Response of Îñ-Synuclein Expression to Amyloid Îò40 and Amyloid Îò42 administration into Rat Brain
Received: 08-Sep-2017 / Accepted Date: 18-Sep-2017 / Published Date: 25-Sep-2017 DOI: 10.4172/2161-0460.1000376
Abstract
Objective: In the present study, we assessed the change in α-synuclein expression upon stereotaxic administration of pre-aggregated amyloid (Aβ40 and Aβ42) in the hippocampus and amygdala of the rat brain.
Method: Forty-eight, male Wistar rats, 6 months of age at the beginning of the experiment, were divided into 8 groups, containing 6 animals in each group. Group 1: saline injected into the hippocampus (Aβ40 Hippocampus Control). Group 2: aggregated-Aβ40injected into the hippocampus (Aβ40 Hippocampus Test). Group 3: saline injected into the amygdala (Aβ40 Amygdala Control). Group 4: aggregated-Aβ40 injected into the amygdala (Aβ40 Amygdala Test). Group 5: 16.7%DMSO in distilled water injected into the hippocampus (Aβ42 Hippocampus Control). Group 6: aggregated-Aβ42 oligomer was injected into the hippocampus (Aβ42 Hippocampus Test). Group 7: 16.7%DMSO in distilled water injected into the amygdala (Aβ42 Amygdala Control). Group 8: aggregated-Aβ42 administered into the amygdala (Aβ42Amygdala Test). Animals of each group were sacrificed by cervical dislocation (n=6) and transcardial perfusion (n=4) for molecular experiments (real-time PCR and western blotting) and histological studies, respectively. Brains were micro-dissected into amygdala, hippocampus, cortex, cerebellum, medulla, and midbrain for the molecular experiments.
Results: We found that there was an increase in the expression of α-synuclein, both at the gene and protein levels, in the hippocampus and cortex of the amyloid injected animals. Aβ42 seemed to produce a quantitatively greater effect than Aβ40.
Conclusion: Therefore, it can be extrapolated that increased expression of amyloid precursor protein (APP) gene can lead to the increase in expression of α-synuclein, leading to greater neurotoxicity in neurodegenerative disorders caused by APP overexpression (such as AD).
Keywords: Alpha-synuclein; Aβ40; Aβ42; Synucleinopathy; Stereotaxy; Rat; Neurotoxicity
Introduction
Alpha-synuclein (α-synuclein) was initially shown to be associated with Alzheimer’s disease (AD) as the non-amyloid component of plaques (NACP) [1] and amyloid beta (Aβ) proteins are known to be implicated in the pathogenesis of AD. It was also found to be present in presynaptic terminals underlying the senile plaques of AD [2]. It also appears to be a constituent of filamentous inclusions of multiple system atrophy [3]. In pathological conditions (neurodegenerative conditions), it is the altered form of wild type α-synuclein that is present. In familial Parkinson’s disease (PD), mutant α-synuclein results from a missense mutation of α-synuclein gene [4]. In sporadic PD, post-translationally modified wild type α-synuclein is present and gene mutations in sporadic PD have not been found. An important point to consider will be how altered α-synuclein is formed in sporadic (idiopathic) conditions.
Physiologically now it appears that a-synuclein is normally expressed in the central nervous system neurons and is localized to the presynaptic terminals, where it is involved in the regulation of synaptic vesicle formation [5] and neurotransmitter release [6]. It has also been implicated in the process of synaptic plasticity during learning [7]. Recently, it has been shown that synucleins can dilate the fusion pore formed during exocytosis, promoting release of certain neurotransmitters [8]. Mutations in α-synuclein may trigger oxidative stress and synaptic damage in neurodegenerative diseases, collectively known as synucleinopathies [7,9-12].
Studies in a cell free system and in a transgenic mouse model expressing β-amyloid (Aβ) peptides and α-synuclein have indicated that Aβ peptides promote aggregation of α-synuclein and its intraneuronal accumulation [13]. In the brain of patients with Parkinson’s disease and Lewy body disease with dementia, Aβ deposition was found to significantly exacerbate α-synuclein abnormalities [14]. Studies in cultured cortical and hippocampal neurons also showed that enhanced Aβ deposition aggravated the α-synuclein abnormalities and lesions [15]. It was further suggested that a direct interaction between Aβ deposition and α-synuclein could increase the toxicity of α-synuclein [15]. There has, however, been little study on whether in in vivo conditions Aβ deposition elevates α-synuclein expression and abnormalities.
In the present study therefore, to investigate the impact of in vivo deposition of Aβ peptides on α-synuclein, we studies the effect of Aβ40 and Aβ42stereotaxically injected into the amygdala and hippocampus on α-synuclein expression and toxicity in the rat brain.
Materials and Methods
Reagents
Aβ42 and Aβ40 were procured in lyophilized powder form, from Sigma Aldrich Chemical Company USA. TaqMan® RNA-to-Ct™ 1-Step Kit was obtained from Thermofisher Scientific (USA). Agarose, acrylamide, bis-acrylamide, PVDF membrane and all other chemicals for western blot were obtained from Sigma Aldrich. Luminata was procured from Thermofisher Scientific (USA). Bovine serum albumin (BSA) was purchased from the Sigma Chemical Co., USA. All other chemicals were obtained from Merck and Hi-media. All chemicals used were of analytical grade. All the antibodies were procured from Abcam.
