Response Surface Methodology for Optimization Laccase Production by Alcaligenes faecalis NYSO Using Agro-industrial Wastes as Co-Substrate
Received: 03-Apr-2019 / Accepted Date: 15-May-2019 / Published Date: 22-May-2019
Abstract
The additives (activators or inhibitors) influencing the Alcaligenes faecalis NYSO (ac: KP859538) laccase productivity were studied in submerged fermentation as well as the exploitation the ultimate benefit from lignocellulosic waste through response surface methodology (RSM) was investigated in this study. Among the various amino acids and vitamins used as a growth factor and or/ nitrogen source, L-glutamine, L-cysteine, L-arginine, and biotin were found to be the most suitable for laccase production. Ethanol addition caused about 40% increment followed by petroleum ether, then acetonitrile and methanol whilst, isopropanol caused a slight decrease in production. Promotion of laccase production was achieved in media supplemented with fast blue, ethidium bromide and azure B by 37-35%. The humic acid, 2,2-azino-di-[3-ethylbenzo-thiazolin-sulphonate] (ABTS) and alkali lignin proved to be the best synthetic inducers for laccase. Among the various wastes used, sugarcane bagasse followed by black liquor and prickly pears peel were found to be the best natural substrates could be used for laccase production with maximum activity (1408 and 1365 U ml-1 min-1), respectively. Considering this trend, pre-formulation media was designed using yeast extract, copper sulfate, and mixture of black liquor and sugarcane bagasse, thereafter, the RSM was adopted to acquire the best process conditions among the selected variables where a five-level Central Composite Design (CCD) was employed to create a polynomial quadratic model creating the relationship between these variables and laccase activity.
Keywords: Agro-industrial wastes; Laccase; Central composite design; Response surface methodology; Alcaligenes faecalis; Phenolic compounds; Submerged fermentation; Bacterial bioremediation
Introduction
With industrialization, the pollution of the environment with hazardous compounds has become a dangerous problem. Recalcitrant and phenolic compounds are associated with wastes from several industrial processes [1]. Pulp and paper industry is considered as one of the most polluter industry around the world [2], large amounts of toxic and intensely colored effluents waste which causing severe water pollution are produced annually. The black liquor and sugarcane bagasse which characterized by a high level of chemical oxygen demand (COD) and high lignin content, are considered as the most important effluents from pulp and paper industry. The primary contributors to the color and toxicity of these effluents are lignin and its derivative. As a result, lignin is high molecular weight compound and resistance to degradation either chemically or biologically [3].
One of the main concerns for the reasonable and sustainable development of planet Earth in the 21st century finds a way to elimination of widely dispersed, anthropogenic, recalcitrant pollutants. Bioremediation is one of the green chemistry strategies which considered the safest, least disruptive and most cost-effective treatment in comparison with traditional physicochemical treatments [4].
Laccases (p-benzenediol: oxygen oxidoreductase, 1.10.3.2) belong to the family of blue multicopper oxidases, which catalyze the one-electron oxidation of four reducing-substrate molecules concomitant with the four-electron reduction of molecular oxygen to water [5]. They have a unique molecular structure of glycoprotein with copper atoms distribution [6]. Researchers in last decades paid more attention toward the study of laccases due to they have a broad range of substrate specificity, which can facilitate industrial purposes and bioremediation processes [7]. As a result of potential capability of laccases for degradation of synthetic azo dyes and dyes of diverse chemical structure, this opens a new era for the biological removal of these azo dyes from nature [8].
As a result of these diversity laccase applications in the industrial and biotechnological field, studies on laccase producing organisms and the optimization of its production are being carried out by many investigators. The ever-increasing demand for this enzyme requires the production process to be economical. Exploitation of inexpensive raw materials for laccase production could be viewed as a solution to make the entire process cost-effective and utilization of natural occurring inducers for further enhancement may add to the benefit was reported by many investigators [9].
The present study was focused on the utilization of Alcaligenes faecalis NYSO (KP859538) laccase for bioremediation of some environmental pollutants to exploit the ultimate benefit from lignocellulosic waste. Efforts were taken to enhance the laccase production by using a wide variety of chemical and natural inducers. The potential of this bacterial strain to utilize various synthetic dyes belonging to different categories was also evaluated.
Materials and Methods
Microorganism and its maintenance
Alcaligenes faecalis NYSO (KP89538) used in the present study was isolated from a discharged effluent of Tanning and leather industry, Alexandria, Egypt and identified by 16S rDNA sequence analysis as described previously [10]. The culture was grown and maintained on buffered LB agar slants.
Medium and cultural conditions for submerged fermentation
In order to study the influence of different inducers on laccase productivity, the inducers were added to buffered optimized media assay.
Laccase activity has been estimated calorimetrically using 2,2-azino-di-[3-ethylbenzo-thiazolin-sulphonate] (ABTS) a substrate with an extinction coefficient (ε) 436=29,300 M-1 cm-1 at 436 nm [11]. One unit of enzyme activity is defined as the amount of enzyme that catalyzes the oxidization 1.0 U mol of ABTS per minute under above standard assay conditions; the activities were expressed in U ml-1 min-1.
Effects of different amino acids and vitamins on laccase production
The effect of some of amino acids and vitamins such as isoleucine, tyrosine, L-arginine, L-glutamine, lysine, phenylalanine, valine, cysteine, glycine, nicotinic acid, thiamin HCl, biotin and histidine-HCl on laccase production was studied at concentration 0.1%. All amino acids and vitamin were sterilized by filtration.
Effects of different solvents on laccase production
1.0% of various solvents such as ethanol, acetone, petroleum ether, isopropanol, methanol, and acetonitrile were used for the study of their influence on laccase production.
Effects of different synthetic dyes on laccase production
Different synthetic dyes were examined in order to characterize their influence on laccase production. Methyl orange, bromophenol blue, Eriochrome blue-black T, phenol red, safranine, fast green, fast blue, Dinitrosalicylic acid, basic fuchsin, azure B, methylene blue, bromocresol purple, bromothymol blue, congo red, malachite green, ethidium bromide, crystal violet, acridine orange, Coamisse blue R250, methyl red and Nile red were used at concentration of 10 μM except ethidium bromide at concentration of 2.5 μM. All dyes were dissolved in 10% DMSO and sterilized by autoclaving except ethidium bromide sterilized by syringe filter.
