Resseguier Method Reduces Neuromuscular Hyper-Excitability and ClinimetricParameters in Patients with Fibromyalgia Syndrome
Received: 04-Mar-2016 / Accepted Date: 03-May-2016 / Published Date: 06-May-2016
Abstract
Background and aim: According to ACR 2011 criteria, Fibromyalgia Syndrome (FMS) is a disorder characterized by widespread musculoskeletal pain for more than 3 months accompanied by fatigue, sleep, memory and mood issues. Recent reviews show that Mind Body Therapies (MBT) are effective to reduce the majority of FMS symptoms. Rességuier Method (RM) is a MBT that aims to obtain patient nonjudgmental awareness and control of bodily perceptions, which is effective in reducing physical and mental disorders in many rheumatic diseases. The aim of this pilot study is to evaluate the effects of RM on FMS clinical and clinimetric parameters and on neuromuscular hyper excitability (NMH), defined as reduction in the excitability threshold assessed by ischemia-hyperpnea test (IHT).
Patients and methods: Fifty-nine patients (pts), 54 women and 5 men, age 50.33 ± 12.15, were treated with RM, 1 h once a week for 8 weeks. At baseline (T0) and at the end of RM treatment (T1) they were evaluated for NMH by IHT. According to IHT results, pts were considered as negative or positive. They were also assessed for pain (Numeric Rating Scale – NRS; Pain and Regional Pain Scale–RPS), disability (Fibromyalgia Impact Questionnaire –FIQ and Health Assessment Questionnaire-HAQ), health related quality of life (SF-36) mood disorders (Hospital Anxiety and Depression Scale-HADS), sleep quality (NRS Sleep) and fatigue (Functional Assessment of Chronic Illness Therapy–fatigue: FACIT-f).
Results: At baseline, IHT was negative in 7 (11.86%) FMS pts and positive in 52 (88.14%), while after RM treatment it resulted negative in 28 (47.46%) pts and positive in 31 (52.54%). Our results show significant improvement for pain, RPS, FIQ, HAQ, HADS, sleep quality, FACIT-f, SF_36 Summary Mental Index (SMI) and Summary Physical Index (SPI) (p<0.01 for all the items).
Conclusions: We found a high prevalence of NMH in FMS patients, as assessed by IHT. We confirmed the efficacy of RMon clinimetric measures of FMS symptoms. Moreover, we showed the RM efficacy on NMH that, according to our results, could be considered as one of the therapeutic targets in FMS chronic pain.
Keywords: Fibromyalgia Syndrome (FMS); Ischemia-Hyperpnea Test (IHT); Electromyography(EMG); Quality of Life (QoL); Rességuier Method (RM); Mind Body Therapies (MBT); Health Related Quality of Life (HRQoL)
4953Introduction
Fibromyalgia Syndrome (FMS) is a condition characterized by chronic widespread pain for more than 3 months and other symptoms such as fatigue, non-restorative sleep, cognitive and somatic symptoms (headache, depressive symptoms, and irritable bowel) [1]. FMS impairs Quality of Life (QoL), social life, working activities and implies mood disorders, such as anxiety and depressive symptoms [2].
As other conditions characterized by chronic pain, FMS represents a huge burden that traditional Western medicine is currently failing to approach efficaciously. Despite many investigation of a wide range of pharmacological and non-pharmacological options, an optimal management of FMS is still unclear [3-5].
The relevance of Mind Body Therapies (MBT) in FMS is supported by recent studies that attribute the pathophysiology of the disease to central sensitization that may explain the different symptoms. FMS, in fact, was recently included in Central Sensitivity Syndromes conditions, characterized by a state of hyper excitement of the central nervous system, due to an amplification of peripheral nociceptive input, that leads to pain exacerbation and excessive sensitivity to peripheral noxious and non-noxious stimuli [6,7].
According to these recent findings, treatment of FMS should target Central Sensitivity changes with centrally acting drugs and with centrally acting non-pharmacological therapies e.g. MBT [7]. Many studies, in fact, showed the efficacy of MBT in approaching the cardinal symptoms of FMS, such as chronic pain, fatigue, difficulty in sleeping and relaxing, depression, anxiety and psychological distress, and thereby, in improving activities of daily living, self-efficacy, Quality of Life (QoL) and finally in reducing disability [6].
Rességuier Method (RM) is a MBT, tailored to patient’s features, that aims to obtain patient nonjudgmental awareness and control of bodily perceptions thus reaching a modulation of responses to pain. RM is based on the continuous attention of the therapist to the patient during all the session. This particular attitude is called “accompanying posture”. In a previous study, we showed RM leading to a significant improvement of QoL, relaxation, sleep and also to a decrease of disability and perceived pain in FMS patients, thus reducing the uptake of analgesics [8].