Amyloid beta aggregation
Aβ42 aggregation was performed as per the protocol of Soto et al. [16]. The lyophilized powder was dissolved in 16.7% dimethylsulfoxide (DMSO) to prepare a 2 nM Aβ42 solution. The Aβ42 was incubated for 48 h at 37°C (without shaking) to induce aggregation. Aβ40 was aggregated into β-sheets following the protocol of Zheng et al. [17]. The lyophilized powder was dissolved in 0.9% saline to a concentration of 5 mg/ml and incubated at 37°C (without shaking) for 1 week to induce aggregation.
Animals
Forty-eight, male Wistar rats, 6 months of age at the beginning of the experiment were taken for this study. Rats were housed in pairs, in standard laboratory cages 8 × 12 × 5-in3 made of polypropylene with stainless-steel covers, and maintained at 23 ± 4°C, under a 12 h light/12 h dark cycle. All experimental protocols were approved by the Committee for the Purpose of Control and Supervision of Experimental Animals (CPCSEA) and the Institutional Animal Ethical Committee (IAEC) of Jawaharlal Nehru University, New Delhi. Each animal was provided ad libitum access to food and water. The health status of each rat was checked by observing various criteria such as: tail sores, posture hunch, grooming, nose red rim, red eye rims, tumors, and teeth [18]. After surgery rats were housed individually and continuously monitored for their health status.
The animals were divided into 8 groups, containing 6 animals in each group (n=6). Group 1: consisted of animals in which saline was injected into the hippocampus (Aβ40 Hippocampus Control; AB40HC). This is the control group for group 2 animals. Group 2: consisted of animals in which Aβ40 oligomer was injected into the hippocampus (Aβ40 Hippocampus Test; AB40HT). Group 3: consisted of animals in which saline was injected into the amygdala (Aβ40 Amygdala Control; AB40AC). This is the control group for group 4 animals. Group 4: consisted of animals in which Aβ40 oligomer was injected into the amygdala (Aβ40 Amygdala Test; AB40AT). Group 5: consisted of animals in which 16.7% DMSO in distilled water was injected into the hippocampus (Aβ42 Hippocampus Control; AB42HC). This is the control group for group 6 animals. Group 6: consisted of animals in which Aβ42 oligomer was injected into the hippocampus (Aβ42 Hippocampus Test; AB42HT). Group 7: consisted of animals in which 16.7% DMSO in distilled water was injected in the amygdala (Aβ42 Amygdala Control; AB42AC). This is the control group for group 8 animals. Group 8: consisted of animals in which Aβ42 oligomer was administered in the amygdala (Aβ42Amygdala Test; AB42AT).
Surgery procedures (Stereotaxic administration of Aβ)
Animals were anesthetized with isoflurane (0.25%) and placed in a stereotaxic apparatus (Surgivet, ISOTEC 4) for administration of Aβ oligomers. The head was shaved, a surgical incision was made in the scalp and the underlying muscles and tissues were cleared to expose the skull surface. A burr hole 0.5 mm in diameter was drilled into the skull bone at the stereotaxically marked site. Stereotaxic co-ordinates for the hippocampus were: -3.0 mm posterior to bregma, -2.0 mm lateral and -3.3 mm ventral to the skull surface; and for amygdala were: -3.0 mm posterior to bregma, -4.6 mm lateral and -8.8 mm ventral to the skull surface. The stereotaxic co-ordinates were calculated using the Paxinos and Watson’s rat brain atlas [19]. Two μl Aβ40 or 3 μl Aβ42 of the before mentioned concentration was delivered bilaterally for the test subjects and corresponding volumes of their respective vehicles were delivered in the control subjects using a cannula and Hamilton syringe. After injection, the burr hole was sealed with bone wax, the incision was stitched up and the animal was kept under observation till recovery.
Animal sacrificing
Animals of each group were sacrificed by cervical dislocation (n=6) for molecular experiments (real-time PCR and western blotting); and transcardial perfusion (n=4) for histological studies (immunohistochemistry and fluorescence studies).
RNA isolation
Brains were quickly taken out and micro-dissected into amygdala, hippocampus, cortex, cerebellum, medulla, and midbrain, according to the stereotaxic atlas of Paxinos and Watson [19].The tissue was thoroughly washed with saline to remove blood and stored at -80°C. Total RNA isolation was performed by the TRIZOL method of RNA isolation. TRIZOL is a monophasic solution of phenol and guanidinium isothiocyanate, which deprotonizes RNA. Briefly, brain tissues were homogenized with TRIZOL reagent (1:1 weight by volume ration) in a polytron homogenizer. Thereafter, chloroform was added for phase separation, (much like extraction with a mixture of phenol, chloroform and isoamyl alcohol). The protein was extracted to the organic phase, DNA resolved at the interface and RNA remained in the aqueous phase. Denatured RNA (2 μg/sample) was separated on 1.5% agarose gel to check for RNA integrity [20].
