res may upregulate the HIF signaling pathway to reduce the viability and proliferation of cancer cells. It has been postulated that targeting the HIF signaling pathway is beneficial in arthritis therapy. Studies have revealed that the expression of HIF-1α decreased during arthritis therapy. It seems, the lung-protective, hepatoprotective and neuroprotective activities of Res are mediated by down regulation of the HIF signaling pathway. Figure 1 provides a summary of the signaling pathway may involve in Res impact.
Acknowledgements
The author would like to express his heartfelt and warm thanks to Bahman Rafiei and Robert Earl Nickerson for their support.
References
- Ashrafizadeh M, Ahmadi Z (2019) Effect of astaxanthin treatment on the sperm quality of the mice treated with nicotine. Rev Clin Med 6: 1-5.
- Ahmadi Z, Mohammadinejad R, Ashrafizadeh M (2019) Drug delivery systems for resveratrol, a non-flavonoid polyphenol: Emerging evidence in last decades. J Drug Deliv Sci Technol 51: 591-604.
- Albers RE, Kaufman MR, Natale BV, Keoni C, Kulkarni-Datar K (2019) Trophoblast-Specific Expression of Hif-1α Results in Preeclampsia-Like Symptoms and Fetal Growth Restriction. Sci Rep 9: 2742.
- Almeida TC, Guerra CC, de Assis BL, de Oliveira ASRD (2019) Antiproliferative and toxicogenomic effects of resveratrol in bladder cancer cells with different TP53 status. Environ Mol Mutagen 60: 740-751
- Brahimi-Horn MC, Pouysségur J (2009) HIF at a glance. J Cell Sci 122: 1055-1057.
- Carpene C, Les F, Casedas G, Peiro C, Fontaine J, et al. (2019) Resveratrol Anti-Obesity Effects: Rapid Inhibition of Adipocyte Glucose Utilization. Antioxidants 8: 74.
- Cho C, Kang LJ, Jang D, Jeon J, Lee H, et al. (2019) Cirsium japonicum var. maackii and apigenin block Hif‐2α‐induced osteoarthritic cartilage destruction. J Cell Mol Med 23: 5369-5379.
- Choi YJ, Heo K, Park HS, Yang KM, Jeong MH, et al. (2016). The resveratrol analog HS-1793 enhances radio sensitivity of mouse-derived breast cancer cells under hypoxic conditions. Int J Oncol 49: 1479-1488.
- Dayan F, Roux D, Brahimi-Horn MC, Pouyssegur J, Mazure NM, et al. (2006). The oxygen sensor factor-inhibiting hypoxia-inducible factor-1 controls expression of distinct genes through the bifunctional transcriptional character of hypoxia-inducible factor-1α. Cancer Res 66: 3688-3698.
- Fallah J, Rini BI (2019) HIF Inhibitors: Status of Current Clinical Development. Curr Oncol Rep 21: 6.
- Farkhondeh T, Samarghandian S, Roshanravan B (2019) Impact of chrysin on the molecular mechanisms underlying diabetic complications. J Cell Physiol 234: 17144-17158.
- Farkhondeh T, Samarghandian S, Pourbagher‐Shahri AM, Sedaghat M (2019) The impact of curcumin and its modified formulations on Alzheimer's disease. J Cell Physiol 234: 16953-16965.
- Firouzi F, Khoei S, Mirzaei HR (2015). Role of resveratrol on the cytotoxic effects and DNA damages of iododeoxyuridine and megavoltage radiation in spheroid culture of U87MG glioblastoma cell line. Gen Physiol Biophys 34: 43-50.
- Fonseca J, Moradi F, Maddalena LA, Ferreira‑Tollstadius B, Selim S, et al. (2019). Resveratrol integrates metabolic and growth effects in PC3 prostate cancer cells involvement of prolyl hydroxylase and hypoxia inducible factor 1. Oncol Lett 17: 697-705.
- Hou CY, Tain YL, Yu HR, Huang LT (2019) The Effects of Resveratrol in the Treatment of Metabolic Syndrome. Int J Mol Sci 20: 535.
- Huang XT, Li X, Xie ML, Huang Z, Huang YX, et al. (2019) Resveratrol: Review on its discovery, anti-leukemia effects and pharmacokinetics. Chem Biol Interact 306: 29-38.
- Huo X, Zhang T, Meng Q, Li C, You B, et al. (2019) Resveratrol Effects on a Diabetic Rat Model with Coronary Heart Disease. Med Sci Monit 25: 540-546.
- Jia R, Li Y, Cao L, Du J, Zheng T, et al. (2019) Antioxidative, anti-inflammatory and hepatoprotective effects of resveratrol on oxidative stress-induced liver damage in tilapia (Oreochromis niloticus). Comp Biochem Physiol C Toxicol Pharmacol 215: 56-66.
- Kim DH, Hossain MA, Kim MY, Kim JA, Yoon JH, et al. (2013) A novel resveratrol analogue, HS-1793, inhibits hypoxia-induced HIF-1α and VEGF expression, and migration in human prostate cancer cells. Int J Oncol 43: 1915-1924.
- Kim DH, Sung B, Kim JA, Kang YJ, Hwang SY, et al. (2017) HS-1793, a resveratrol analogue, downregulates the expression of hypoxia-induced HIF-1 and VEGF and inhibits tumor growth of human breast cancer cells in a nude mouse xenograft model. Int J Oncol 51: 715-723.
Citation: Rafiei H (2022) Resveratrol-Mediated Protection via Hypoxia-Inducible Factor. Arch Sci 6: 138. DOI: 10.4172/SCIENCE.1000127
Copyright: © 2022 Rafiei H. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.
Share This Article
Open Access Journals
Article Tools
Article Usage
- Total views: 1658
- [From(publication date): 0-2022 - Apr 04, 2025]
- Breakdown by view type
- HTML page views: 1323
- PDF downloads: 335