As musculoskeletal pain is a cardinal symptom of FMS, electromyography (EMG) was used in muscle evaluation: EMG changes were observed both in involved and uninvolved skeletal muscles [9,10].
EMG and, above all, ischaemia-hyperpnea test (IHT), may detect neuromuscular hyper excitability (NMH), defined as a reduction in the excitability threshold, that may be nonspecific or related to tetany [11,12].
Vitali et al. demonstrated a higher prevalence of NMH, assessed with IHT, in FMS in comparison with rheumatoid arthritis. Bazzichi et al. reported 25.8% of IHT positive patients in FMS also presenting a higher incidence of depressive and panic disorders and a lower number of tender points than in IHT negative patients [13,14].
A recent study of our group demonstrated that 62.5% of 145 patients suffering from FMS were positive to IHT. Moreover, IHT positive patients had a lower Health Related Quality of Life (HRQoL) and a higher fatigue than IHT negative patients [15]. Thus, IHT positivity seemed to identify FMS patients with a more severe disease.
The principal aim of the present pilot study was to evaluate the effect of RM treatment on NMH, assessed by IHT [8].
Thus, the aims of the study were to evaluate:
• the prevalence of NMH, assessed by IHT in FMS patients;
• the effects of RM on NMH, assessed by IHT;
• the efficacy of RM on clinical and clinimetric parameters in order to confirm its utility as a tailored treatment on FMS patients.
Patients And Methods
Eighty FMS patients, 8 men and 72 women, were recruited, consecutively from January 2015 to June 2015, from the outpatient clinic of the Division of Rheumatology, Department of Experimental and Clinical Medicine, University of Florence.
Inclusion criterion was the diagnosis of FMS according to the American College of Rheumatology [1].
Exclusion criteria were changes in thyroid function (Free triiodothyronine (FT3), free thyroxine (FT4), Thyroid-stimulating hormone (TSH), Thyroperoxidase antibodies (TPOAb), thyroglobulin antibodies (TgAb) and TSHAb receptor (TRAb)), electrolytes ((Magnesium (Mg) and Potassium (K)), Calcium (Ca), Parathyroid hormone (PTH) and 25-OH vitamin D3.
Patients recruited signed a written informed consent for all the procedures. All the procedures were in accordance with Helsinki Declaration of 1975/83 and the study was approved by local Ethical Committee.
At baseline (T0), patients were evaluated for demographic characteristics (sex, age) and disease duration. All patients were tested by standard EMG and IHT to evaluate neuromuscular hyperexcitability. They performed RM treatment, 1 h once a week for 8 weeks, with the same physiotherapist (CDF). They continued their pharmacological treatments (antidepressant agents by 13 pts, anxiolytic medications by 15 pts, anticonvulsant drugs by 5 pts and land exercise was continued by 4 pts, NSAIDs and analgesics only as needed) and throughout the period of the study they were asked not to start any new pharmacological therapy.
Patients were assessed by clinic and clinimetric tools at T0 and at the end of the study (T1) for pain, sleep, disability, fatigue, QoL and mood disorders.
Assessment
Electromyography (EMG) and Ischaemia-hyperpnea test (IHT)
EMG assesses the electrical potential formed in a muscle during its contraction (motor unit action potential (MUAP)), which reflects the activity of a group of motor units.
EMG/NCS and IHT were performed with Synergy electromyography equipment (VIASYS Health Care UK, Manor Way, Old Woking, Surrey, GU22 9JU, UK, 2007) by using surface electrodes for NCS and coaxial needles for EMG and IHT.
For IHT a coaxial needle was inserted in the first dorsal interosseous dorsal muscle of the left hand. The examination consisted of 2 phases:
1. Ischaemia phase (5 min), caused by applying the cuff of the sphygmomanometer (blood pressure cuff) to the lower third of the arm with a pressure of 200 mgHg.
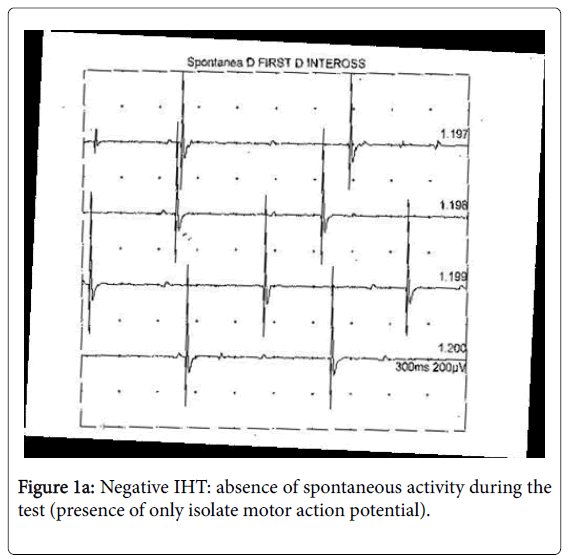
2. Hyperpnea phase (3 min), immediately after ischaemia, executed by inviting the patient to perform a deep hyperventilation with a frequency of around 40 breaths a minute. In normal subjects, no spontaneous activity is present in the muscle at rest and during IHT (Figure 1a).