Real-time PCR (RT-PCR)
The total RNA from the tissue was extracted and was converted to cDNA. The quantitation of this cDNA was monitored as the amplification was proceeding, i.e., in real time, with the help of fluorescent dye, and not at the end of the reaction like in the conventional PCR. Quantitative RT-PCR was performed for α-synuclein RNA using TaqMan® RNA-to- Ct™ 1-Step Kit, with gene specific primers (Table 1). Real Time PCR was performed on Applied Biosystems 7500. Cycling condition of 48°C for 15 min, 95°C for 10 min, 95°C for 15 min (for primer annealing and elongation) for 40 cycles and 60°C for 1 min for 40 cycles, per the manufactures protocol. Real Time data were normalized with GAPDH.
| Primer | Sequence (5’-3’) |
|---|---|
| Alpha-synuclein Forward primer | TGCTGTGGATATTGTTGTGG |
| Alpha-synuclein Reverse primer | AGGTGCGTAGTCTCATGCTC |
| GAPDH Forward primer | GTGGACCTCATGGCCTACAT |
| GAPDH Reverse primer | TGTGAGGGAGATGCTCAGTG |
Table 1: Sequence of primers used for real time-polymerase chain reaction.
Preparation of tissue homogenates
Animals of each group were sacrificed by cervical dislocation after the behavioral experiments. Brains were quickly taken out and micro-dissected into cerebellum, medulla, midbrain, hippocampus and cortex, according to the stereotaxic atlas of Paxinos and Watson [19]. The tissue was thoroughly washed with saline to remove blood and stored at -80°C. Tissue samples were homogenized in 50 mM Tris (pH 7.4) with a Potter-Elevehijam type homogenizer fitted with Teflon plunger. The homogenate was diluted 1:10 (with Tris, pH 7.4, buffer) and centrifuged at 6000 rpm for 5 min in a refrigerated centrifuge (Sorvall RCS or RC5C). The resulting pellet (P1), consisting of nuclear and cellular material, was discarded. The supernatant (S1), containing mitochondria, synaptosomes, microsomes and cytosol, was further ultracentrifuged at 25,000 rpm for 25 min to form mitochondrial pellet (P2). The resulting supernatant (S2) was used as such as cytosolic fraction. All tests were performed using the cytosolic fraction. Protein estimation was performed by Bradford’s method [21] using BSA as standard.
Western blotting
SDS-PAGE followed by Western blotting is an analytical protein detection technique. The SDS denatures the protein, which are separated by the number of polypeptides on a PAGE gel. These separated proteins can then be transferred onto a membrane and the desired protein can be detected and quantitated by specific anti-bodies. Bio-Rad PAGE apparatus was used to separate the denatured proteins. Briefly, proteins (10-30 μg) from the tissue homogenate was separated on 15% SDSPAGE, transferred to PVDF membrane (Whatman, Sigma-Aldrich) for 1 h at 4°C. Overnight incubation with primary antibodies used for detection (anti-α-synuclein and anti-β-actin) was performed at 4°C. This was followed by incubation with secondary antibody for 1 h at room temperature and detected using enhanced chemiluminescence kit (Thermo Scientific, USA). Blocking (skimmed milk) and buffer washing were performed after ever antibody step.
Histology
Tissue processing
All the animals (test and control) were anesthetized with ketamine (50 mg/kg i.p.). Each rat was transcardially perfused with physiological saline solution and then fixed with a fixative containing 4% paraformaldehyde in 0.1 M phosphate buffer (pH 7.4). Perfusion was performed by infusing the saline solution into the left ventricle of the animal’s heart, as the left ventricle channels blood to the systemic circulation through the aorta. Simultaneously, an incision was made in the right atrium, which receives blood from the entire body via the inferior and superior vena cava, to wash out all the blood and thereafter, the perfusate from the system. For the rats, 200 ml of saline solution (approximately four times the total blood volume) was used over a period of 6-8 minutes to wash out all the blood. Thereafter, 200-300 ml of the fixative was perfused for 15 min. Post perfusion, the brain was removed and fixed in 10% formalin solution for 7 days. Following formalin-fixation, the brain was put in gradients of sucrose (10% to 30%) for sucrose embedding. Sucrose is a cryopreserving and thus protects the tissue integrity upon cryosectioning. Cryosectioning was performed using Leica (CM 1860 UV) cryotome; sections 15 μm in thickness were cut and mounted on gelatin coated slides. The sections were stored (mounted on gelatin slides) at -20°C till further processing/ staining.