Effects of different synthetic substrates and inducers on laccase production
In order to study the effect of putative phenolic and aromatic inducers on enzyme production, various aromatic and phenolic compounds such as guaiacol (2-methoxy phenol), veratryl alcohol (3,4-dimethoxy benzyl alcohol), catechol (benzene-1,2-diol), pyrogallol (benzene-1,2,3-triol), para-anisidine (p-methoxy aniline), vanillic acid (4-hydroxy,3-methoxy benzoic acid), o-toluidine, syringaldehyde, p-dimethylamino-benzaldehyde, 4-aminophenol, 4-aminobenzoic acid, p-phenylenediamine, benzophenol, 4 nitrophenol, alkali lignin, tannic acid, benzoic acid, kojic acid, hydroquinone, humic acids, benzaldehyde, Tween 80, phenol, inuline, aniline, mercaptoacetic acid, ascorbic acid, sodium azide, dimethyl sulfoxide (DMSO) and ABTS were exploited at concentration 1.0 mM at the time of inoculation under sterile conditions. Catechol, vanillic acid, and alkali lignin were dissolved in sterile water while all the other inducers were dissolved in 10% DMSO.
Effects of different lignocellulosic residues on laccase production
To investigate the effects of natural inducers on Alcaligenes faecalis NYSO (KP859538) laccase production, a range of agricultural wastes such as wheat bran, wheat straw, rice straw, rice bran, sugarcane bagasse, corn cobs, banana stalk, grape seed, grape skin, green tea, yellow corn, pear peel, oat, prickly pears peel, pomegranate peel, hazelnuts peel, black liquor, sugarcane pith and orange peel were exploited at concentration of 1% (w/v) [12]. Banana stalks, prickly pears peel, sugarcane bagasse, green tea, pear peel, and pomegranate peel, which collected from the fruit opened market were washed and chopped into small chips. The chips were sun-dried, oven dried (50°C) to constant weight, ground to 40 mm mesh size, and stored in plastic jars to keep the material moisture-free [13]. While orange peel the chips was socked in 18.17 mM KOH for 1.0 hour then, sundried/ oven dried (50°C) to constant weight, ground to 40 mm mesh size and stored in plastic jars. The wheat bran, wheat straw, rice straw, rice bran, yellow corn, corn cobs, hazelnuts peel and oat are obtained from Agriculture Institute Research-Alexandria were air-dried and cut into 10-20 mm lengths. Black liquor was obtained from pulp and paper factory, Alexandria, Egypt.
Utilization of agro-waste materials for evaluation of culture conditions
In order to obtain the ultimate benefit from lignocellulosic wastes, pre-formulation media was designed. Sugarcane bassage and black liquor were selected for evaluation of culture conditions. This study carried out in four trials experiment compared with control; the first trial consist of same ratio 1:1 of 1.0% sugarcane bagasse and black liquor, 0.8 mM copper sulfate and 0.8% yeast extract dissolved in 0.1 M glycine-NaOH buffer pH 11.0. The second trial consists of the same component as the first trial except yeast extract was omitted, whilst, the third trial had the same media composition as the first trial but cupper sulfate was deleted. In the fourth trial, only consist of 1.0% sugarcane bagasse and black liquor. Finally, the control trial is the previous optimized media (previous submitted). The inoculated flasks were incubated at 30°C in a shaker incubator (200 rpm) for 24 hrs. The culture was filtered through cheese cloths for remove of agrowaste materials residual from cultural media and laccase activity was estimated in bacterial cell pellets.
Response surface methodology (Central composite design): Using central composite design was adopted to find the optimum levels of the significant variables (Copper sulfate, yeast extract, and selected agrowaste material) and the effects of their mutual interactions on enzyme production. A total of 20 trials were carried out. Each independent variable was studied at five different levels coded as (-2, -1, 0, +1 and +2 respectively) [14]. The center point of the design was replicated six times for the estimation of error. The CCD and the coded levels of each factor were shown in Table 1. The experimental design matrix used for the study is shown in Table 2. The JMP program was used for experimental design, data analysis, and quadratic model building. Laccase yield obtained was taken as the experimental values of the dependent variable or response (Y), while predicted values of the response were obtained from quadratic model fitting techniques. The optimal values of the three parameters were achieved by solving the obtained polynomial equation. In addition to it, three-dimensional plots were constructed for visual observation of the trend of maximum response and the interactive effects of the significant variables on the response by STATISTICA 5.0 software. The response was fitted by a second order model to be correlated with the independent parameters. The correlation between the three parameters and the response (laccase activity) was described by the following predictive quadratic polynomial equation:
| Variable | Variable code | Low level (-2) g/l | (-1) g/l | Middle level (0) g/l | (+1) g/l | High level (+2) g/l |
|---|---|---|---|---|---|---|
| Yeast extract | X1 | 3 | 6 | 9 | 12 | 15 |
| CuSO4.2H2O | X2 | 0.075 | 0.15 | 0.225 | 0.3 | 0.375 |
| Mixture of agro-wastes | X3 | 6 | 12 | 18 | 24 | 30 |
Table 1: The levels of variables chosen for the Box-Behnken optimization experiment.
| Trials | X1 (Y.E.) | X2 (CuSO4) | X3 (waste) | Activity (Uml-1min-1) |
|---|---|---|---|---|
| 1 | 0 | 2 | 0 | 503.7543 |
| 2 | 1 | 1 | -1 | 829.3515 |
| 3 | 1 | -1 | -1 | 712.628 |
| 4 | 0 | 0 | -2 | 509.8976 |
| 5 | -1 | -1 | 1 | 411.6041 |
| 6 | 0 | 0 | 0 | 511.9454 |
| 7 | 0 | 0 | 2 | 530.3754 |
| 8 | -2 | 0 | 0 | 303.0717 |
| 9 | 0 | -2 | 0 | 573.3788 |
| 10 | -1 | 1 | 1 | 337.884 |
| 11 | -1 | -1 | -1 | 243.686 |
| 12 | 0 | 0 | 0 | 550.8532 |
| 13 | 1 | -1 | 1 | 620.4778 |
| 14 | 0 | 0 | 0 | 532.4232 |
| 15 | 0 | 0 | 0 | 526.2799 |
| 16 | 1 | 1 | 1 | 628.6689 |
| 17 | -1 | 1 | -1 | 346.0751 |
| 18 | 0 | 0 | 0 | 466.8942 |
| 19 | 2 | 0 | 0 | 1044.369 |
| 20 | 0 | 0 | 0 | 522.1843 |
Table 2: Matrix designed for Alcaligenes faecalis NYSO (KP859538) central composite factorial experimental design.