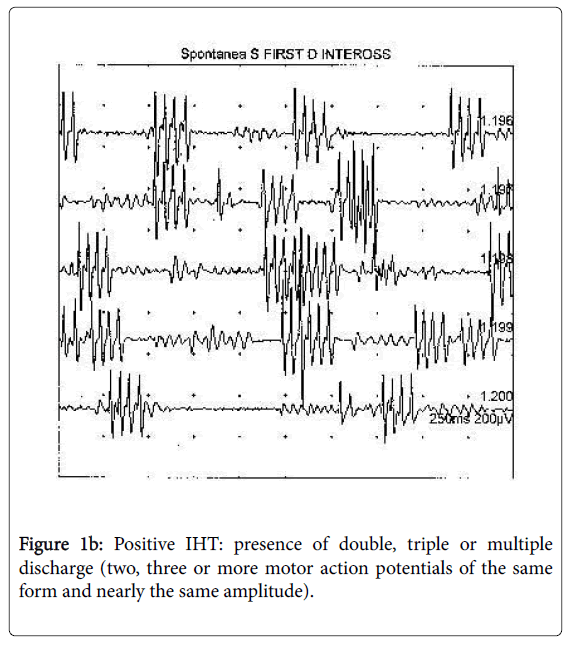
The fasciculation potential, a spontaneous involuntary discharge of an individual motor unit, may be defined as singlet. Spontaneous MUAPs that fire in groups of 2, 3, or multiple potentials are known as doublets, triplets, and multiplets, respectively. These potentials and the singlets, often seen together, represent the spontaneous depolarization of a motor unit or of its axon.
We considered IHT as positive only after the observation of coupled discharges (doublets, triplets or multiplets) (Figure 1b) [16]. The carpal spasm (tetany), which can be observed in the hands of some patients during ischaemia hase, is not supported by repetitive discharges (multiplets), but by EMG, activity (sub-interferential pattern) similar to that observed during voluntary recruitment. When doublets, triplets and multiplets are not associated to carpal spasms or tetany, the condition is defined as not specific NMH.
Clinimetric assessment
Anxiety and depression were assessed by Hospital Anxiety and Depression Scale (HADS). It is a reliable and valid self-report questionnaire for identifying and quantifying psychological distress [17]. It consists of two subscales: HADS-A (anxiety) and HADS-D (depression). HADS includes seven questions for each dimension, whose scores ranges from 0 (no depression, no anxiety) to 21 (maximal depression or anxiety). Evaluation was based on cut-off score >8 in both subscales [18].
Pain was rated with an auto-administered Number Rating Scale (NRS 0-10), which measures current pain intensity on a 0-10 scale (0=no pain, 10=worst pain) [19].
Regional pain scale (RPS) is a measure of the number of painful body regions [20].
Sleep quality was assessed by Number Rating Scale (NRS 0-10), used to measure the quality of sleep reported last week (0=the worst perceived sleep and 10=the best-perceived sleep).
Disability: Fibromyalgia Impact Questionnaire (FIQ) and Health Assessment Questionnaire (HAQ) were used to assess disability. The first is a specific self-administered instrument designed to evaluate the overall impact of FMS over many dimensions (physical functioning, work status, depression, anxiety, sleep, pain, stiffness, fatigue, and wellbeing). It consists of 10-items and it is scored between 0 and 100, with higher scores representing higher disability [21,22].
HAQ is a self-report questionnaire referring to global disability, rated from 0 (no difficulty) to 3 (unable to do) [23].
Fatigue was evaluated by Functional Assessment of Chronic Illness Therapy-Fatigue (FACIT-F) that assesses physical and functional consequences of fatigue in performing activities of daily living. Total score ranges 0-52, high scores representing less fatigue [24].
Health related quality of life (HRQoL), was assessed by Italian version of Medical Outcomes Survey Short Form 36 (SF-36) which is a self-report questionnaire with 36 items organized into 8 domains then combined into summary physical index (SPI) and summary mental index (SMI). For all the scales, the score is 0-100, with higher scores corresponding to better and lower scores to worse HRQoL (range 0–100) [25].
Rehabilitation
Rességuier Method (RM)
RM is a MBT that aims to obtain patient nonjudgmental awareness and control of bodily perceptions, in particular nociception. Its mainstay is the therapeutic relationship between therapist and patient, based on the continuous attention to the patient during all the session (regarded as “accompanying posture”). During the individual session, the therapist maintains and continuously monitors the state of attention and awareness of the patient by verbal and manual contacts leading to perform bodily active and conscious movements and respiratory exercises always respectful of the pain threshold and tailored for the patients in different positions (supine, sitting and standing) [26,27].