Immunohistochemistry (IHC)
α-Synuclein IHC was performed to show its localization in the cell, and show the regions of the neuron in which there was increased expression of this protein. Cryosections were used for immunohistochemical localization of α-synuclein in neurons. Briefly, sections were taken out from -20°C and left at room temperature (37°C) for 1 h, followed by denaturation in 1% triton-100, which is a mild detergent. Thereafter, to inhibit any endogenous peroxidase activity, the sections were incubated in the dark in 1% hydrogen peroxide (H2O2). The sections were next incubated in 10% normal goat serum (NGS) at room temperature for 90 min to prevent non-specific binding. The sections were then incubated in primary antibody (1:1000 for anti-α- synuclein), overnight at 4°C in the humid chamber. The slides were again normalized to room temperature for 1 hour after incubation with primary antibody, before incubation with biotinylated secondary antibody (IgG 1:100) at room temperature for 2 h. The slides were next incubated in streptavidin-peroxidase (1:100) for 2 h at room temperature. A 10-15-min incubation was then given in DAB (0.25% in 1% H2O2) or till the sections turn brownish in color. The slides were then washed in running tap water, followed by two washes in dH2O. After ever step, the slides were washed thrice with PBS. Finally, the slides were dehydrated by ascending grades of alcohol, cleared with xylene and mounted with DPX.
Fluorescence imaging
Fluorescence studies on α-synuclein were performed to show its localization in the cell, and show the regions of the neuron in which there is increase in the expression of this protein. The cryo-sections were visualized with the 561 laser for TRITC (561nm wavelength). The slides were viewed under the Nikon clips Ti confocal microscope. The images were analyzed using NIS-Elements 4.0. Briefly, sections were taken out from -20°C and left at room temperature (37°C) for 1 h, followed by denaturation in 1% triton-100, which is a mild detergent. Thereafter, the sections were next incubated in 10% normal goat serum (NGS) at room temperature for 90 min to prevent non-specific binding. The sections were then incubated in primary antibody (1:1000 diluted anti-α-synuclein), in humid chamber for 1 hour at room temperature (37°C). The slides were next incubated with TRITC-conjugated secondary antibody (IgG 1:100) at room temperature for 1 hour in humid chamber. After every step, the slides were washed thrice with PBS. Finally, the slides were mounted with fluoromount (with DAPi) and stored in the dark at 4°C until visualization.
Statistical Analysis
Data were expressed as mean ± standard deviation (S.D.). Statistical comparison was performed by Student’s t-test. All tests were performed using Sigma Plot software version 11.0.
Results
α-Synuclein gene expression
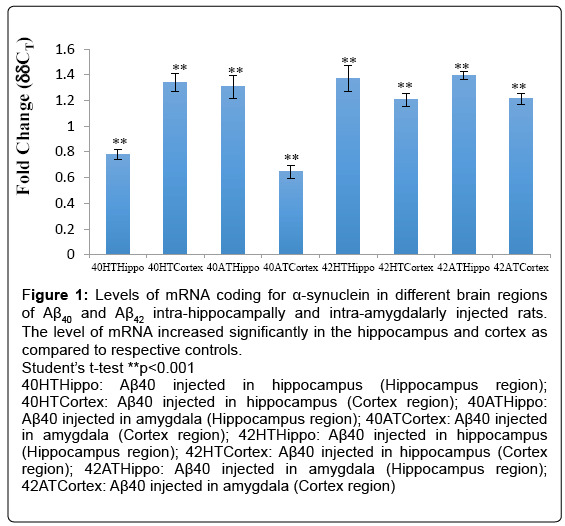
The level of α-synuclein was found altered, i.e., increased only in the hippocampus and the cortex and in the rest of the regions the levels in the test animals were found to be similar to those in the respective controls (Figure 1). In the cortex and hippocampus thus there was an upregulation of the expression of α-synuclein gene as compared to their respective controls.
Figure 1: Levels of mRNA coding for α-synuclein in different brain regions of Aβ40 and Aβ42 intra-hippocampally and intra-amygdalarly injected rats. The level of mRNA increased significantly in the hippocampus and cortex as compared to respective controls.
Student’s t-test **p<0.001
40HTHippo: Aβ40 injected in hippocampus (Hippocampus region); 40HTCortex: Aβ40 injected in hippocampus (Cortex region); 40ATHippo: Aβ40 injected in amygdala (Hippocampus region); 40ATCortex: Aβ40 injected in amygdala (Cortex region); 42HTHippo: Aβ40 injected in hippocampus (Hippocampus region); 42HTCortex: Aβ40 injected in hippocampus (Cortex region); 42ATHippo: Aβ40 injected in amygdala (Hippocampus region); 42ATCortex: Aβ40 injected in amygdala (Cortex region)
The upregulation of the α-synuclein gene expression in the hippocampal region as estimated by the ddCT values was found to be 0.78-fold in Aβ40 intra-hippocampally-injected rats (**p<0.001), 1.31- fold in Aβ40 intra-amygdalarly-injected rats (**p<0.001), 1.37-fold in Aβ42 intra-hippocampally-injected rats (**p<0.001) and 1.40-fold in Aβ42 intra-amygdalarly-injected rats (**p<0.001) (Figure 1).
The upregulation in the cortex as estimated by the ddCT values was found to be 1.34-fold in Aβ40 intra-hippocampally-injected rats, 0.65- fold in Aβ40 intra-amygdalarly-injected rats, 1.21-fold in Aβ42 intra-hippocampally-injected rats, and 1.21-fold in Aβ42 intra-amygdalarlyinjected rats (Figure 1).
α-Synuclein protein expression
The α-synuclein protein levels were increased in both the intrahippocampally and intra-amygdalarly Aβ40 and Aβ42 injected animals as compared to their respective controls by western blot analysis, immunohistochemistry and fluorescence studies.
