Research Article Open Access
Response to Hydrolysed Collagen Protein Supplementation in a Cohort of Pregnant and Postpartum Women
Leon Baginski1, Marc Winter2, Thomas S Bailey3, Scott Capobianco4, Marsha Granese5, Freddie Granger MA6, Kurt Miller7, Kerry Price8, Sara Ramirez9, Craig Salcido10, Frank Turner11* and Mary O’Toole12
1Mission Obstetrics and Gynaecology, Mission Viejo, California and Orange Coast Women’s Medical Group, Laguna Hills, California, USA
2University of California College of Medicine, Orange Coast Women's Medical Group, Laguna Hills, CA, USA
3University of Arizona Medical School, Mission OBGYN Medical Group, Mission Viejo CA, USA
4Medical School, Mission OBGYN Medical Group, Mission Viejo, CA, USA
5St. George's University, Mission OBGYN Medical Group, Mission Viejo, USA
6Mission Ob/Gyn Medical, Mission Viejo California, USA
7University of San Diego School of Medicine, Mission OBGYN Medical Group, Mission Viejo, USA
8University of Chicago Pritzger School of Medicine, Orange Coast Women's Medical Group, Laguna Hills, CA, USA
9University of Chicago Medical School, Mission OBGYN Medical Group, Mission Viejo, CA, USA
10University of New York Medical College, Mission OBGYN Medical Group, Mission Viejo, CA, USA
11Innovative Research Associates 415 Elmwood Ave, Sharon Hill, PA, USA
12Chicago Medical School, Orange Coast Women's Medical Group, Laguna Hills, CA, USA
- *Corresponding Author:
- Frank Turner
Innovative Research Associates 415 Elmwood Ave
Sharon Hill, PA, USA
Tel: 5403168030
E-mail: neuroma7@msn.com
Received Date: August 21, 2016; Accepted Date: September 26, 2016; Published Date: September 30, 2016
Citation: Baginski L, Winter M, Bailey TS, Capobianco S, Granese M, et al. (2016) Response to Hydrolysed Collagen Protein Supplementation in a Cohort of Pregnant and Post-Partum Women. J Preg Child Health 3: 275. doi:10.4172/2376-127X.1000275
Copyright: © 2016 Bakginski L, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Pregnancy and Child Health
Abstract
There is overwhelming evidence that links maternal nutrition during pregnancy with fetal outcomes. This study evaluated the safety and efficacy of Protiva treatment responses in pre and postpartum female subjects between the ages of 19 years and 43 years who entered into this open label study. The protocol was designed to take a twice daily collagen protein supplementation drink during the third trimester and for 10 weeks postpartum. The outcomes were measured against a control group of patients who did not take the protein supplementation. The differences between mean score for the physical health domain of WHOQOL-BREF scale in the Protiva group and the control group were different and met statistical significance (p=0.0003). The study found that 100% of control patients and 95% of Protiva patients had initial serum protein levels below the normal median range and 27% of control patients and 33% of Protiva patients were below normal range. With collagen protein supplementation we were able to demonstrate improved quality of life and wound healing rates and 100% improvement in the protein levels for Protiva patients and a 72% improvement over control patients. Protiva Pregnancy and Protiva New Mom was determined to be safe and well tolerated when taken during pregnancy and post-partum during the study.
Keywords
Pregnancy protein; Protiva; Breast feeding
Introduction
There is over whelming evidence that links maternal nutrition during pregnancy with fetal outcomes [1]. Pregnancy is associated with physiologic changes that result in increased plasma volume and red blood cells and decreased concentrations of circulating nutrientbinding proteins and micronutrients. There is an increased requirement for vitamins and minerals during pregnancy relative to the non-pregnant state [2]. The demand for protein during the second and third trimester of pregnancy increases to 1.1 g/kg/day or approximately 71 g, amounting to more than a 50% increase in protein that is necessary for fetal growth and maternal milk production [3].
Normal blood total protein levels in a non-pregnant woman are between 6.0 and 8.3 grams per deciliter (g/dL) [4]. In a study by Rahman et al. [5], it was demonstrated that serum protein levels fluctuate during the three trimesters of pregnancy and they are further impacted on the basis of parity and dietary protein consumption. The mean total serum protein during first, second and third trimesters were found to be 6.85, 6.60 and 6.81 g%, respectively which were lower than the mean value obtained in a group of non-pregnant women (7.55 g%). While some clinical laboratories currently report total protein levels below 6.0 (g/dL) as normal in pregnancy, there is no data to support that this is indeed “normal” or that it should be considered safe. The levels of total protein and albumin that are necessary to maximize fetal and maternal outcomes are poorly understood and certainly open for debate.
Many women find it difficult to consume the recommended amount of protein during pregnancy. Physicians and caregivers are often short on time and find it difficult to address the many dietary concerns and restrictions that face the gravid patient. Women looking for additional protein during pregnancy may find protein powders an easy and convenient alternative to other forms of unprocessed protein. Unfortunately, there is insufficient data regarding the impact on these drinks on either the mother or the fetus to routinely recommend them during pregnancy. Without efficacy or safety information, physicians are hard pressed to recommend for or against supplementation. This study was undertaken to answer some of these questions and hopefully guide caregivers in understanding the increased nutrient needs during pregnancy and the benefits of protein supplementation. It was also prudent to continue the study into the postpartum period and during breast feeding to assess any impact on lactation so as to help guide pediatricians with respect to new-born responses to maternal supplementation.