The purpose of the session is to obtain patient awareness and control of perceptions, derived from individual parts of the body. This may allow the patient to modulate the response to pain perception. The capacity to perceive the body and to regulate the sensations and the emotion is obtained by the instruments of RM as follows:
1. Verbal contact of the therapist. The therapist asks the patient about her/his perception of specific body segments, particularly of painful areas. Guided by the therapist, the patient describes the perceived characteristics of these areas in terms of dimensions, weight, consistency and symmetry.
2. Manual contacts of the therapist on the patient, essential to promote patient perception of specific areas.
3.“Petite gymnastique”, consisting of exercises performed during the sessions:
• Exercises of conscious respiration;
• Active and conscious movements of head, trunk, upper and lower limbs, firstly in a supine position, then sitting and standing. The therapist chooses the appropriate movements and exercises, tailoring them to the patient.
4. Home exercises, consisting of the movements of “Petite gymnastique” chosen by the therapist and tailored on the patient, are performed daily (30 min/day) both during the treatment period, and during follow-up.
Data analysis
Statistical analysis: Data are presented as Mean ± Standard Deviations (M ± SD) for continuous variables and as numbers and percentages for binomial variables. Continuous variables were tested with the Kolmogorov–Smirnov test for normality.
Due to normal distribution of the data, student’s T-test for repeated measures was used to compare variables at baseline (T0) and after RM (T1), in order to detect effect of the treatment (SPSS statistical package, version 20.0 for Windows, SPSS, Chicago, USA). The differences were considered significant for P<0.05 (IC=95%).
Minimal clinically important difference (MCID): We used MCID in order to quantify if an individual patient within a particular treatment group has a clinically significant response and if it can be used to assess the mean percentage change within a particular treatment group. MCID accounts for the varying baseline levels of functional impairment [28]. The analysis indicates that a >14% change in the FIQ total score is clinically relevant, and results of these analyses should enhance the clinical utility of the FIQ in research and practice [29]. A reduction in HAQ of >0.25 from baseline was used to determine the proportion of patients with improvements in HAQ that met or exceeded the MCID at each time point [30].
Results
Eighty FMS patients were enrolled: 21 of them did not perform IHT after RM. 21 of them did not complete the ischaemia/hyperpnea testing. 11 of them felt better and refused to continue the study, 6 of them did not finished RM treatment and 4 of them refused to perform IHT. Therefore, 21 patients were excluded from the study. Thus, we assessed 59 FMS patients (54 women and 5 men; mean age: 50.33 ± 12.15 years; 9.51 ± 10.43 disease duration).
Prevalence of IHT in FMS patients at baseline and after RM treatment
At T0, 7 (11.86%) patients were negative and 52 (88.14%) were positive while after the RM treatment 28 (47.46%) patients were negative and 31 (52.54%) were positive.
The 28 patients with negative IHT at T1 consisted in 4 patients negative also at T0 and 24 patients previously positive. The 31 patients with positive IHT at T1 were in 28 patients already positive at T0 and 3 previously negative patients.
Comparison of clinimetric parameters before and after MR treatment
The results shows a statistically significant improvement (p<0.05) by comparing HADS A, HADS D, HADS TOT, Sleep Quality, Pain Intensity, HAQ, FACIT-f, FIQ, SF_36 SMI and RPS data at baseline (T0) with T1 (p<0.001 for all comparisons). Also SF_36 SPI improved significantly (p<0.01) (Table 1). In addition, disability, assessed by HAQ and FIQ, improved in FMS patients after MR treatment according to MCID (minimal clinically important difference). More than 58% of the patients reported a clinically significant improvement in HAQ and more than 82% improved according to FIQ.