Western blotting
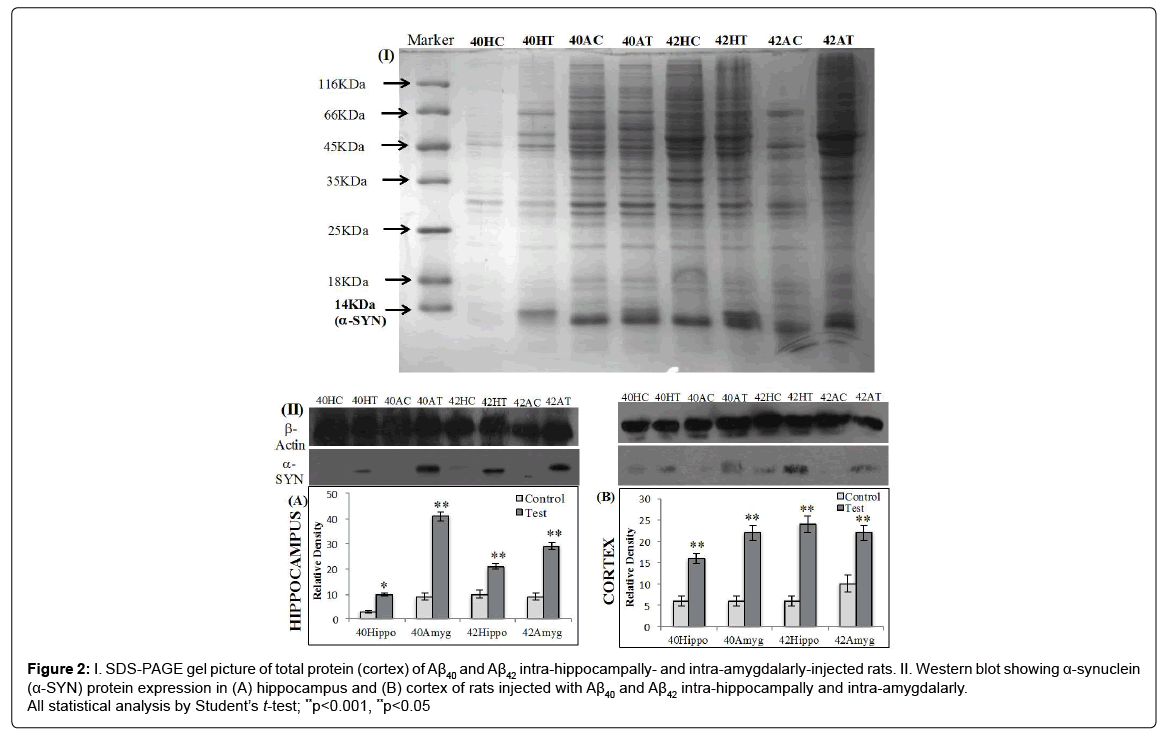
The upregulation of α-synuclein protein as estimated by western blot analysis in the hippocampus was found to be 3.3-fold in Aβ40 intra-hippocampally-injected rats, 4.5-fold in Aβ40 intra-amygdalarlyinjected rats, 2.1-fold in Aβ42 intra-hippocampally-injected rats and 3.2-fold in Aβ42 intraamygdalarly-injected rats (Figure 2A).
Figure 2: I. SDS-PAGE gel picture of total protein (cortex) of Aβ40 and Aβ42 intra-hippocampally- and intra-amygdalarly-injected rats. II. Western blot showing α-synuclein (α-SYN) protein expression in (A) hippocampus and (B) cortex of rats injected with Aβ40 and Aβ42 intra-hippocampally and intra-amygdalarly. All statistical analysis by Student’s t-test; **p<0.001, **p<0.05
The upregulation of α-synuclein protein as estimated by western blot analysis in the cortex was found to be 4.6-fold in Aβ40 intrahippocampally- injected rats, 3.6-fold in Aβ40 intra-amygdalarlyinjected rats, 2.2-fold in Aβ42 intra-hippocampally-injected rats, and 4-fold in Aβ42 intra-amygdalarly-injected rats (Figure 2B).
Immunohistochemistry
Furthermore, IHC and fluorescence studies were also performed to localize the presence of α-synuclein in the neurons.