Y=β0+β1X1+β2X2+β3X3+β12(X1X2)+β13(X1X3)+β23(X2X3)+β11X12+β22 X22+β33X32
where Y is the predicted response (laccase activity U ml-1 min-1); β0 is the model intercept; X1, X2, and X3 are the independent variables, β1, β2, and β3 are linear coefficients; β12, β13, and β23 are cross product coefficients; and β11, β22, and β33 are the quadratic coefficients. The quality of fit of the polynomial model equation was expressed by a coefficient of determination, R2.
The enzyme activity data were subjected to multiple linear regressions using the JMP program to estimate the t-values, P-values, and confidence levels expressing the P-values as a percentage.
Validation of the model: The RSM model was validated further for predicted versus actual responses. Each experiment was carried out in triplicate, and the results were compared with the predicted responses by the mathematical model.
Determination of lignin residual
Residual of lignin (Klason lignin) from biodegradation of sugarcane bagasse according to the previous experiment was estimated by the method described by Templeton and Ehrman [15]. 1.0 oven dried gram of sample was mixed with 20 ml of sulfuric acids (72%) and the mixture swirled at room temperature overnight. 540 ml distilled water was added and the sample was heated by autoclave at 100°C for 4.0 hrs. After cooling to room temperature, samples were separated into liquid and solid fractions by filtration (Whatman #1). The filter paper was washed with hot water for 1.0 hour. Filter paper that contained lignin residues was muffled at 450°C for 30 min, and then at 800°C for 45 min for determining the ash content.
Lignin %=weight of lignin-the weight of its ash/weight of the dry raw material.
Results and Discussion
Effects of different amino acids and vitamins on laccase production
Among the various amino acids and their analogs studied in this work, the maximum laccase production was stimulated in the presence of L-glutamine, L-cysteine and L-arginine by 1.84, 1.49 and 1.34-fold, respectively (Table 3). A slight stimulation was observed in the presence of thiamin HCl, tyrosine and isoleucine by 10, 9.3 and 8.6%; respectively greater than control cultures. It has been found that shortchain amino acids with up to four carbons, like L-glycine, caused a slight reduction in laccase production, (0.92%) compared to the control culture. In contrast, the presence of histidine-HCl in bacterial culture did not stimulate the laccase production but caused a sharp decline in laccase titer (93.2%). Laccase titer was gone down approximately to four-fifths, three fifths and one-fifth time less than control culture when the cultures supplemented with nicotinic acid, phenylalanine, and valine; respectively. Also, it was noticed that the laccase production was promoted in presence of biotin and lysine in culture media by about 1608 and 1466 U ml-1 min-1; respectively, more than that obtained from culture control (1198 U ml-1 min-1). This find may be in accordance with others citation that reported the enhancement of laccase production in presence of L-glutamine, tyrosine, L-cystine, lysine, and isoleucine [16]. Contrary to other finding recorded that the L-valine, L-arginine, L-cysteine, and biotin caused repression of laccase titer [17,18] and phenylalanine, nicotinic acid and L-histidine HCl were recorded as enhancers for laccase production.
| Amino acids (0.1%) | Laccase activity (U ml-1 min-1) | Laccase activity (%) |
|---|---|---|
| Control | 1198.635 | 100 |
| L-Glutamine | 2214.334 | 184.738 |
| L-Cysteine | 1788.396 | 149.2027 |
| L-Arginine | 1608.191 | 134.1686 |
| Biotin | 1583.618 | 132.1185 |
| Lysine | 1466.212 | 122.3235 |
| Thiamin HCl | 1318.771 | 110.0228 |
| Tyrosine | 1310.58 | 109.3394 |
| Isoleucine | 1302.389 | 108.656 |
| Glycine | 1187.713 | 99.08884 |
| Nicotinic acid | 1067.577 | 89.06606 |
| Phenylalanine | 764.5051 | 63.78132 |
| Valine | 354.9488 | 29.61276 |
| Histidine-HCl | 81.91126 | 6.833713 |
Table 3: Effect of different amino-acids on laccase production by Alcaligenes faecalis NYSO (KP859538).
Effects of different solvents on laccase production
Addition of organic solvents, with respect to their concentration, showed varied effects on laccase production. The cultures with ethanol expressed more laccase activity than the other treatments exhibiting an increase in activity and reaching a peak of 1679 U ml-1 min-1 in compared with culture control without any solvent (1198 U ml-1 min-1). The causes of increase laccase production in the presence of ethanol were previously explained by Chhaya et al. [16] as a result of its dual role, i.e., gene expression and inhibition of protease activity. Moreover, the results (Table 4) show that petroleum ether, acetonitrile, and methanol enhanced the laccase activity by 1.307, 1.23 and 1.18-fold than that found by control trial. On the other hand, isopropanol caused a slight decline in laccase titer about 4.4% reduction was noticed, while a sharp reduction in laccase production was achieved by acetone, more than one-half of enzyme activity was lost. The present results are consistent with that obtained by other investigators who recorded that laccase production was increased in presence of ethanol and methanol, and suppressed in presence of acetone [16,19], but in contrast to result obtained by Sharma et al. [20] and Singh et al. [21] who recorded that the ethanol and methanol caused reduction in laccase production, while, acetone and isopropanol caused no change in laccase production.
| Solvents (1.0%) | Laccase activity (U ml-1min-1) | Laccase activity (%) |
|---|---|---|
| Control | 1198.635 | 100 |
| Ethanol | 1679.181 | 140.0911 |
| Petroleum ether | 1567.235 | 130.7517 |
| Acetonitrile | 1474.403 | 123.0068 |
| Methanol | 1419.795 | 118.451 |
| Isopropanol | 1146.758 | 95.67198 |
| Acetone | 693.5154 | 57.85877 |
Table 4: Effect of different solvents on laccase production by Alcaligenes faecalis NYSO (KP859538).