| T0 | T1 | P | |
|---|---|---|---|
| Clinimetric Parameter |
Media ± DS | Media ± DS | |
| HADS-A | 10.93 ± 4.76 | 7.74 ± 4.26 | <0.001 |
| HADS-D | 9.13 ± 5.0 | 5.56 ± 3.68 | <0.001 |
| HADS-TOT | 20.05 ± 8.69 | 13.62 ± 6.94 | <0.001 |
| Sleep Quality | 4.17 ± 2.147 | 6.65 ± 2.13 | <0.001 |
| Pain | 5.71 ± 2.71 | 3.06 ± 2.7 | <0.001 |
| FIQ | 57.46 ± 18.1 | 29.83 ± 19.59 | <0.001 |
| HAQ | 0.84 ± 0.71 | 0.33 ± 0.59 | <0.001 |
| FACIT | 23.57 ± 10.66 | 13.66 ± 9.29 | <0.001 |
| SF-36 SMI | 35.33 ± 12.4 | 44.21 ± 9.9 | <0.001 |
| SF-36 SPI | 35.64 ± 11.4 | 42.36 ± 10.46 | <0.01 |
| RPS | 12.9 ± 4.74 | 6.56 ± 6.025 | <0.001 |
Table 1: HADS A (Hospital Anxiety and Depression Scale Anxiety), HADS D (Hospital Anxiety and Depression Scale Depression), HADS TOT (Hospital Anxiety and Depression Scale Total score), FIQ (Fibromyalgia Impact Questionnaire), HAQ (Health Assessment Questionnaire), FACIT-f (Functional Assessment Chronic Illness Fatigue), SF_36 SMI (SF36 Summary Mental Index); SF36 SPI (SF_36 Summary Physical Index), RPS (Regional Pain Scale).
Discussion
The results of this study showed that 88.14% of our FMS patients were positive to IHT, demonstrating a high prevalence of NMH.
As all subjects (positive and negative to IHT) presented normal calcium metabolism, NMH was not linked to hypocalcaemia and/or hypoparathyroidism.
On the basis of bioptic, metabolic, and molecular studies, muscles are considered as involved in FMS. Muscle changes may initiate or perpetuate sensitization of muscle nociceptors and provide a tonic afferent input to central nervous system (CNS) that ultimately leads to central sensitization.
EMG, used in different studies to evaluate muscular physiopathology in FMS, showed altered muscle fibres conduction velocity (CV) in involved muscles [9] and high muscle membrane CV in muscles not clinically involved in FMS [24], suggesting an overall muscular membrane disorder [10,25].
IHT may detect NMH, defined as a reduction in the threshold of excitability of skeletal muscles [11,12]. only two studies analyzed NMH in FMS by IHT. Vitali et al. showed a higher prevalence of NMH in FMS in comparison with Rheumatoid Arthritis [13], while Bazzichi et al. [14] diagnosed spasmophilia as a comorbid and distinct condition in 25.8% of FMS patients. In this study, the diagnosis of spasmophilia was based on the positive results of IHT and on the presence of at least 1/4 of the following clinical symptoms: cramps and/or tetanic seizures, paraesthesia, tachycardia and/or dyspnea, asthenia, and dizziness.
However, as Wolfe et al. included in 2010 diagnostic ACR criteria paraesthesia, muscle stiffness and contractures, cramps, seizures or convulsions, tachycardia, dyspnoea, asthenia and dizziness, in our opinion, these symptoms could be part of the wide array of FMS manifestations rather than being representative of spasmophilia [31].
NMH, as defined by positivity at IHT, is present in the majority (88.14%) of our group of FMS patients. The reason of IHT positivity in patients with FMS is not known.
FMS is characterized by excessive sensitivity to many stimuli including pressure, chemicals and ischemia. Thus, it can be assumed that ischemia and hyperpnea, during the IHT, cause a progressive increase of activation in the second order neurons, projecting to the brain. This mechanism produces the hyperexcitable state within the CNS by involving finally ventral horn neurons that can produce discharge of doublets, triplets and multiplets [6].
Moreover, in our FMS patients IHT positivity always begins in the hyperpnea phase which causes also a high degree of psychological distress in the majority of FMS patients. It is known that psychological distress increase central sensitization and the subsequent increased motor activity [32].
The diagnosis of FMS is still difficult, underestimated and often delayed [33]; therefore, an easily performed and reproducible test, able to confirm or facilitate the diagnosis, is needed. Our results suggest that NMH is common in patients with FMS and that IHT could be helpful in diagnosing and also in characterizing FMS. In a previous study of ours, in fact, NMH positivity identified patients, in which FMS symptoms caused a higher distress, leading to QoL impairment and to increased fatigue [15]. Then IHT positivity may also be useful in better tailoring treatment interventions, particularly non pharmacological treatments such as MBT, dealing efficaciously with pain, depression, anxiety, psychological distress, fatigue and QoL [34]. In our sample, in fact, at baseline approximately 11% were negative at IHT, then after RM treatment 46% of positive patients turn into negative.
Moreover, in agreement with the literature, our FMS patients demonstrated at baseline, impaired HRQOL, disability, fatigue and pain, while after MR treatment, at T1, patients significantly improved in Mood, Sleep Quality, Pain, Disability, Fatigue, HRQOL in respect to T0 [15].
The relevance of centrally acting non-pharmacological therapies e.g. MBT in FMS is supported by recent studies that attribute the disease to central sensitization that explains all symptoms of FMS [7]. About this, many reviews report positive results of MBT and cognitive behavioral therapies in inducing a long lasting improvement of the key symptoms of FMS [4,33].