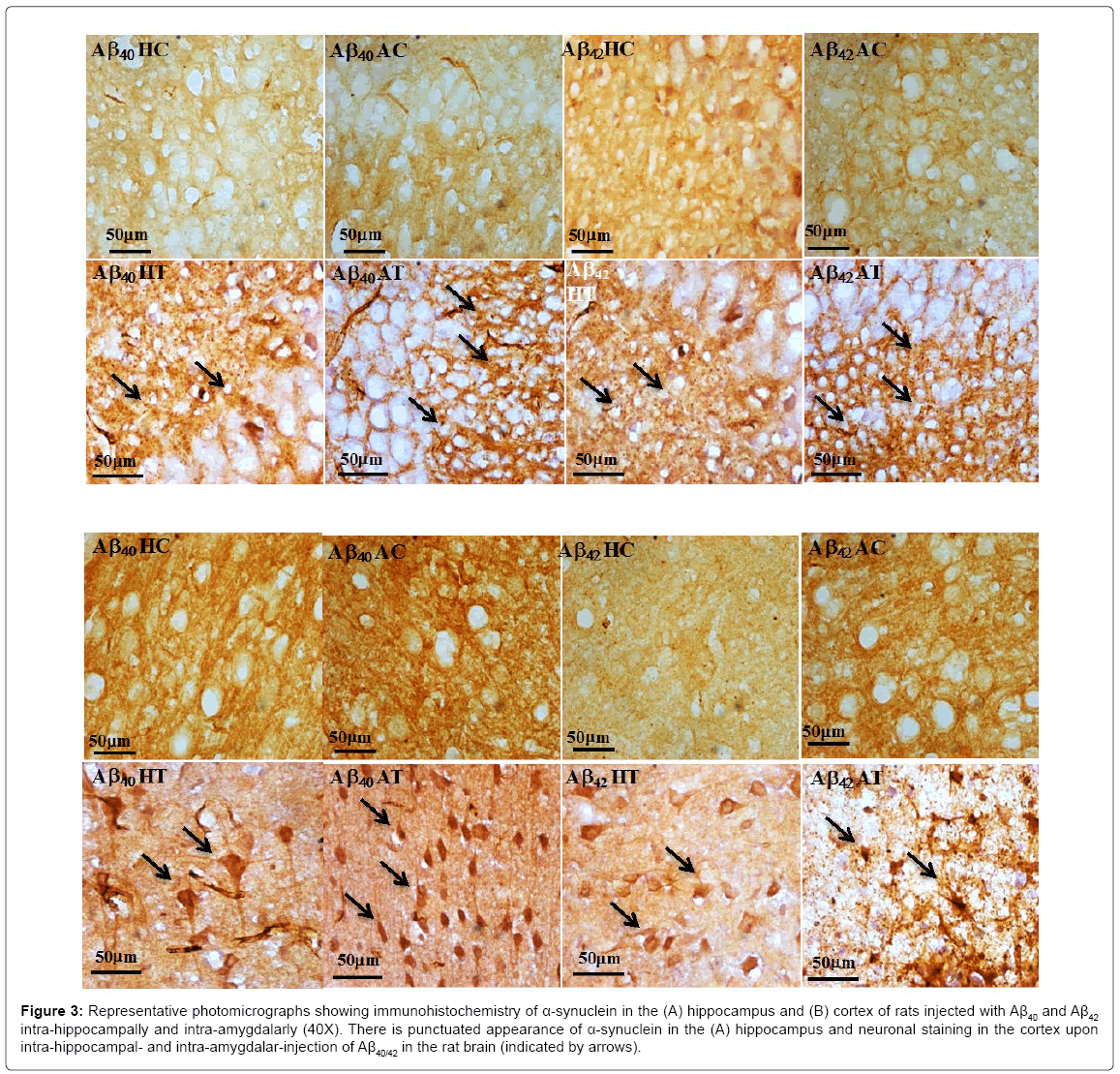
In the cortical neurons of the amyloid-injected animals, α-synuclein was densely localized in the perikaryal area of the neuron. In the hippocampal neurons, α-synuclein protein was found localized more towards the periphery of the perikaryal membrane, hinting towards its putative function in vesicle transport. The amyloid-injected rats, both intra-hippocampally and intra-amygdalarly, showed increases in the α-synuclein protein expression as compared to their respective controls (Figure 3).
Figure 3: Representative photomicrographs showing immunohistochemistry of α-synuclein in the (A) hippocampus and (B) cortex of rats injected with Aβ40 and Aβ42 intra-hippocampally and intra-amygdalarly (40X). There is punctuated appearance of α-synuclein in the (A) hippocampus and neuronal staining in the cortex upon intra-hippocampal- and intra-amygdalar-injection of Aβ40/42 in the rat brain (indicated by arrows).