Effects of different synthetic dyes on laccase production
The dye decolorization studies showed great variation in the ability of Alcaligenes faecalis NYSO (KP859538) to degrade dyes belonging to diverse categories. As well as laccase production varied greatly with a different dye-containing media (Table 5). The laccase titer had induced by 37.5, 37.5, 35.3, 34.8, 26.19, 25.2, 24.1, 21.6, 19.5 and 14.1% more than that control culture when ethidium bromide (phenanthridinium) and fast blue (benzenediazonium), Azure B (N,N,N′-trimethylthionin), bromophenol blue (triphenylmethane), congo red (azo), Dinitrosalicylic acid, bromocresol purple, methyl orange (azo), Eriochrome black T (azo), basic fuchsin and phenol red (triphenylmethane); respectively was added to culture media. However, bromothymol blue (triphenylmethane) was not shown a significant effect on laccase production only 6.0% more than control culture. Laccase production went down when fast green, safranine (heterocyclic), Coamisse blue R250, methyl red, acridine orange, and crystal violet were used by 8.5%, 15.5, 28.3 and 31.5%, 48.1% and 75%; respectively. In addition to this, a sharp decline was noticed by using the Nile red, methylene blue (heterocyclic) and malachite green (triphenyl-methane), only 51, 43 and 19 U ml-1 min-1; respectively of enzyme titer had retained in comparison with control culture (1198 U ml-1 min-1).
| Synthetic Dyes (10 µM) | Laccase activity (U ml-1 min-1) | Laccase activity (%) |
|---|---|---|
| Control | 1198.635 | 100 |
| Ethidium bromide (2.5 µM) | 1649.147 | 137.5854 |
| Fast blue | 1649.147 | 137.5854 |
| Azure B | 1621.843 | 135.3075 |
| Bromophenol blue | 1616.382 | 134.8519 |
| Congo red | 1512.628 | 126.1959 |
| Dinitrosalicylic acid | 1512.628 | 126.1959 |
| Bromocresol purple | 1501.706 | 125.2847 |
| Methyl orange | 1488.055 | 124.1458 |
| Eriochrome blue black T | 1458.02 | 121.6401 |
| Basic fuchsin | 1433.447 | 119.59 |
| Phenol red | 1367.918 | 114.123 |
| Bromothymol blue | 1272.355 | 106.1503 |
| Fast green | 1097.611 | 91.57175 |
| Safranine | 1012.969 | 84.51025 |
| Coamisse blue R250 | 860.0683 | 71.75399 |
| Methyl red | 821.843 | 68.56492 |
| Acridine orange | 622.5256 | 51.93622 |
| Crystal violet | 300.3413 | 25.05695 |
| Nile red | 51.87713 | 4.328018 |
| Methylene blue | 43.68601 | 3.644647 |
| Malchite green | 19.11263 | 1.594533 |
Table 5: Effect of different synethetic dyes on laccase production by Alcaligenes faecalis NYSO (KP859538).
Comparing the obtained results with those cited by the other investigators, it was found that the ethidium bromide was the only dye reported to increase laccase activity in fungi and bacteria [20]. Bromophenol blue, congo red, basic fuchsin, Dinitrosalicylic acid, phenol red, and methyl orange were recorded as promotors for laccase production [18,22] which matched the results of the present study. On the other hand, Eriochrome black T (azo), methyl orange (azo), bromophenol blue (triphenylmethane), congo red (azo) and bromothymol blue (triphenylmethane) caused repressed to laccase production [20], unlike the findings of the results of the present study. It was found that through the study of other investigators the malachite green, and the crystal violet were used for induction of laccase production [23].
Effects of different synthetic substrates and inducers on laccase production
The different inducers were added in an equal concentration (1.0 mM) to the medium to investigate their effect on laccase production by Alcaligenes faecalis NYSO (KP859538), as shown in Table 6. The results indicated that the humic acid (1752 U ml-1 min-1), provided a 1.46-fold increase in laccase activity in relation to control (1198 U ml-1 min-1). Also, the laccase titer was increased in presence of ABTS, alkali lignin and Tween 80 by approximately 42.8, 36.9 and 35.9%; respectively more than that obtained from control trial which free from any inducer. The phenolic compound such as phenol (1556 U ml-1 min-1) also proved to be a promising inducer for laccase production by this strain. Furthermore, vanillic acid, mercaptoacetic acid, inuline, DMSO, ascorbic acid and guaiacol stimulated with a moderate increase in laccase production to a certain extent.
| Synthetic substrates (1.0 mM) | Laccase activity (U ml-1 min-1) | Laccase activity (%) |
|---|---|---|
| Control | 1198.635 | 100 |
| Humic acids | 1752.901 | 146.2415 |
| ABTS | 1711.945 | 142.8246 |
| Alkali lignin | 1640.956 | 136.9021 |
| Tween 80 | 1630.034 | 135.9909 |
| Phenol | 1556.314 | 129.8405 |
| Vanillic acid | 1534.471 | 128.0182 |
| Mercaptoacetic acid | 1474.403 | 123.0068 |
| Inuline | 1449.829 | 120.9567 |
| DMSO | 1351.536 | 112.7563 |
| Ascorbic acid | 1337.884 | 111.6173 |
| Guaiacol | 1318.771 | 110.0228 |
| Benzoic acid | 1288.737 | 107.5171 |
| Catechol | 1269.625 | 105.9226 |
| Tannic acid | 1261.433 | 105.2392 |
| Syringaldazine | 1253.242 | 104.5558 |
| Sodium azide | 1215.017 | 101.3667 |
| 4-aminobenzoic acid | 1179.522 | 98.40547 |
| Pyrogallol | 1160.41 | 96.81093 |
| Veratryl alcohol | 1113.993 | 92.9385 |
| Kojic acid | 1094.881 | 91.34396 |
| O-tolidine | 1092.15 | 91.11617 |
| p-dimethylaminobenzaldehyde | 999.3174 | 83.3713 |
| Hydroquinone | 969.2833 | 80.8656 |
| Aniline | 881.9113 | 73.57631 |
| 4- nitrophenol | 865.529 | 72.20957 |
| p-anisidine | 245.7338 | 20.50114 |
| Benzaldhyde | 185.6655 | 15.48975 |
| Benzophenol | 38.22526 | 3.189066 |
| 4-aminophenol | 10.9215 | 0.911162 |
| p.phylenediamine | 0 | 0 |
Table 6: Effect of different synthetic inducers on laccase production by Alcaligenes faecalis NYSO (KP859538).