According to literature, MBT seem to be suitable and efficacious in FMS patients, by acting both on somatic and on central-derived symptoms; these methods that integrate physical, psychological, emotional, spiritual and behavioral elements, promote the rebalancing of the mind and body, often impaired in these patients. MBT, such as Tai Chi, Yoga and Qi Gong are strongly recommended by the German guidelines [35]. Tai Chi is proved safe and efficacious in FMS patients in which it reduces sleep disorders, psychological distress and fatigue and improves QoL [4,33,36,37]. In FMS, Qi Gong is effective and useful in improving local tenderness (tender points), pain, disability, psychological health and HRQoL [4,38-40]. Mindfulness Based Stress Reduction program may help in coping with stress and in improving mood and disability, while interventions based on different Yoga programs improved disability, pain, fatigue, mood and coping strategies [4,41].
In our study, the significant improvement of all the clinimetric parameters suggests a positive effect of RM in the treatment of FMS patients.
In contrast with techniques directly inducing relaxation, and somewhat similarly to Mindfulness Meditation, RM promotes a nonjudgmental awareness to sensations and emotions as they arise that, in turn, induces self-observation and thoughtful responses to pain.
Acting on these mechanisms, RM in FMS breaks the vicious circle of chronic pain-stress typical of the disease, and may lead to a more attentive vision on the immediate experience.
In a previous study [8], we assessed the efficacy of RM, in the treatment of FMS patients; the results showed a significant improvement of QoL, a significant decrease of disability and perceived pain in patients with reduction of the analgesics uptake. It is important to note that the benefits achieved during the treatment are maintained at follow-up.
These results were confirmed also in another study [42], in which an integrated treatment with RM and Qi Gong in FMS patients led to a significant improvement in pain, disability, quality of life, tenderness, anxiety. RM also ameliorated sleep and QG improved depression.
Furthermore, in the present study, a significant improvement of disability is also confirmed by the MCID values for HAQ and FIQ. 58.54% of FMS patients showed a clinically significant improvement according to HAQ score, and more than 80% showed a significant reduction of disability according to FIQ score (which is a specific tool in FMS).
RM, as other MBT, in FMS patients help to cope with pain and to disconnect the affective response to pain, thus decreasing pain catastrophizing and the consequent emotional distress.
It can therefore be assumed that RM influence IHT results in FMS patients by reducing psychological factors that contribute to central sensitization. Gracely et al. found that catastrophic thoughts in FMS are associated also with increased activity in several motor brain areas [32]. RM approach finally can rebalance the neuronal motor activity increased in FMS patients by emotional distress.
RM is efficaciously rapid and safe, because easy to be tailored and respectful of pain threshold, extremely low in FMS patients.
The feasibility of a rehabilitation treatment should guarantee a high adherence to the program and reduce the probability of dropouts. In our study, differently from other MBT, applied in FMS, the adherence to RM program is complete. Thus, it could be an useful rehabilitative tool in FMS patients, also as a “first step intervention” when other techniques are difficult to be used [4,43].
In the management of FMS patients, MBT, and in particular RM, in order to maintain a long-term effect, should be used at regular cycles and integrated, in a multicomponent approach, with medical treatment and educational measures.
Another recent study of ours [8] assessed the efficacy of 'Body movement and perception' a method that integrates RM with low impact exercises derived from soft gymnastics. The results showed an improvement of many symptoms of FMS (pain, fatigue, irritability, well-being, quality of movement, postural self-control, ability to relax mind and body, movement perception and tender point scores); this method is useful when performed after RM treatment in patients unable to deal with standard training programs.
Our study has some limitations, such as the low number of the patients and the absence of a control group and follows up.
Further investigations on wider cohorts of patients and with longitudinal design are required to confirm the utility of IHT as a diagnostic and grading tool in order to design tailored interventions for FMS patients.
Conclusion
In conclusion, we confirm in FMS patients the prevalence of NMH, assessed by IHT, and the efficacy of RM. This latter is an important non pharmacological treatment, useful not only in improving FMS symptoms, but also in reducing NMH, that could be considered as another therapeutic target in FMS.
References
- Wolfe F, Clauw DJ, Fitzcharles MA, Goldenberg DL, Häuser W, et al. (2011) Fibromyalgia criteria and severity scales for clinical and epidemiological studies: a modification of the ACR Preliminary Diagnostic Criteria for Fibromyalgia. J Rheumatol 38: 1113-1122.
- Consoli G, Marazziti D, Ciapparelli A, Bazzichi L, Massimetti G, et al. (2012) The impact of mood, anxiety, and sleep disorders on fibromyalgia. Compr Psychiatry 53: 962-967.
- Bernardy K, Klose P, Busch AJ, Choy EHS,  Häuser W (2013) Cognitive behavioural therapies for fibromyalgia. Cochrane Database Sys Rev 9: CD009796.