Aevum Life Science has developed two novel protein products Protiva Pregnancy and Protiva New Mom, each specifically formulated with hydrolyzed collagen as the protein source. Hydrolyzed collagen was chosen because animal studies have demonstrated that high levels of certain amino acids were associated with reduced litter size and the health of offspring [6,7]. Among these amino acids that were studied, Tryptophan was found to have the greatest negative impact on fetal development. Collagen protein contains no Tryptophan and has the lowest concentration of the other detrimental amino acids in comparison to other protein sources. Hydrolyzed collagen is also easily digested and absorbed because of the low molecular weight of the peptides produced during hydrolysis. Adequate protein is a requirement for proper wound healing from collagen synthesis, angiogenesis, fibroblast proliferation and maintenance of tissue oncotic pressure. Only hydrolyzed collagen peptides have been demonstrated to be chemotactic to dermal fibroblasts which are an essential component in the healing process of damaged tissue [8].
In anticipation of abdominal or vaginal delivery, proper nutrition pre and post-delivery is important in order to have a positive effect on tissue healing [9]. Patients preparing to undergo surgery need peak immune function to prevent infection, peak clotting function to reduce bleeding and bruising. Adequate protein intake is necessary for collagen formation and repair as well as other critical functions [10].
Studies of postoperative nutritional support have demonstrated reduced morbidity and reduced length of hospital stay [11]. Huisman et al. were able to demonstrate that low pre-surgical serum protein levels were a predictor of increased risk for post-operative complications [11-13].
Proper healing after delivery, whether vaginal or by cesarean, requires adequate macro and micronutrients. Not only is protein and important component of the healing process but vitamins and minerals also play a critical role. Vitamins A, C, E and D as well as the minerals Copper and Zinc and Calcium have all been shown to play an important role in proper wound repair [14-21]. Their role in the healing process involves angiogenesis, induction of endothelial growth factors with proliferation and differentiation of human keratinocytes, fibroblast proliferation as well as collagen maturation and stabilization. Prospective randomized clinical trials support the use of supplementation of Vitamin C, Vitamin E and trace elements in critically ill patients for proper healing to take place [22].
This study was designed to: 1) determine the blood protein levels in a group of women in their third trimester who were well nourished with access to both adequate macro and micronutrients. 2) Provide supplementation of protein with vitamins and minerals during pregnancy, delivery and into the postpartum period. 3) Evaluate the potential outcome differences of patients taking supplemental protein and vitamins and minerals compared to a control group. 4) To see if there were any negative effects of collagen protein on fetal or maternal outcomes.
Participants
Female subjects between 18 and 50 years of age in their final trimester of pregnancy were considered eligible to participate after evaluation of the inclusion/exclusion criteria and completion of screening procedures.
Materials
The materials utilized in this study included Protiva Pregnancy and Protiva New Mom.
Study patients were instructed to mix Protein Pregnancy and Protiva New Mom with 8-10 oz of cold water in a blender or shaker bottle and to avoid mixing them with milk or other protein containing products.
Protiva Pregnancy has been specifically formulated for pregnant women to provide 15 g of Hydrolyzed Collagen protein per serving. Protiva Pregnancy contains no artificial colors or flavors, and is gluten, lactose, soy and preservative free. One scoop provides 25% of the RDA of Vitamin A, 80% of Vitamin C, 100% of Vitamin D, 50% of Calcium, 30% of Zinc and 50% of Copper.
Protiva New Mom has been specifically formulated for women following delivery and while breastfeeding and contains 15 g of hydrolyzed collagen protein per serving. Protiva New Mom also contains no artificial colors or flavors, and is gluten, lactose, soy and preservative free. It provides 50% of the RDA of Vitamin A, 625% of Vitamin C, 100% of Vitamin D 50% of Vitamin E, 50% of Calcium, 30% of Zinc and 50% of Copper. It also contains the additional amino acids L-Leucine, L-Isoleucine and L-Valine.
Methods
Patient population selection
Study subjects were screened at 2 investigative sites in the United States. Screening assessments were conducted, and if patients were accepted into the study, study participants received Protiva Pregnancy mailed to their homes. Screening procedures included: medical and surgical history including medication history, review of inclusion/ exclusion criteria, physical examination (including height, weight and BMI), vital signs (BP and HR). For patients who were selected into the study, laboratory tests (chemistry and hematology) were taken and the World Health Organization Quality of Life (WHOQOL)-BREF was administered and completed prior to starting on the product. Subjects returned to the site at 6 weeks post-delivery (Visit 2) and 10 week’s post-delivery (Visit 3).
The following assessments were performed during those visits: physical examination (including height, weight and BMI), vital signs (BP and HR), review of adverse events (AEs) and concomitant medications, laboratory tests (chemistry and hematology), Patient Global Satisfaction with Treatment Scale (only completed for treatment group), WHOQOL-BREF, REEDA Scale (only for subjects that had an incision or laceration with or without repair at delivery) and Protiva Pregnancy and Protiva New Mom accountability/ compliance assessment for the treatment group.