However, inducing effect was not observed clearly when the other aromatic compounds were used such as benzoic acid, catechol, tannic acid, and syringaldazine; whereas, an increase in laccase induction by 7.5, 5.9, 5.2 and 4.5% were obtained using these compounds; respectively. Sodium azide had not shown any significant effect on laccase production. It can be clearly seen that laccase level was affected slightly by 4-aminobenzoic acid, pyrogallol, veratryl alcohol, kojic acid and o-toluidine where laccase titer was little bit return back to 1.6, 3.2, 7.1, 8.7 and 8.9% respectively, which is less than that obtained from control trial. In the same way, p-dimethylaminobenzaldehyde, hydroquinone, aniline, and 4- nitrophenol exhibited a moderate repression of laccase production by four and two-fifth, four-fifth, three and seven-fifth, and three and five-fifth; respectively, less time than the control trial. On the contrary, a strong repressed for laccase activity was observed in presence of p-anisidine, benzaldehyde, benzophenol, and 4-aminophenol in cultivation media, where laccase was retained only about 245, 185, 38 and 10.0 U ml-1 min-1; respectively, from its activity obtained from the control trial (1198 U ml-1 min-1). While the bacterium failed to grow in the p.phenylenediamine supplemented medium, consequently, the laccase production was fully inhibited. Results obtained in the present study are in a good agreement with that obtained by other investigators [18,24] but inconsistent with that obtained by Malhotra et al. [20].
Effects of different lignocellulosic residues on laccase production
Results cited in Table 7 showed that among the various agricultural wastes screened for laccase production, sugarcane bagasse was found to be the most suitable substrate as it supported maximum laccase production (1408 U ml-1 min-1) in comparison with control trial (1198 U ml-1 min-1). Similarly, black liquor, prickly pears peel, corn cob, and sugarcane pith exerted a stimulatory effect on Alcaligenes faecalis NYSO (KP859538) laccase production, compared to the control media. Black liquor and prickly pears peel were found to be effective next to sugarcane bagasse; they had nearly the same induction effect on laccase titer (1365 U ml-1 min-1). This result was considered acceptable, since black liquor, which contains lignin from pulp and paper bleaching, induce laccase activity as reported by Mongkolthanaruk et al. [24].
| Natural substrates (1.0%) | Lignin content (%) (36,80,90) | Laccase activity (U ml-1 min-1) | Laccase activity (%) |
|---|---|---|---|
| Control | 0.0 | 1198.635 | 100 |
| Sugarcane bagasse | 25 | 1408.874 | 117.5399 |
| Black liquor | 17 | 1365.188 | 113.8952 |
| Prickly pears peel | 2.4 | 1365.188 | 113.8952 |
| Corn cob | 4.5-6.6 | 1294.198 | 107.9727 |
| Sugarcane pith | 16.1 | 1277.816 | 106.6059 |
| Rice straw | 9.9 | 1220.478 | 101.8223 |
| Rice bran | 5 | 1209.556 | 100.9112 |
| Orange peel | 12 | 1053.925 | 87.92711 |
| Oat | 13.7 | 1018.43 | 84.96583 |
| Wheat straw | 14.5 | 996.587 | 83.14351 |
| Wheat bran | 11.4 | 832.7645 | 69.47608 |
| Corn yellow | 18 | 800 | 66.7426 |
| Pear | 29.8 | 791.8089 | 66.05923 |
| Apple | 32 | 660.7509 | 55.12528 |
| Peanut shell | 41.2 | 622.5256 | 51.93622 |
| Bannana stalk | 8 | 603.413 | 50.34169 |
| Grape skin | 5 | 455.9727 | 38.041 |
| Hazelnuts peel | 51.3 | 174.744 | 14.57859 |
| Pomegranate peel | 4.5 | 8.191126 | 0.683371 |
| Grape seed | 44 | 0.0 | 0.0 |
| Green tea | 5-6 | 0.0 | 0.0 |
Table 7: Effect of different natural inducers on laccase production by Alcaligenes faecalis NYSO (KP859538).
The media supplement with corn cob and sugarcane pith characterized by tiny induction effect, where laccase level slightly climbed up by 7.9 and 6.6% more than control trial. However, the rice straw and rice bran had not significant effect on laccase production; about 1.9 and 0.9% more increase than the control trial was obtained. Other worth mentioned before, the orange peel, oat, wheat straw caused little bite reduction in laccase production to about 12.1, 15.1 and 16.9% less than control trial. Whereas the laccase productions by wheat bran, corn yellow, pear, apple and pea nut did not show any increment in the laccase production but they were retained only about 69, 66.7, 66, 55 and 51% respectively of its activity when compared to the control trial. Laccase activity reduction reached 50, 38 and 14% was obtained in media supplement with banana stalk, grape skin, and hazelnuts peel respectively. On the other hand, the lowest activity was obtained with pomegranate peel when used as a substrate. No detectable increase in laccase activity was found when grape seed and green tea were used as a substrate.
The present study results are in disagreement with observations reported by other investigators [9,17,25] who found that rice straw, wheat bran, peanut shell, banana peel, and black grapes were identified as promising substrates for laccase production, while the other substrates like corn cops, rice straw, and sugarcane bagasse caused low laccase production. However, the observation documented by other investigators [26,27] are compatible with the results of this study in term of apple and wheat bran caused decline in laccase production.
Utilization of sugarcane bagasse and black liquor for media formulation
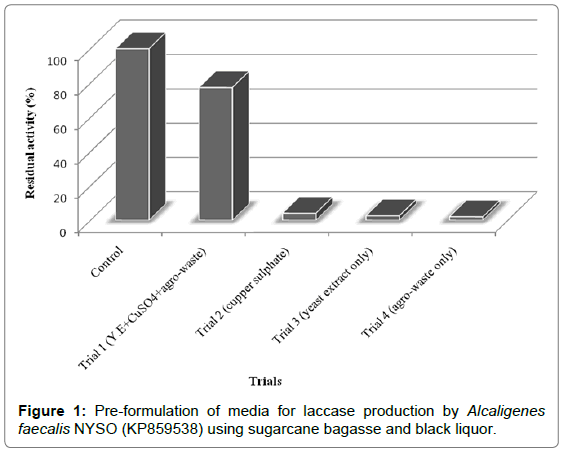
The exploitation of the ultimate benefit from lignocellulosic waste was investigated by using the most important and the cheapest agrowaste as a substrate for the formulation of suitable and simple media for bacterial growth and laccase production. The recorded results (Figure 1) revealed that the media composition of trial one, which consisted of (yeast extract, cupper sulfate, and mixture sugarcane bagasse and black liquor), was considered the most suitable media for bacterial growth and production of laccase (1138 U ml-1 min-1) in comparison with control trial (1471 U ml-1 min-1). However, enzyme titer was reduced to about 22.7% from initial activity but the ultimate goal of this study is looking for a strategy to formulate simple media with maximizing utilization available agro-waste, to avoid their accumulation in the environment. About 96, 97.5 and 98% reduction in initial laccase activity was obtained from trial two, three and four; respectively, this result reflected components of media trial number one are complemented each other and necessary for both bacterial growth and enzyme production.