- Del Rosso A, Maddali Bongi S (2016) Mind body therapies in rehabilitation of patients with rheumatic diseases. Complement Ther Clin Pract 22: 80-86.
- Busch AJ, Schachter CL, Overend TJ, Peloso PM, Barber KA (2008) Exercise for fibromyalgia: a systematic review. J Rheumatol 35: 1130-1144.
- Kindler LL, Bennett RM, Jones KD (2011) Central sensitivity syndromes: mounting pathophysiologic evidence to link fibromyalgia with other common chronic pain disorders. Pain Manag Nurs 12: 15-24.
- Yunus MB (2015) Editorial review: an update on central sensitivity syndromes and the issues of nosology and psychobiology. Curr Rheumatol Rev 11: 70-85.
- Maddali Bongi S, Di Felice C, Del Rosso A, Galluccio F, Landi G, et al. (2010) The efficacy of the Rességuier method in the treatment of fibromyalgia syndrome: a randomised controlled trial. Clin Exp Rheumatol 28: S46-S50.
- Gerdle B, Ostlund N, Grönlund C, Roeleveld K, Karlsson JS (2008) Firing rate and conduction velocity of single motor units in the trapezius muscle in fibromyalgia patients and healthy controls. J Electromyogr Kinesiol 18: 707-716.
- Bazzichi L, Dini M, Rossi A, Corbianco S, De Feo F, et al. (2009) Muscle modifications in fibromyalgic patients revealed by surface electromyography (SEMG) analysis. BMC Musculoskelet Disord 10: 36.
- Ronchi O, Lolli F, Lori S, Nuti Ranucci E, Grippo A (1994) The ischaemia-hyperpnea test in the evaluation of neuronal hyperexcitability syndrome. Electromyogr Clin Neurophysiol 34: 289-294.
- Mogyoros I, Kiernan MC, Burke D, Bostock H (1997) Excitability changes in human sensory and motor axons during hyperventilation and ischaemia. Brain 120 : 317-325.
- Vitali C, Tavoni A, Rossi B, Bibolotti E, GianniniC, et al. (1989) Evidence of neuromuscular hyperexcitability features in patients with primary fibromyalgia. Clin Exp Rheumatol 7: 385-390.
- Bazzichi L, Consensi A, Rossi A, Giacomelli C, De Feo F, et al. (2010) Spasmophilia comorbidity in fibromyalgia syndrome. Clin Exp Rheumatol 28: S94-99.
- Maddali Bongi S, Del Rosso A, Lisa D, Orlandi M, De Scisciolo G (2015) Ischemia-hyperpnea test is useful to detect patients with fibromyalgia syndrome. Eur J Rheumatol DOI:10.5152/eurjrheum 2015 0094.
- Macefield G, Burke D (1991) Paraesthesiae and tetany induced by voluntary hyperventilation. Increased excitability of human cutaneous and motor axons. Brain 114 : 527-540.
- Bennett R (2005) The Fibromyalgia Impact Questionnaire (FIQ): a review of its development, current version, operating characteristics and uses. Clin Exp Rheumatol 23: S154-162.
- Sarzi-Puttini P, Atzeni F, Fiorini T, Panni B, Randisi G, et al. (2003) Validation of an Italian version of the Fibromyalgia Impact Questionnaire (FIQ-I). Clin Exp Rheumatol 21: 459-464.
- Ranza R, Marchesoni A, Calori G, Bianchi G, Braga M, et al. (1993) The Italian version of the Functional Disability Index of the Health Assessment Questionnaire. A reliable instrument for multicenter studies on rheumatoid arthritis. Clin Exp Rheumatol 11: 123-128.
- Wolfe F (2003) Pain extent and diagnosis: development and validation of the regional pain scale in 12,799 patients with rheumatic disease. J Rheumatol 30: 369-378.
- Apolone G, Cifani S, Mosconi P (1997) Questionario sullo stato di salute SF-36. Traduzione e validazione della versione italiana: risultati del progetto IHRQoLA. Metodologia e Didattica Clinica 5: 86-94.
- Zigmond AS, Snaith RP (1983) The hospital anxiety and depression scale. Acta Psychiatr Scand 67: 361-370.
- Cella D, Yount S, Sorensen M, Chartash E, Sengupta N, et al. (2005) Validation of the Functional Assessment of Chronic Illness Therapy Fatigue Scale relative to other instrumentation in patients with rheumatoid arthritis. J Rheumatol 32: 811-819.
- Klaver-Krol EG, Zwarts MJ, Ten Klooster PM, Rasker JJ (2012) Abnormal muscle membrane function in fibromyalgia patients and its relationship to the number of tender points. Clin Exp Rheumatol 30: 44-50.