Study Design
This open-label study was designed to assess the efficacy and safety of: 1) Protiva Pregnancy during the third trimester of pregnancy and 2) Protiva New Mom during the first 10 weeks following delivery. The duration of this study was 14 weeks. Study patients were instructed to consume Protiva Pregnancy twice daily (30 g) in their third trimester of pregnancy until delivery and then switch to Protiva New Mom twice daily until their 10 week postpartum visit. A control group of 27 subjects were included in the study that did not receive either of the Protiva products. Clinical outcomes such as blood protein levels, complication rates, and change in body mass index (BMI) were measured. The clinical study statistically evaluated the improvement in blood protein levels of study subjects from Screening to End of Study. Blood protein levels were drawn on patients between weeks 30-32 of pregnancy.
Evaluation of recovery following delivery was measured by the REEDA scale change in scores (5 items with a score ranging from 0 to 3, higher score representing a greater level of tissue trauma with a maximum of 15) was assessed using the non-parametric Wilcoxon Rank Sum test.
Statistical Analysis
Statistical analyses were performed based on the treatment received by the study subject. Primary and secondary endpoints are presented by treatment received. The last observation carried forward (LOCF) method was used for missing data in the primary analysis.
Statistical analysis of the study results included a Full Analysis Set (FAS) as all subjects in the treatment group who received both Protiva Pregnancy and Protiva New Mom had completed at least one postdelivery assessment; and all subjects in the control group who completed at least one post-delivery assessment were included in the FAS.
The safety population included all subjects who took any of the Protiva products. The efficacy population was comprised of all subjects who took any Protiva product, and had both baseline and at least 1 post-delivery assessment. The completed efficacy population was comprised of all subjects who received both Protiva Pregnancy and Protiva New Mom, completed 10 weeks of Protiva New Mom and completed all protocol specified study assessments.
Descriptive analyses of clinical laboratory tests, vital signs, BMI and physical examinations were done on all subjects in the safety population.
Ethics
The protocol was reviewed by independent Institutional Review Board (IRB). Prior to the initiation of the clinical trial, the Principal Investigators obtained written and dated approval by the IRB for the protocol and the informed consent form. The study was conducted in compliance with IRB, informed consent regulations and International Conference on Harmonization (ICH) Good Clinical Practice (GCP). The Principal Investigator was responsible for performing the study in accordance with the protocol and GCP/ICH guidelines and for collecting, recording and reporting the data accurately and properly. The principle investigator has not received financial support from the sponsor Aevum Life Science to perform this study. Prior to enrolment in the study, an IRB approved written informed consent was obtained from each subject.
Results
Patient disposition
The safety and efficacy analysis was conducted on 142 subjects enrolled into the study. Of the 142 subjects, 109 completed the study (i.e., returned for Visit 3), 6 subjects were lost to follow-up between Visits 1 and 3, 17 subjects demonstrated noncompliance or lack of cooperation, and 10 subjects had other reasons for discontinuation such as moving out of the area.
Demography and baseline characteristics
One hundred and forty two (n=142) subjects were enrolled in the trial are shown in Table 1 below, all were female, 132 (93%) were Caucasian, and their mean age+SD [min, max] was 31.5+5.11 (19, 44) years.
| Characteristics | All Subjects (N=142) |
|---|---|
| Age (years) | |
| Mean (SD) | 31.5 (5.11) |
| Median | 32 |
| Minimum, Maximum | 19, 43 |
| Gender | |
| Female N (%) | 142 (100%) |
| Race | |
| Caucasian N (%) | 132 (93%) |
| Black N (%) | 2 (1.4%) |
| Asian N (%) | 6 (4.2%) |
| Native American N (%) | 2 (1.4%) |
| Height (in) | |
| Mean (SD) | 64.8 (2.81) |
| Median | 65 |
| Minimum, Maximum | 54, 71 |
| Weight (lb) | |
| Mean (SD) | 176.4 (32.20) |
| Median | 170 |
| Minimum, Maximum | 104, 286 |
Table 1: Demography of the safety population.
Efficacy results
A total of 115 subjects were assigned to Protiva treatment and 27 to no treatment at 2 centers and 113 (91 Protiva and 22 controls) completed the primary endpoint evaluations at least at one of the postdelivery visits 2 or 3.
Efficacy analyses results are shown in Table 2 were obtained from a total of 113 (91 Protiva and 22 controls) who completed the primary endpoint evaluations in at least one of the post-delivery visits 2 or 3.
| PPS Population | FAS Population | ||||
|---|---|---|---|---|---|
| Control Group (N=11) | Protiva Group (N=37) | Control Group (N=22) | Protiva Group (N=91) | ||
| Total Protein (g/dL) | |||||
| Screening | n | 11 | 37 | 22 | 90 |
| Observed Mean (SD) | 6.1 (0.29) | 6.2 (0.42) | 6.1 (0.25) | 6.1 (0.39) | |
| Visit 2 | n | 11 | 37 | 18 | 78 |
| Observed Mean (SD) | 6.8 (0.40) | 7.0 (0.35) | 6.8 (0.37) | 7.0 (0.35) | |
| Mean Change (SD) | 0.7 (0.41) | 0.8 (0.40) | 0.7 (0.38) | 0.9 (0.37) | |
| Visit 3 | n | 11 | 37 | 20 | 72 |
| Observed Mean (SD) | 6.8 (0.47) | 7.0 (0.39) | 6.9 (0.43) | 7.1 (0.42) | |
| Mean Change (SD) | 0.7 (0.46) | 0.8 (0.42) | 0.8 (0.44) | 0.9 (0.38) | |
Table 2: Improvement in nutritional status: Total protein, (PPS and FAS Population) (SD=Standard Deviation).