The present study proved the utility of sugarcane bagasse and black liquor as an inexpensive and easily available raw material for laccase production, and convenient with combinations study carried to explore the interaction between the two agricultural residues [28]. Additionally, the employed lignocellulosic residues are abundant and renewable, which are suitable for both growth and laccase enzyme induction [29].
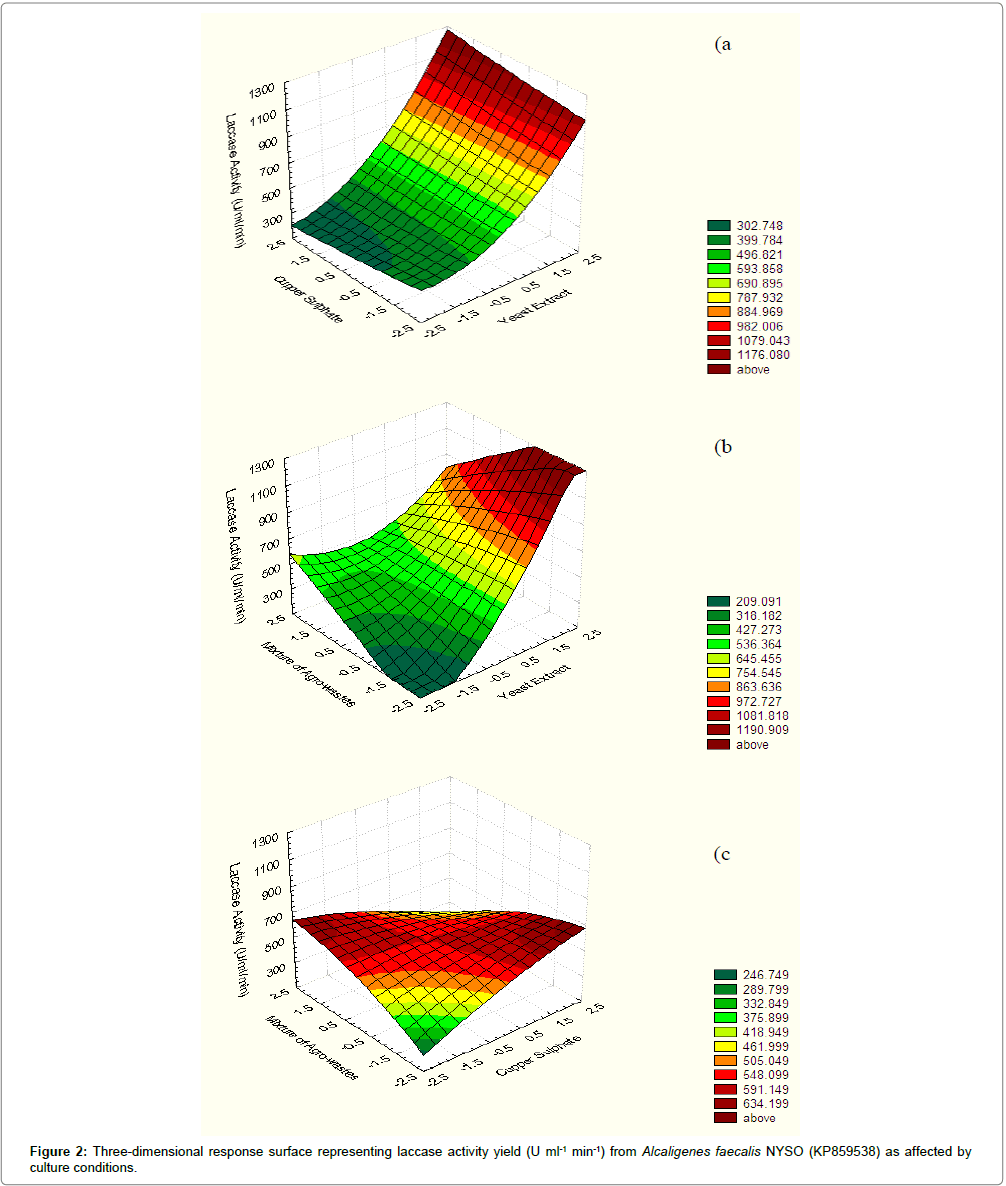
Response surface methodology (Central Composite Design): RSM in terms of CCD was applied to achieve the interactions between independent variables with each other along with the possible maximal levels of the process outcome at five different levels viz; -2, -1, 0, +1, +2 as presented in Table 1. Table 2 depicts the design matrix of the coded variables together with the experimental results of the enzyme activity. The main results of this study are presented in Figure 2, which represents the expected laccase response and the correlation between variables in three-dimensional plots. It can be clearly observed from Figure 2a and 2b that the effects of pairs of factors were additive since there are low interactions between the yeast extract-cupper sulfate and yeast extract – a mixture of agro-wastes. Figure 2c showed nonadditive effects of cupper sulfate and a mixture of agro-wastes due to the significant interaction between them. By additive of the twofactor effects, it is meant that the effect of one factor on the response does not depend on the level of the other factor. In Figure 2b it is obvious that maximum laccase activity was attained at higher levels of yeast extract (15 g/L) and lower levels of cupper sulfate (0.075 g/L). Figure 2b illustrates that increasing yeast extract concentration in the medium independent on a mixture of agro-wastes concentration led to increases in laccase activity. The optimum point deduced from Figure 2 is in accordance with the mathematically calculated optimum point. For predicting the optimal point, within experimental constraints, a second-order polynomial function was fitted to the experimental results of laccase activity (non-linear optimization algorithm):
Y=509.99+183.40X1+0.895X2-5.759X3+12.03X1X2-56.57X1X3- 35.58X2X3+34.60X12+0.814X22-3.79X32
Where, X1, X2, and X3 are yeast extract, CuSO4.5H2O, and a mixture of agro-wastes; respectively. At the model level, the correlation measures for the estimation of the regression equation are the multiple correlation coefficients R and the determination coefficient R2. The closer the value of R is to 1.0, the better is the correlation between the measured and the predicted values. The value of the determination coefficient in this experiment R2=0.966 for laccase activity, is a measure of fit of the model, indicates that about 3.4% of the total variations created by variables are not explaining laccase activity.