- Casale R, Rainoldi A (2011) Fatigue and fibromyalgia syndrome: clinical and neurophysiologic pattern. Best Pract Res Clin Rheumatol 25: 241-247.
- Resseguier JP (2015) Riabilitazione integrata: il metodo Resseguier in “La riabilitazione multidisciplinare del malato reumaticoâ€, Firenze 2015, Editore Maddali e Bruni, pp 153-156.
- Doganay Erdogan B, Leung YY, Pohl C, Tennant A, Conaghan PG (2016) Minimal Clinically Important Difference as Applied in Rheumatology: An OMERACT Rasch Working Group Systematic Review and Critique. J Rheumatol 43: 194-202.
- Bennett RM, Bushmakin AG, Cappelleri JC, Zlateva G, Sadosky AB (2009) Minimal clinically important difference in the fibromyalgia impact questionnaire. J Rheumatol 36: 1304-1311.
- Strand V, Balbir-Gurman A, Pavelka K, Emery P, Li N, et al. (2006) Sustained benefit in rheumatoid arthritis following one course of rituximab: improvements in physical function over 2 years. Rheumatology (Oxford) 45: 1505-1513.
- Wolfe F, Clauw DJ, Fitzcharles MA, Goldenberg DL, Katz RS, et al. (2010) The American College of Rheumatology preliminary diagnostic criteria for fibromyalgia and measurement of symptom severity. Arthritis Care Res (Hoboken) 62: 600-610.
- Gracely RH, Geisser ME, Giesecke T, Grant MA, Petzke F, et al. (2004) Pain catastrophizing and neural responses to pain among persons with fibromyalgia. Brain 127: 835-843.
- Maddali Bongi S, Del Rosso A (2011) Mind Body Therapies in the Rehabilitation Program of Fibromyalgia Syndrome. New Insights into Fibromyalgia 10: 169-186.
- Langhorst J, Klose P, Dobos GJ, Bernardy K, Häuser W (2013) Efficacy and safety of meditative movement therapies in fibromyalgia syndrome: a systematic review and meta-analysis of randomized controlled trials. Rheumatol Int 33: 193-207.
- Ablin J, Fitzcharles MA, Buskila D, Shir Y, Sommer C (2013) Treatment of Fibromyalgia Syndrome: Recommendations of Recent Evidence-Based Interdisciplinary Guidelines with Special Emphasis on Complementary and Alternative Therapies. Evid Based Complement Alternat Med Article ID 485272.
- Taggart HM, Arslanian CL, Bae S, Singh K (2003) Effects of T'ai Chi exercise on fibromyalgia symptoms and health-related quality of life. Orthop Nurs 22: 353-360.
- Wang C, Schmid CH, Rones R, Kalish R, Yinh J, et al. (2010) A randomized trial of tai chi for fibromyalgia. N Engl J Med 363: 743-754.
- Chen KW, Hassett AL, Hou F, Staller J, Lichtbroun AS (2006) A pilot study of external qigong therapy for patients with fibromyalgia. J Altern Complement Med 12: 851-856.
- Haak T, Scott B (2008) The effect of Qigong on fibromyalgia (FMS): a controlled randomized study. Disabil Rehabil 30: 625-633.
- Stephens S, Feldman BM, Bradley N, Schneiderman J, Wright V, et al. (2008) Feasibility and effectiveness of an aerobic exercise program in children with fibromyalgia: results of a randomized controlled pilot trial. Arthritis Rheum 59: 1399-1406.
- Thieme K, Flor H, Turk DC (2006) Psychological pain treatment in fibromyalgia syndrome: efficacy of operant behavioural and cognitive behavioural treatments. Arthritis Res Ther 8: R121.
- Maddali Bongi S, Del Rosso A, Di Felice C, Calà M, Giambalvo Dal Ben G (2012) Rességuier method and Qi Gong sequentially integrated in patients with fibromyalgia syndrome. Clin Exp Rheumatol 30: 51-58.
- Maddali Bongi S, Del Rosso A (2015) Il trattamento riabilitativo della fibromialgia. Evidenze scientifiche. In “La riabilitazione multidisciplinare del malato reumaticoâ€, Firenze Editore Maddali e Bruni 331-336.
Citation: Susanna MB, Khadija EA, Caterina DF, Svetlana M, Angela DR, et al. (2016) Resseguier Method Reduces Neuromuscular Hyper- Excitability and Clinimetric Parameters in Patients with Fibromyalgia Syndrome. Fibrom Open Access 1: 108.
Copyright: ©2016 Susanna MB, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Open Access Journals
Article Usage
- Total views: 12999
- [From(publication date): 7-2016 - Jul 04, 2025]
- Breakdown by view type
- HTML page views: 12062
- PDF downloads: 937