For the endpoint of total blood protein, based on treatment or no treatment, efficacy analysis for the total blood protein, was based on the difference in blood protein levels of the Protiva group and the Control group from Screening (Visit 1) to End of Study (Visit 3). The primary endpoint was analyzed and the change from baseline values was used for the lab chemistry endpoints. If a subject withdrew from the study without undergoing the early termination assessments, the data available up until that time was used for analysis. Missing values were imputed using LOCF imputation method.
Physical Health Results
The differences between mean score for the physical health domain of WHOQOL-BREF scale in the Protiva group and the control group was statistically significant at the End of Study, i.e., visit 3 (Table 3: P=0.0003 for FAS population and Table 4: p=0.0001 for PPS population), meaning significant improvement in the physical health of subjects in the Protiva group compared to the control group. The mean score of all four domains increased from screening to visit 3 in the Protiva group meaning improvement in quality of life, while the mean score of all four domains decreased from screening to visit 3 in the control group. The number of responders of PGS scores for each of the two post-delivery visits clearly shows much higher percentages of subjects with a satisfaction rating of very satisfied (Visit 2: 46.1% vs. 7.4%; Visit 3: 42.6% vs. 3.7%) and satisfied (Visit 2: 22.6% vs. 0; Visit 3: 19.1% vs. 0) in the Protiva group compared to the Control group.
| Visit | WHOQOL-BREF domains | Control Group | Protiva Group | p-value* | ||
|---|---|---|---|---|---|---|
| Screening | Physical Health | n | 22 | 90 | 0.1774 | |
| Mean (SD) | 27.9 (3.94) | 26.7 (4.47) | . | |||
| Median | 29 | 27 | . | |||
| Minimum, Maximum | 17 , 34 | 12 , 35 | . | |||
| Psychological | n | 22 | 91 | 0.1627 | ||
| Mean (SD) | 25.5 (2.61) | 24.3 (3.48) | . | |||
| Median | 26 | 24 | . | |||
| Minimum, Maximum | 21, 29 | 9 , 30 | . | |||
| Social Relationships | n | 22 | 90 | 0.5981 | ||
| Mean (SD) | 12.8 (1.60) | 12.5 (1.82) | . | |||
| Median | 13 | 12 | . | |||
| Minimum, Maximum | 10, 15 | 6, 15 | . | |||
| Environment | n | 22 | 91 | 0.2451 | ||
| Mean (SD) | 34.4 (3.99) | 33.5 (3.75) | . | |||
| Median | 35 | 33 | . | |||
| Minimum, Maximum | 25, 40 | 23, 40 | . | |||
| Visit 2 | n | 18 | 80 | |||
| Mean (SD) | 25.4 (6.22) | 28.5 (3.40) | ||||
| Median | 26 | 29 | ||||
| Minimum, Maximum | 14, 34 | 18, 34 | ||||
| n | 18 | 78 | ||||
| Mean (SD) | 23.9 (3.49) | 24.8 (3.29) | ||||
| Median | 25 | 25 | ||||
| Minimum, Maximum | 19, 29 | 15, 30 | ||||
| n | 18 | 78 | ||||
| Mean (SD) | 11.9 (2.03) | 12.6 (1.69) | ||||
| Median | 12 | 12 | ||||
| Minimum, Maximum | 8, 15 | 8, 15 | ||||
| n | 17 | 80 | ||||
| Mean (SD) | 33.7 (3.57) | 34.6 (3.83) | ||||
| Median | 35 | 35 | ||||
| Minimum, Maximum | 29, 39 | 24, 40 | ||||
| Visit 3 | n | 19 | 71 | |||
| Mean (SD) | 24.8 (5.48) | 30.0 (3.22) | ||||
| Median | 23 | 30 | ||||
| Minimum, Maximum | 16, 35 | 16, 35 | ||||
| n | 19 | 70 | ||||
| Mean (SD) | 23.7 (2.94) | 24.8 (3.79) | ||||
| Median | 24 | 25 | ||||
| Minimum, Maximum | 18, 29 | 11, 30 | ||||
| n | 19 | 71 | ||||
| Mean (SD) | 12.5 (1.90) | 12.7 (2.02) | ||||
| Median | 12 | 13 | ||||
| Minimum, Maximum | 9, 15 | 7, 15 | ||||
| n | 19 | 71 | ||||
| Mean (SD) | 34.2 (4.61) | 34.8 (3.76) | ||||
| Median | 35 | 34 | ||||
| Minimum, Maximum | 26, 40 | 23, 40 | ||||
*P-values are calculated using Wilcoxon-Mann-Whitney U-test; SD=Standard Deviation
Table 4: Summary of the WHOQOL-BREF domains and comparisons between treatments (PPS population).