Fluorescence studies
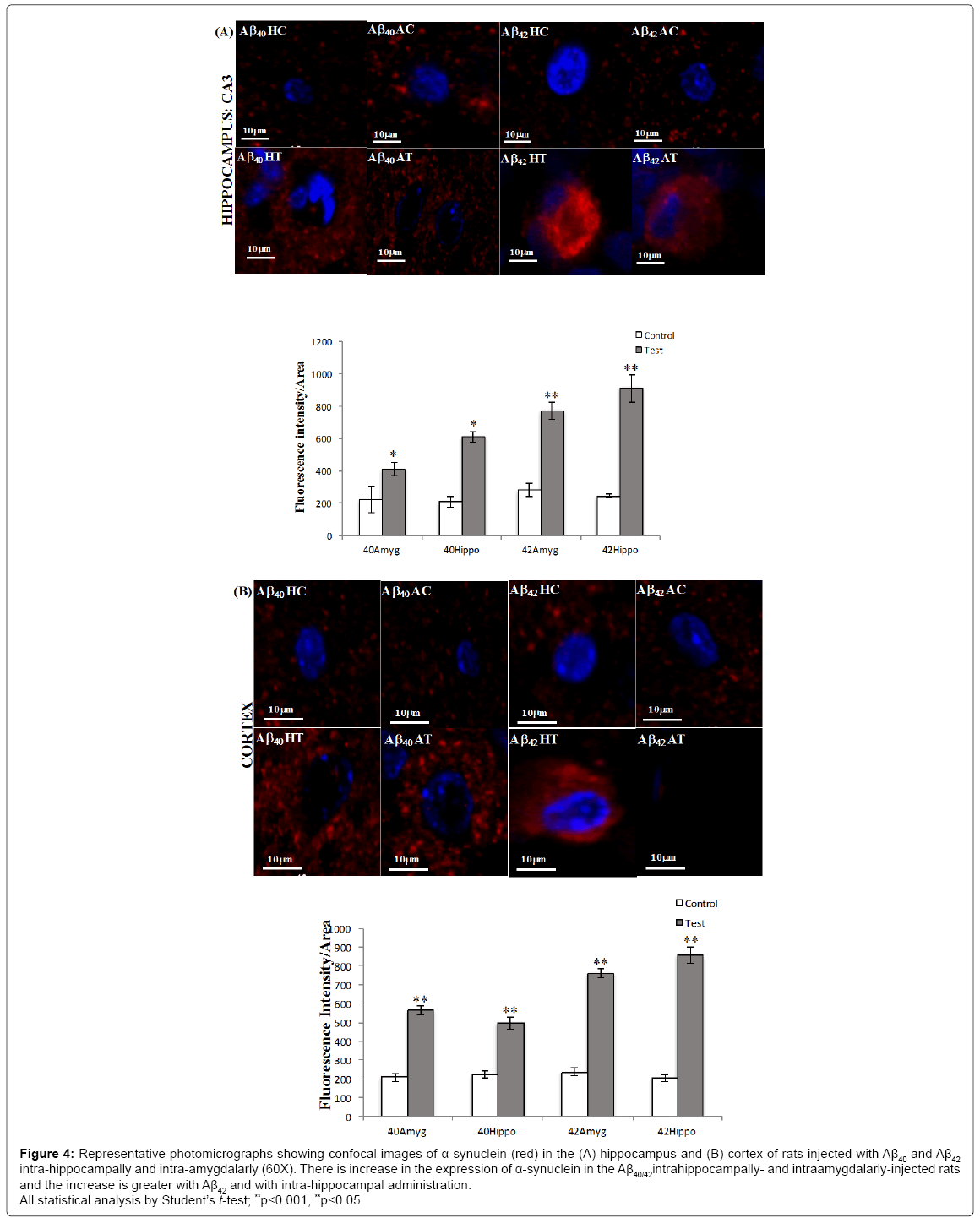
The upregulation of α-synuclein protein as estimated by fluorescence intensity quantification in the hippocampus was found to be 1.4- fold in Aβ40 intrahippocampally-injected rats, 1.7-fold in Aβ40 intraamygdalarly- injected rats, 1.5-fold in Aβ42 intrahippocampally-injected rats and 1.6-fold in Aβ42 intra-amygdalarly-injected rats (Figure 4A).
Figure 4: Representative photomicrographs showing confocal images of α-synuclein (red) in the (A) hippocampus and (B) cortex of rats injected with Aβ40 and Aβ42 intra-hippocampally and intra-amygdalarly (60X). There is increase in the expression of α-synuclein in the Aβ40/42intrahippocampally- and intraamygdalarly-injected rats and the increase is greater with Aβ42 and with intra-hippocampal administration.
All statistical analysis by Student’s t-test; **p<0.001, **p<0.05
The upregulation of α-synuclein protein as estimated by fluorescence intensity quantification in the cortex was found to be 1.8-fold in Aβ40 intra-hippocampally-injected rats, 2.4-fold in Aβ40 intra-amygdalarlyinjected rats, 2.3-fold in Aβ42 intra-hippocampally-injected rats, and 2.3-fold in Aβ42 intra-amygdalarly-injected rats (Figure 4B).
Thus, the results indicate that there was Aβ-induced increase in the expression of α-synuclein, both at the gene level and at the protein level in the hippocampus and cortex. Both, intra-hippocampally- and intraamygdalarly- injected Aβ40 and Aβ42 showed unregulated α-synuclein in the neurons. IHC and fluorescence studies demonstrated that the increase in protein expression was confined to the periphery of the perikaryal area of the neurons, in the cortex and hippocampus of the rat brain.
Discussion
The results obtained demonstrate that there are Aβ40/42-induced increases in the expression of α-synuclein at the gene level as well as the protein level in the hippocampus and the cortex of the rat.
These results are similar to those obtained by a previous study by Chen et al. [22], who showed that aged rats exposed to bacterial amyloid protein (Curli) had enhanced α-synuclein aggregation in the brain.