The analysis of variance using the ANOVA test in the central composite experiment was generated which gives p=0.0001. Since the p-value indicated in the ANOVA table is less than 0.05, it is concluded that there is a statistically significant relationship among the studied variables at 95% confidence level (p=0.05) (Table 8).
| Term | Estimate | Std Error | t Ratio | Prob>|t| | Confidence level (%) |
|---|---|---|---|---|---|
| Intercept | 509.9907 | 18.4104 | 27.70123 | <.0001 | |
| X1&RS | 183.4044 | 11.53933 | 15.89386 | <.0001 | 99.9 |
| X2&RS | 0.895904 | 11.53933 | 0.077639 | 0.939647 | 7 |
| X3&RS | -5.75939 | 11.53933 | -0.49911 | 0.628499 | 37.2 |
| X1*X2 | 12.03072 | 16.31907 | 0.737218 | 0.47793 | 52.3 |
| X1*X3 | -56.57 | 16.31907 | -3.46649 | 0.006057 | 99.395 |
| X2*X3 | -35.5802 | 16.31907 | -2.18028 | 0.05422 | 94.6 |
| X1*X1 | 34.60285 | 9.205199 | 3.759056 | 0.003728 | 99.63 |
| X2*X2 | 0.814459 | 9.205199 | 0.088478 | 0.931244 | 6.9 |
| X3*X3 | -3.79305 | 9.205199 | -0.41206 | 0.688993 | 31.2 |
Table 8: Statistical analysis of central composite design showing coefficient values, t- and p-values for each variable on laccase activity.
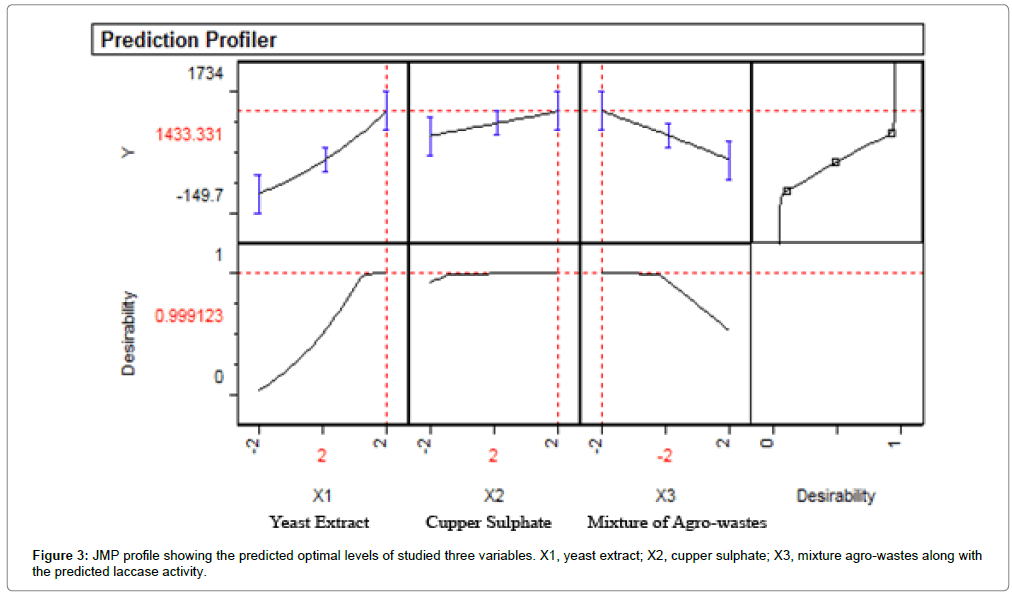
The optimal levels of the five studied variables as obtained from the maximum point of polynomial model were estimated using the JMP programme and Solver function of Microsoft Excel tools, and found to be: yeast extract, 15 g/l; CuSO4.5H2O, 0.375 g/l and mixture of agrowastes, 6 g/l with prediction calculated enzyme activity equal to 1433.33 U ml-1 min-1 (Figure 3). Results obtained in this study are in accordance with others findings [30,31] recorded that the bagasse fibers were dignified in an eco-friendly method using the laccase enzyme and the delignification process was optimized using surface response methods.
Verification model: In order to determine the accuracy of the quadratic polynomial, a verification experiment was carried out under predicted optimal conditions monitoring growth and enzyme activity in the optimized medium. The bench-scale experiments show that experimental laccase activity was 1386.31 U ml-1 min-1. The calculated model accuracy was 96.74% and this high degree of accuracy is evidence of the model validation under the following optimal conditions: 15 g/l; CuSO4.5H2O, 0.375 g/l and mixture of agro-wastes, 6 g/l, and buffered condition pH 11.0 under cultivation temperature of 30°C and incubation time of 24 hrs.
According to method described by Templeton and Ehrman [15] for determination of residual of lignin (Klason lignin) from biodegradation of sugarcane bagasse, the result referred that the laccase enzyme from Alcaligenes faecalis NYSO (KP859538) had the capacity to degrade the sugarcane bagasse to about 3.2% according to previously described growth condition, where lignin content for raw sugarcane bagasse before cultivation of bacterial strain was approximately 27.8%, while, the lignin content after cultivation of bacteria strain for 24 hrs incubation time was 24.6%.
Conclusion
The present study focused on the utilization of Alcaligenes faecalis NYSO (KP859538) laccase for bioremediation of some environmental pollutants to exploit the ultimate benefit from lignocellulosic waste. A five-level CCD was employed to create a polynomial quadratic model correlating the relationship between the three variables and laccase activity. It was found that the following optimal conditions: 15 g/l; CuSO4.5H2O, 0.375 g/l and a mixture of agro-wastes, 6 g/l, and buffered condition pH 11.0 under cultivation temperature of 30°C and incubation time of 24 hrs for utilization of agro waste with maximum laccase production around 1386.31 U ml-1 min-1. Efforts were taken to enhance the laccase production by using a wide variety of chemical and natural inducers. Among the various inducers used as a growth factor L-glutamine, ethanol and humic acid were found to be the most suitable for laccase production. The potential of this bacterial strain to utilize various synthetic dyes belonging to different categories was also evaluated. It was found that the promotion of laccase production was achieved in media supplemented with fast blue, ethidium bromide and azure B by 37-35%.
Financial Support
This work is financially supported by ULIXES project.
Acknowledgments
Authors would like to acknowledge Dr. Yasser R Abdel-Fattah for funding and scientific supporting.
References
- Desai SS, Nityanand C (2011) Microbial laccases and their applications: a review. Asian J Biotechnol 3: 98-124.
- Lawrence KW, Yung-Tse H (2006) Treatment of pulp and paper mill wastes. In: Waste Treatment in the process Industries. CRC Press, Taylor & Francis Group, New York, USA, pp: 453-497.