Safety Results
The Protiva products were well tolerated.
• There were no Serious Adverse Events reported during the study.
• The most frequently reported adverse events are summarized by system organ class and preferred term in Table 5 below were mastitis in 4.2%, depression in 4.2%, reproductive system and breast disorders in 3.5%, renal and urinary disorders in 2.8% and urinary tract infection in 2.8% of subjects.
| Primary System Organ Class | Adverse Event (Preferred Term) | Control Group (N=27) | Protiva Group (N=115) | Total (N=142) |
|---|---|---|---|---|
| Overall Total, n (%) | 8 (29.6) | 31 (27.0) | 39 (27.5) | |
| Blood and Lymphatic System Disorders | - | 2 (1.7) | 2 (1.4) | |
| Lymphadenopathy | - | 1 (0.9) | 1 (0.7) | |
| Thrombocytics | - | 1 (0.9) | 1 (0.7) | |
| Gastrointestinal Disorders | 2 (7.4) | 1 (0.9) | 3 (2.1) | |
| Abdominal pain | 1 (3.7) | - | 1 (0.7) | |
| Hemorrhoids | 1 (3.7) | 1 (0.9) | 2 (1.4) | |
| Gastrointestinal Disorders - Blood and lymphatic system disorders | 1 (3.7) | - | 1 (0.7) | |
| Oral pain | 1 (3.7) | - | 1 (0.7) | |
| Gastrointestinal Disorders - Surgical and Medical Procedures | - | 1 (0.9) | 1 (0.7) | |
| Abdominal pain | - | 1 (0.9) | 1 (0.7) | |
| Infections and Infestations | 2 (7.4) | 14 (12.2) | 16 (11.3) | |
| Breast infection | - | 1 (0.9) | 1 (0.7) | |
| Candida infection | - | 1 (0.9) | 1 (0.7) | |
| Cellulitis | - | 1 (0.9) | 1 (0.7) | |
| Cervicitis infection | - | 1 (0.9) | 1 (0.7) | |
| Cystitis | 1 (3.7) | 2 (1.7) | 3 (2.1) | |
| Group B Streptococcal infection | 1 (3.7) | - | 1 (0.7) | |
| Mastitis | - | 6 (5.2) | 6 (4.2) | |
| Urinary tract infection | 1 (3.7) | 3 (2.6) | 4 (2.8) | |
| Injury, Poisoning and procedural complications | - | 2 (1.7) | 2 (1.4) | |
| Seroma | - | 2 (1.7) | 2 (1.4) | |
| Investigations - Endocrine disorders | - | 1 (0.9) | 1 (0.7) | |
| Blood Prolactin increased | - | 1 (0.9) | 1 (0.7) | |
| Investigations - Hepatobiliary disorders | - | 1 (0.9) | 1 (0.7) | |
| Hepatic enzyme increased | - | 1 (0.9) | 1 (0.7) | |
| Investigations - Metabolism and Nutrition disorders | 1 (3.7) | - | 1 (0.7) | |
| Weight increased | 1 (3.7) | - | 1 (0.7) | |
| Psychiatric Disorders | 1 (3.7) | 5 (4.3) | 6 (4.2) | |
| Depression | 1 (3.7) | 5 (4.3) | 6 (4.2) | |
| Renal and Urinary Disorders | 1 (3.7) | 3 (2.6) | 4 (2.8) | |
| Dysuria | 1 (3.7) | 1 (0.9) | 2 (1.4) | |
| Hematuria | - | 2 (1.7) | 2 (1.4) | |
| Reproductive System and Breast Disorders | 2 (7.4) | 3 (2.6) | 5 (3.5) | |
| Bleeding | - | 1 (0.9) | 1 (0.7) | |
| Lactation disorder | 1 (3.7) | - | 1 (0.7) | |
| Menorrhagia | - | 1 (0.9) | 1 (0.7) | |
| Menstruation irregular | - | 2 (1.7) | 2 (1.4) | |
| Vaginal pain | 1 (3.7) | - | 1 (0.7) | |
| Reproductive System and Breast Disorders - Generaldisorders and administration site conditions | - | 1 (0.9) | 1 (0.7) | |
| Pelvic pain | - | 1 (0.9) | 1 (0.7) | |
| Skin and Subcutaneous Tissue Disorders | - | 2 (1.7) | 2 (1.4) | |
| Pruritus | - | 2 (1.7) | 2 (1.4) | |
Table 5: Summary of treatment emergent adverse events (Safety population).
• There were no apparent treatment related changes in the vitals and the BMI.
There were no death or serious adverse events reported during the study period covered by this clinical study report.
Blood Protein Level Results
One hundred percent (100%) of the control patients presented with an initial blood protein level below the mean pregnancy average of 6.6 (g/dL) and 27% had an initial blood protein level below 6.0 (g/dL). Ninety-Five per cent (95%) of the study patients that took Protiva, had an initial blood protein level below the mean pregnancy average of 6.6 (g/dL) and 33% had an initial blood protein level below 6.0 (g/dL).
