Responses on Chip and pin Raman Spectroscopy in Brief
Received: 01-Apr-2023 / Manuscript No. jabt-23-95329 / Editor assigned: 03-Apr-2023 / PreQC No. jabt-23-95329 / Reviewed: 17-Apr-2023 / QC No. jabt-23-95329 / Revised: 21-Apr-2023 / Manuscript No. jabt-23-95329 / Accepted Date: 27-Apr-2023 / Published Date: 28-Apr-2023 QI No. / jabt-23-95329
Abstract
In the presence of moisture, hydrolysis and polycondensation mechanisms cause the alkoxysilane groups linked to the ends of polymer chains to cross-link silyl-modified polymers. Three separate physical states can be distinguished during these processes (viscous, skin effect and cross-linked state). To establish the open time for the adhesive business, it is crucial to understand how these states evolve at each reaction time. This information is typically gathered manually. Automation of this monitoring could reduce human error and be used to screen a large number of catalysts or for extremely lengthy cross-linking reactions. Hence, by observing the decrease in intensity of the Si-OCH3 groups during chemical reactions, a contactless microprocessing technology was established to link these physical states with an optical technology, Raman spectroscopy. Using the least amount of chemical components possible, this online characterisation approach can also be utilised to compare the efficacy of various catalysts for the cross-linking of silylmodified polymers.
Keywords
Physical States; Micro Contactless Monitoring; Raman Spectroscopy; Cross-Linking Silyl Modified Polymers
Introduction
Key components utilised as binders for elastic adhesives [1] [2], composites [3] [4], and electrolytes [5], as well as for coating [6] [3] applications, are silyl-modified polymers (SMPs). These hybrid polymers are also showing up more frequently in aerospace and aviation applications [6]. Because of its adaptable features, including Young’s modulus, tensile strength, thermal properties, gas barrier, and photonic properties, they can be employed in numerous other applications. Alkoxysilane groups connect to the ends of polymer chains via hydrolysis and polycondensation mechanisms in the presence of moisture, which causes cross-linking of SMPs [1], [5]. During cross-linking, there are various intermediary phases. For many applications, it is crucial to monitor these intermediate states in real time to understand how polymers behave. For adhesive adhesives, for example, it’s important to understand the application time (viscous state), setting time (skin effect), and time to obtain maximum properties (cross-linked state). In the literature, it is reported that a variety of analytical techniques, such as 29Si NMR, FTIR, and Raman spectroscopy have been employed to describe the intermediate products produced during the hydrolysis and condensation reactions of alkoxysilanes. We provide a contactless method for characterising the various physical states produced during the cross-linking of various SMPs that is based on Raman spectroscopy.
Materials and Methods
Adhesive Materials Used in Experiments Silane-based substances called silyl modified polymers combine organic and inorganic components to create new, very effective sealant polymers. SMP1 and SMP2, two silyl-terminated prepolymers that were purchased from BOSTIK, were employed in this work. In Figure 1, SMP1 is a polyether based on dimethoxysilane with a urethane moiety, and SMP2 is polyurethane based on trimethoxysilane.
Commercial Chemicals
Triethylamine (TEA, 99% wt), 1, 5-Diazabicyclo [4.3.0] non-5-ene (DBN), and 1, 8-Diazabicyclo [5.4.0] undec-7-ene (DBU, 98% wt) were acquired from Sigma Aldrich and used directly after purchase without further purification.
Instruments Using Raman
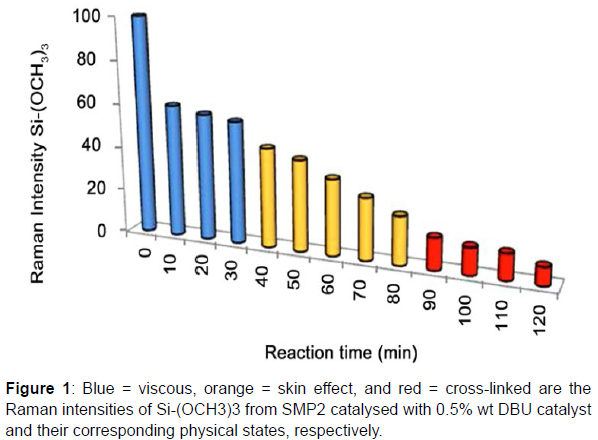
A confocal microscope and LabRAM HR 800 (Jobin-Yvon) with a 632.8 nm from a He-Ne laser were used to acquire the Raman spectra. A grating with 600 grooves/mm and an air-cooled CCD detector were used to detect the signal, producing a spectral resolution of 4 cm-1. The peak at 521 cm-1 of the silicon sample is used to test the spectrometer’s calibration before recording the spectra of a new sample. The surface of the combination containing silyl modified polymers and catalyst was focused for our study using a 100 x objective in the 100 - 3600 cm1 range, and more preferably in the 400 - 750 cm1 regions. The slit and whole specifications were established at 125 m and 1100 m, respectively. One spectrum measurement took one minute, broken down into a 30-second acquisition time and two accumulations. To track the evolution of Si-OCH3 groups during the hydrolysis and polycondensation reactions, this acquisition was repeated for SMP1 and SMP2 over the course of an hour and two hours, respectively. Measurements were made at a temperature of 23°C (+/- 1°C) and a moisture content of 55% H2O (+/- 5%). To ascertain the initial intensity of Si-OCH3 prior to the cross-linking, initial spectra of each silyl modified prepolymer (SMP1 and SMP2) were conducted without the use of a catalyst. The final intensity of Si-OCH3 groups was calculated using the spectra of each cross-linked silyl modified polymer (SMP1 and SMP2, aged 3 months). Prior to integration with the software LabSpec V4.04, all spectra were baseline adjusted. The integration was carried out with the following parameters: a gaussien/lorentzien ratio of 0.5 and w (FWHM = full-width half maximum) fixed at. The area or intensity of the Si-OCH3 peak can be utilised to track the tendency of a chemical reaction after the automatic fitting technique. Due to the high resolution of Raman spectra, findings with area and intensity produce identical results. Yet, the fitting method based on peak intensity was quicker than the method based on peak area (approximately 5 to 10 times in our case). Consequently, samples containing various mixtures of catalysts were tested in order to swiftly compare and assess in real time the physical states (viscous, skin effect, or gum appearance).