- Wu J, Xiao YZ, Yu HQ (2005) Degradation of lignin in pulp mill wastewaters by white-rot fungi on biofilm. Bioresource Technology 96: 1357-1363. â€
- Riva S (2006) Laccases: blue enzymes for green chemistry. Trends Biotechnol 24: 219-226. â€
- Galhaup CS, Goller C, Peterbauer K, Strauss J, Haltrich D (2002) Characterization of the major laccase isoenzyme from Trametes pubescens and regulation of its synthesis by metal ions. Microbiology 148: 2159-2169.
- Gianfreda L, Xu F, Bollag JM (1999) Laccases: a useful group of oxidoreductive enzymes. Bioremediat J 3: 1-25.
- Chhaya U, Gupte A (2010) Optimization of media components for laccase production by litter dwelling fungal isolate Fusarium incarnatum LD-3. J Basic Microbiol 50: 43-51.
- Tavares AP, Cristóvão RO, Gamelas JA, Loureiro JM, Boaventura RA, et al. (2009) Sequential decolourization of reactive textile dyes by laccase mediator system. J Chem Technol Biotechnol 84: 442-446.
- Niladevi KN, Prema P (2008) Effect of inducers and process parameters on laccase production by Streptomyces psammoticus and its application in dye decolourization. Bioresour Technol 99: 4583-4589. â€
- Abdelgalil SA, Morsi AA, Reyed RM, Soliman NA (2018) Application of Experimental Designs for Optimization the Production of Alcaligenes Faecalis Nyso Laccase. J Sci Ind Res 77: 713-722.
- Niku-Paavola ML, Raaska L, Itävaara M (1990) Detection of white-rot fungi by a non-toxic stain. Mycol Res 94: 27-31
- Arora DS, Gill PK (2000) Laccase production by some white rot fungi under different nutritional conditions. Bioresour Technol 73: 283-285. â€
- Irshad M, Asgher M, Sheikh MA, Nawaz H (2011) Purification and characterization of laccase produced by Schyzophylum commune IBL-06 in solid state culture of banana stalks. BioResources 6: 2861-2873. â€
- Box GE, Behnken DW (1960) Some new three level designs for the study of quantitative variables. Technometrics 2: 455-475. â€
- Templeton D, Ehrman T (1995) Determination of acid-insoluble lignin in biomass. In: Laboratory Analytical Procedures No. 003. National Renewable energy Laboratory, Golden, Colorado, USA.
- Chhaya U, Gupta A (2013) Effect of different cultivation conditions and inducers on the production of laccase by the litter-dwelling fungal isolate Fusarium incarnatum LD-3 under solid substrate fermentation. Ann Microbiol 63: 215-223.
- Mahmoud MG, Rifaat HM, El Sayed OH, El Beih FM, Selim MS (2013) Effect of inducers and process parameters on laccase production by locally isolated marine Streptomyces lydicus from Red Sea, Egypt. Int J Chem Tech Res 5: 15-23. â€
- Singh G, Batish M, Sharma P, Capalash N (2009) Xenobiotics enhance laccase activity in alkali-tolerant γ-proteobacterium JB. Braz J Microbiol 40: 26-30. â€
- Kaira G, Dhakar K, Pandey A (2015) A psychrotolerant strain of Serratia marcescens (MTCC 4822) produces laccase at wide temperature and pH range. AMB Express 5: 1-8. â€
- Malhotra K, Sharma P, Capalash N (2004) Copper and dyes enhance laccase production in γ-proteobacterium JB. Biotechnol Lett 26: 1047-1050. â€
- Singh G, Sharma P, Capalash N (2009) Performance of an alkalophilic and halotolerant laccase from. γ-proteobacterium JB in the presence of industrial pollutants. J Gen Appl Microbiol 55: 283-289.
- Shah MP (2015) Microbial decolorization of dyes by laccase. †Int J Curr Microbiol Appl Sci 4: 1-14.
- Qiao M, Wei K, Ding J, Liu Z, Zhang KQ, et al. (2011) Decolorizing activity of malachite green and its mechanisms involved in dye biodegradation by Achromobacter xylosoxidans MG1. J Mol Microbiol Biotechnol 20: 220-227. â€
- Mongkolthanaruk W, Tongbopit S, Bhoonobtong A (2012) Independent behavior of bacterial laccases to inducers and metal ions during production and activity. Afr J Biotechnol 11: 9391-9398.
- Jing D, Wang J (2012) Controlling the simultaneous production of laccase and lignin peroxidase from Streptomyces cinnamomensis by medium formulation. Biotechnol Biofuels 5: 1-7. â€
- Sampoorna Laxmi MV, Khan MM (2010) Effect of natural phenolic and lignin rich inducers on the production of laccases by Streptomyces griseus MTCC 4734. Int J Eng Sci Techn 2: 2130-2132. â€
- Niladevi KN, Sukumaran RK, Prema P (2007) Utilization of rice straw for laccase production by Streptomyces psammoticus in solid-state fermentation. J Ind Microbiol Biotechnol 34: 665-674. â€
- Bakkiyaraj S, Aravindan R, Arrivukkarasan S, Viruthagiri T (2013) Enhanced laccase production by Trametes hirusta using wheat bran under submerged fermentation. Int J Chem Technol Res 5: 1224-1238. â€
- Fu K, Fu S, Zhan H, Zhou P, Liu M, et al. (2013) A newly isolated wood-rot fungus for laccase production in submerged cultures. BioResources 8: 1385-1397. â€
- Singhal A, Choudhary G, Thakur IS (2013) Optimization of growth media for enhanced production of laccase by Cryptococcus albidus and its application for bioremediation of chemicals. J Environ Eng Sci 8: 340-351. â€
- Bhanu Rekha V (2013) Enhancing the absorbency of bagasse through enzymatic delignification. J Fashion Technol Textile Eng 1: 1-5.
Citation: Abdelgalil SA, Attia AM, Reyed RM, Soliman NA (2019) Response Surface Methodology for Optimization Laccase Production by Alcaligenes faecalis NYSO Using Agro-industrial Wastes as Co-Substrate. J Bioremediat Biodegrad 10: 462.
Copyright: © 2019 Abdelgalil SA, et al. This is an open-a ccess article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Usage
- Total views: 3880
- [From(publication date): 0-2019 - Dec 04, 2025]
- Breakdown by view type
- HTML page views: 2913
- PDF downloads: 967