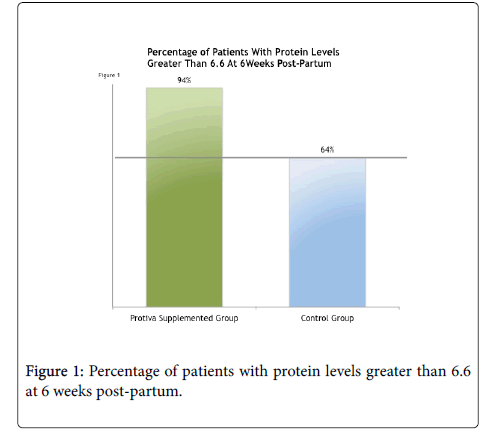
At six weeks post-delivery, 94% of the patients taking Protiva improved their blood protein levels above the mean pregnancy average of 6.6 (g/dL) as seen in Figure 1 below. This compares to 64% of the control group who improved blood protein levels above the mean pregnancy average of 6.6 (g/dL).
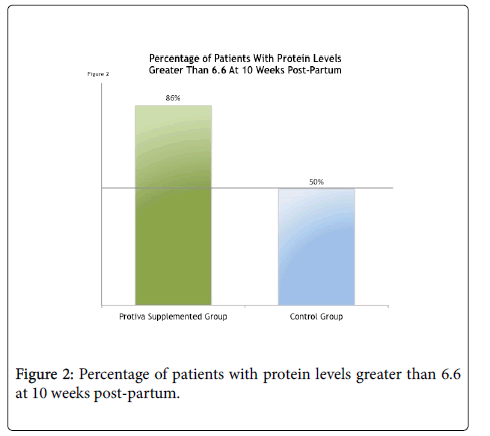
At ten weeks post-delivery, 86% of study patients who took Protiva maintained their blood protein levels above the mean of 6.6 (g/dL) as seen in Figure 2 below. This compares to only 50% of the control group.
After 14 weeks of treatment, 100% of the study patients who took Protiva demonstrated improved blood protein levels in subsequent visits, compared to only 86% of the control patients.
Discussion
Pregnancy and lactation place increased nutritional demands on the pregnant woman. There is an overall increased need for calories to achieve adequate gestational weight gain. The need for protein likewise also increases for proper fetal and placental growth and development. The amount of increase depends upon the woman’s starting weight and body composition as well as current protein intake [23]. The average non-pregnant woman needs approximately 46 g of protein daily and this increase to 71-76 g/d in pregnancy [24-27]. Approximately 400 g of protein is turned over in 24 h and of this 25% is replaced by dietary intake [28]. It is impossible to accurately determine for any individual their dietary and nutrient requirement. Current measure of height, weight and BMI, although reasonable markers for malnutrition, are poor for assessing inadequate nutrition or poor dietary habits [29]. Most studies done on maternal protein and calorie supplementation have been performed in countries or regions of the world where both caloric and protein restriction are common [30-33]. It has been widely assumed that industrialized countries, with abundantly available calories and protein should have adequate maternal nutrition and certainly adequate protein intake. Studies, however, have shown that women do not increase consumption of protein to proper levels as pregnancy progresses [27]. Dietary advice by caregivers and physicians is time consuming and difficult to standardize. Furthermore, there are often many barriers to getting pregnant woman to consume increased volumes of whole protein foods. Studies addressing dietary intervention and counselling have disappointing compliance rates and lower than expected achievement of target endpoints [33-36].
Our current study looked to address a way to determine the underlying protein status in a group of healthy women from a community where food and protein resources are abundant, and then determine if protein supplementation would have an impact on the measurement parameters and pregnancy outcomes. What we found with respect to underlying protein and albumin levels was very interesting. The fact that the great majority of patients had serum levels below the normal median and even completely below the normal range was not necessarily new. It was surprising that in an affluent area with
nutritional abundance that such a high percentage would fall into this low range. With collagen protein supplementation we were able to demonstrate a 94-100% improvement in protein levels in study patients. The control patients showed a much lower improvement even with dietary advice and overall the protein group showed a 72% improvement over control patients. It is not entirely clear why protein levels decease during pregnancy, but simple hemodilution cannot explain all of the decrease as levels begin to go down as early as the first trimester [37] while other serum protein of hepatic origin remain stable or increase [38]. What is known is that protein is important for many aspects of fetal/placental growth and maternal well-being during and after childbirth.
Our study also showed an improvement in overall quality of life scores and wound healing indices as measured by the WHOQOLBREF and REEDA pre and post study evaluations. We did not anticipate that patients who entered the study apparently healthy and without underlying depressive disorders, would have such dramatic improvement in both of these outcomes. It is understood that amino acids are important to many body and brain functions and that inadequate protein intake can worsen depression or mental illness [39]. To what degree protein supplementation helps with quality of life assessments has not been studied in pregnant women and this report makes for interesting future direction of study for pregnant patients. The addition of the vitamins A, C, E and the minerals Zinc, Copper and Calcium are necessary for the different phases of wound healing and have studies showing their efficacy and importance. Vitamin D has been shown to be of value both for the mother and fetus [40] however we did not intend to measure maternal or fetal effects of supplementation alone. Studies looking at the use of supplemental vitamins have had mixed results and there is little evidence that vitamin supplements improve fetal outcomes when looking at stillbirth, neonatal death, preterm birth, etc. [40,30]. Their use in our study was an adjunct to protein supplementation for proper healing of vaginal lacerations, episiotomies or caesarean section incisions. Based on the responses from the patient REEDA evaluations it appears that there was indeed a significant improvement in the supplementation compared to the control group.