The Findings and Discussion
Usually, SMPs cure in two responses. The end polymeric alkoxysilane groups hydrolyze in the presence of moisture in the first step, catalysing their conversion into silanol groups. The silanol groups combine with additional silanols or alkoxy-silanes in the second process, where a catalyst is present, to generate Si-O-Si bonds. Condensation and hydrolysis happen virtually simultaneously and compete with one another. The silyl modified prepolymer is initially viscous and stays so throughout the hydrolysis procedure. This is a match for the viscous state (state 1). The creation of dimeric structures raises the viscosity but does not result in the production of a threedimensional network. A skin effect develops as polymer chains lengthen or form rings as a result of the creation of many Si-O-Si bonds during the polycondensation process (state 2). The result is a cross-linked state (state 3), which corresponds to a three-dimensional structure and has properties similar to gum.
Conclusions
In order to describe the physical states of SPM prepolymers during moisture curing, a novel spectroscopic technique was created. The alkoxysilane groups’ high Raman intensity enables rapid, repeatable assessment of the physical states of viscosity, skin effect, and crosslinking. The adhesive business can characterise the physical state and ascertain the open time using this technology in an appealing and effective manner. One can foresee using this non-contact optical approach for online or on-site applications for adhesives thanks to commercially accessible tiny Raman spectrometers. Furthermore, a method for performing real-time measurements has already been developed that integrates fiber-optic Raman spectroscopy with microreactor technology. We may envision combining the HTS methodology with the Raman method created in this study to produce a potent quantitative method based on our recent study on the development of a high-throughput semi-quantitative screening technique to compare and find catalysts for adhesive compounds that work well. For applications like repositionable samples or samples that are usable after a certain period of time, this technique could be utilised to find new catalysts for adhesive materials with certain time windows permanent adherence.
Acknowledgements
The funding from CNRS, ANRT, BOSTIK, and TOTAL is gratefully acknowledged by the authors.
References
- Bongiorno D, Di Stefano V, Indelicato S, Avellone G, Ceraulo L, et al. (2021)Bio‐phenols determination in olive oils: Recent mass spectrometry approaches. Mass Spectrometry Reviews: 21744.
- Wang S, Blair IA, Mesaros C (2019) Analytical methods for mass spectrometry-based metabolomics studies. Advancements of Mass Spectrometry in Biomedical Research: 635-647.
- Jang KS, Kim YH (2018) Rapid and robust MALDI-TOF MS techniques for microbial identification: a brief overview of their diverse applications. Journal of Microbiology 56:209-216.
- Kim E, Kim J, Choi I, Lee J, Yeo WS, et al. (2020) Organic matrix-free imaging mass spectrometry. BMB reports 53:349.
- Wang Y, Han Y, Hu W, Fu D, Wang G (2020) Analytical strategies for chemical characterization of bio‐oil. Journal of separation science 43:360-371.
- Ishii K, Zhou M, Uchiyama S (2018) Native mass spectrometry for understanding dynamic protein complex. Biochim Biophys Acta Gen Subj 1862:275-286.
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Citation: Nomi MB (2023) Responses on Chip and pin Raman Spectroscopy in Brief. J Anal Bioanal Tech 14: 511.
Copyright: © 2023 Nomi MB. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Open Access Journals
Article Usage
- Total views: 926
- [From(publication date): 0-2023 - Apr 07, 2025]
- Breakdown by view type
- HTML page views: 707
- PDF downloads: 219