Of note was that there was no difference in adverse events between the two groups and no apparent impact on breast feeding or other post-delivery parameters with regards to breastfeeding or new-born problems. This should reassure paediatricians and lactations specialists who encounter patients taking collagen protein supplementation and how they counsel them about stopping or continuing with the supplementation.
This study appears to be the first of its kind to look at the physical and quality of life of mothers taking collagen protein supplementation during pregnancy. It is also unique in measuring the response to supplementation with respect to wound healing after delivery. The fact that there were no adverse outcomes in the study group points to the safety of collagen protein supplementation during pregnancy. There are no theoretical risks to collagen as it is comprises 65% of the protein content in animals and thus is a major constituent of what is consumed when eating animal proteins in the diet. The form provided for the study group was easy to consume, readily available and highly effective in supplementing the pregnant woman’s diet to improve maternal outcomes and at the same time provide adequate protein to her developing fetus. When protein is limited in the diet, there is the concern that the fetus may be at risk for both short and long term health consequences [41-45]. This study did not address fetal growth,neonatal parameters or long term infant health. However, it would appear prudent that since fetal health is directly dependent on maternal health and nutrition, that providing proper nutrition to the mother would maximize the potential for a healthy fetus and newborn.
Key Message
Pregnancy and lactation place increased nutritional demands on the pregnant woman. The need for protein also increases, for proper fetal and placental growth and development. The average non-pregnant woman needs approximately 46 g of protein daily and this increase to 71-76 (g/dL) in pregnancy.
This study found the great majority of patients had initial serum levels below the normal median and even completely below the normal range. With collagen protein supplementation we were able to demonstrate improved quality of life and wound healing and 100% improvement in protein levels in study patients and a 72% improvement over control patients.
Acknowledgement
Marsha Kmec who's tireless help to retrieve difficult to find references was critical to the success of this project Carolyn Pratchett RNP for her support and leadership at site #2 Elena Mata de la Garza for her incredible phlebotomy skills.
Contributor Statement
Leon Baginski, M.D is the principle investigator, first author and editor for this original research article. Marc Winter MD is the second author for this original article. Mary O’Toole, M.D. is the principle investigator, site number 2. Frank Turner, DPM is the editor and medical writer for this original research article. Innovative Research Associates is the contracted CRO for this original research article.
References
- ASPEN Board of Directors and the Clinical Guidelines Task Force (2002) Guidelines for the use of parenteral and enteral nutrition in adult and pediatric patients. JPEN J Parenter Enteral Nutr 26: 1-138.
- Pathak P, Kapil L (2004) Institute of Medicine Food and Nutrition Board 2001, 2000, 1998, 1997.
- Butte NF (2000) Carbohydrate and lipid metabolism in pregnancy: Normal compared with gestational diabetes mellitus. Am J ClinNutr 71: 1256-1261.
- Klein S (2011) Protein-energy malnutrition. Cecil Medicine. Saunders Elsevier p: 222.
- Rahman SH, Khairunnessa (1978) Change of serum protein level during pregnancy and the impact of parity and diet on it. Bangladesh Med Res Counc Bull 4: 16-20.
- Wu G, Bazer FW, Satterfield MC, Li X, Wang X, et al. (2013) Impacts of Arginine nutrition on embryonic and fetal development in mammals. Amino Acids 45: 241-256.
- Matsueda S, Niiyama Y (1982) The effects of excess amino acids on maintenance of pregnancy and fetal growth in rats. J NutrSciVitaminol28: 557-573.
- Postlethwaite AE, Seyer JM, Kang AH (1978) Chemotactic attraction of human fibroblasts to type I, II and III collagens and collagen-derived peptides. ProcNatlAcadSci 75: 871-875.
- Cerantola Y, Grass F, Cristaudi A, Demartines N, Schäfer M, et al. (2011) Perioperative nutrition in abdominal surgery:Recommendations and reality. Gastroenterology Research and Practice p: 7
- (2014) Academy of nutrition and dietetics nutrition care manual.
- Askanazi J, Starker PN, Olsson C, Hensle TW, Lockhart SH, et al. (1986) Effect of immediate post-operative nutritional support on the length of hospitalization. Ann Surg 203: 236-239.
- Huisman MG, Audisio RA, Ugolini G, Montroni I, Vigano A, et al. (2015) Screening for predictors of adverse outcome in onco-geriatric surgical patients: A multicenter prospective cohort study. Eur J SurgOncol 41: 844-851.
- Uppal S, Igwe E, Rice LW, Spencer RJ, Rose SL (2015) Frailty index predicts severe complications in gynecologic oncology patients. GynecolOncol 137: 98-101.
- Escott-Stump S (2012) Nutrition and diagnosis related care. Philadelphia, PA. Wolters Kluwer Health/Lippincot Williams and Wilkins 955: 800-812.
- MacKay D, Miller AW (2003) Nutritional support for wound healing. Alter Med Rev 8: 359-377.
- Sakae K, Yanagisawa H (2014) Oral treatment of pressure ulcers with polaprezinc (zinc Lcarnosine complex): 8 week open-label trial. Biol Trace Elem Res 158: 280-288.
- Raju KS, Alessandri G, Ziche M, Gullino PM (1982) Ceruloplasmin, copper ions and angiogenesis. J Natl Cancer Inst69: 1183-1188.
- Alessandri G, Raju K, Gullino PM (1984) Angiogenesis in vivo and selective mobilization of capillary endothelium in vitro by heparin-copper complex. Microcir Endothelium Lymphatics 1: 329-346.
- Alessandri G, Raju K, Gullino PM (1983) Mobilization of endothelium in vitro induced by effector of angiogenesis in vivo. Cancer Res 43: 1790-1797.
- Sen CK, Khanna S, Venojarvi M, Trikha P, Ellison EC, et al. (2002) Copper induced vascular endothelial growth factor expression and wound healing. Am J Physio Heart CircPhysiol 282: 1821-1827.
- Lansdown AB (2002) Calcium: A potential central regulator in wound healing in the skin. Wound Repair Regen 10: 271-285.
- Fukushina R, Yamazaki E (2010) Vitamin C requirement in surgical patients. CurrOpinClinNutrMetab Care 13: 669-676.
- World Health Organization (2007) Protien and amino acid requirements in human nutritio: Report of a joint WHO/FAO/UNU expert consultation. Geneva: World Health Organisation.
- Hytten FE, Leitch I (1971) Te physiology of pregnancy. Oxford, United Kingdom: Blackwell Scientific Publications.
- Energy Protien requirements (1985) Report of a joint FAO/WHO/UNU Expert Consultation. World Health Organ Tech Rep Ser 724: 1-206.
- Institute of Medicine (US) (1990) Nutrition during pregnancy. Washington, DC: National Academic Press.
- Godfrey K, Robinson S, Barker DJP, Osmond C, Cox V (1996) Maternal nutrition in early and late pregnancy in relation to placental and fetal growth. BMJ 312: 410-414.
- Zakim K, Boyer B (1998) Hepatology: A textbook of liver disease. WB Sanders 2: 1.
- Halder M, Halder SQ (1984) Assessment of protien-calorie malnutrition. ClinChem 30: 1286-1299.
- Imdad A, Yakoob MY, Bhutta ZA (2011) The effect of folic acid, protein energy and multiple micronutrient supplements in pregnancy on stillbirths. BMC Public Health Suppl 3: 4.
- Imdad A, Bhutta ZA (2012) Maternal nutrition and birth outcomes: Effect of balanced protein-energy supplementation. PaediatrPerinatEpidemiol Jul 1: 178-190.
- Yakoob MY, Menezes EV, Soomro T, Haws RA, Darmstadt GL, et al. (2009) Reducing still-births: Behavioral and nutritional interventions before and during pregnancy. BMC Pregnancy Childbirth 1: 3.
- Kramer MS, Kakuma R (2003) Energy and protein intake during pregnancy p: 4.
- (2015) Health Promotion Practice 13: 816-825.
- Broekhuizen K, Althuizen E, van Poppel MN, Donker M, van Mechelen W (2012) Health PromotPract 13: 816-825.
- Piirainen T, Isolauri E, Lagstrom H, Laitinen K (2006) Impact of dietary counseling on nutrient intake during pregnancy: A prospective cohort study. British Journal of Nutrition96: 1095-1104.
- Maher JE, Goldenberg RL, Tamura T, Cliver SP, Hoffman HJ, et al. (1993) Albumin levels in pregnancy: A hypothesis-decreased levels of albumin are related to increased levels of alpha-fetoprotein. Early Hum Dev 34: 209-215.
- Abbassi-Ghanavati M, Greer LG, Cunningham FG (2009) Pregnancy and laboratory studies: A reference table for clinicians. ObstetGynecol 114: 1326-1331.
- Sathyanarayana TS, Asha MR, Ramesh BN, Jagannatha KS (2008) Understanding nutrition, depression and mental illnesses. Indian J Psychiatry 50: 77-82.
- (2016) Drug and therapeutics bulletin 54: 81-84.
- Grantham-McGregor S, Baker-Henningham H (2005) Review of the evidence linking protein and energy to mental development. Public Health Nutrition 8: 1191-2011.
- Mi J, Law C, Zhang KL, Os mond C, Stein C, Barker D (2000) Effects of infant birth weight and maternal body mass index in pregnancy on components of the insulin resistance syndrome in China. Ann Intern Med pp: 132.
- Farley DM, Choi J, Dudley DJ, Li C, Jenkins SL, et al. (2010) Placental amino acid transport and placental leptin resistance in pregnancies complicated by maternal obesity. Placenta 31: 718-24.
- Ornoy A (2011) Prenatal origin of obesity and their complications: Gestational diabetes, maternal overweight and the paradoxical effects of fetal growth restriction and macrosomia. ReprodToxicol 32: 205-212.
Relevant Topics
Recommended Journals
Article Tools
Article Usage
- Total views: 40552
- [From(publication date):
October-2016 - Apr 03, 2025] - Breakdown by view type
- HTML page views : 39196
- PDF downloads : 1356