In amygdala, despite Aβ-injection, α-synuclein did not increase in the region. Though not many studies have investigated the α-synuclein levels in the amygdala, Flores-Cuadrado et al. [23] showed that α-synuclein positive cells are present mostly in the basolateral and cortical amygdala in the A53T transgenic mice, which overexpress α-synuclein. They also showed that there were no increases in the number of α-synuclein positive neurons in the amygdala over 43 weeks between the transgenics and the controls. There was however, a significant decrease in the α-synuclein positive neurons after 43 weeks in the A53T mice, as compared to the controls. Thus, mice overexpressing α-synuclein did not show any increase in the expression of α-synuclein in the amygdala, rather a significant decrease after prolonged period in the α-synuclein positive neurons.
There have however been reports of increased α-synuclein gene and protein expression in the amygdala upon abstinence from alcohol [24]. Downregulation of α-synuclein mRNA level together with increased α-synuclein protein level was reported upon morphine-withdrawal [25] in mice. Thus, α-synuclein in the amygdala could be responsible for coping with alcohol and drug withdrawal. One plausible manner in which α-synuclein could provide protection to neurons in conditions of abstinence could be via suppression of dopamine neurotransmission during withdrawal or by mediating some unknown adaptations.
Furthermore, in intra-amygdalarly-injected animals, there is increase in α-synuclein gene and protein expression in the hippocampus and cortex. The results obtained support previous findings by Masliah et al. [13], who showed that increase in cerebral human (hSYN) in hSYN/ hAPP transgenic mice at the protein level. Moreover, they showed that Aβ also promoted accumulation of hSYN into neurons leading to 1.6- fold higher hSYN inclusions in the neocortical neurons in hSYN/hAPP mice as compared to age matched hSYN mice. They also suggested that Aβmay promote hSYN fibrillization in vivo. However, as transgenic mice were used for the study, it could not be ascertained whether Aβ was involved in producing the observed effects or some other hAPP product. In the in vitro studies Aβ42 was administered extracellularly was able to affect synuclein intracellularly, but the in vivo data was still lacking. Study performed in vitro in adult rat neurons by Majd et al. [26] also showed the increase α-synuclein protein levels in the presence of Aβ42.
Thus, although there have been previous studies demonstrating an increase in synuclein expression at the gene and the protein level, the confirmation of the said phenomenon after in vivo administration of Aβ was lacking. Also, region specific changes in synuclein expression were also required.
Therefore, to build upon the results obtained from the mRNA expression studies, the estimation of the protein in the specific regions was performed by western blot analysis. In the present study, there was an increase in the expression of α-synuclein protein in the hippocampus and cortex of the rat brain. The estimation of protein levels and localization of α-synuclein was performed by immunohistochemistry and fluorescence/confocal studies. In the IHC and fluorescent studies also, an increase in the α-synuclein protein was observed in the hippocampus and cortex of the intra-hippocampally and intraamygdalarly Aβ40- and Aβ42-injected rat.
Another argument to explain the increased in expression of α-synuclein can be its role in protection of neurons from amyloidtoxicant-induced degeneration. α-Synuclein has been proposed to be neuroprotective in the physiological conditions. Recently, a study had shown that intracellular injections of α-synuclein in APP transgenic mice suppressed Aβ deposition and reduced plaque formation [27]. Manning-Bog et al. [28] had shown that over expression of α-synuclein in mice leads to the protection of dopaminergic neurons from paraquat-induced neurodegeneration. Furthermore, Masliah et al. [29] and Matsuoka et al. [30] have shown that overexpression of α-synuclein in transgenic mice may not induce neuronal damage; nor exacerbate MPTP-induced neurodegeneration [31].
There are also in vitro findings that support the possibility of α-synuclein playing a protective role. α-Synuclein transfected neuronal cell lines have been shown to be resistant to H2O2-induced oxidative stress as well as to apoptotic stimuli [32,33]. α-Synuclein null mice derived primary mesencephalic cultures are more susceptible to rotenone-induced dopaminergic cell death [34].
There is also suggestion of a positive relationship between α-synuclein expression and neuronal plasticity and recovery, from observations made during brain development [7,35] and models of developmental target injury [36,37]. Albeit, there is degeneration of neurons as a consequence of amyloid-injection into the rat brain, the increase in α-synuclein expression can be explained in a different light. Rather than being an expression of a degenerative process, the results obtained may be representative of the expression of a synaptic plasticity response. Thus, the increase in α-synuclein expression could be to combat the excessive oxidative stress, afford protection to the degenerating neurons, and also generation of synaptic plasticity response.
The present results indicate that, the increase in expression of α-synuclein is greater with Aβ42-as compared to Aβ40-injection in the rat brain, demonstrating greater neurotoxic potential of Aβ42.
α-Synuclein is predominantly a pre-synaptic protein. Thus, it is important to be cognizant of the fact that the mRNA level measured in one brain region may give rise to the protein in another brain region, depending on the termination point of the axons. Reciprocally, the protein levels estimated in a particular region might be due to neuronal projections from outside the region under investigation and not from the neurons within the specific region.
The increase in expression of α-synuclein is greater in the cortex as compared to the hippocampus. This could be explained by the fact that the cortex is more richly supplied with neuronal projections as compared to the hippocampus. Thus, there would be greater influx of α-synuclein protein in the cortex as compared to the hippocampus. Also, the pyramidal neurons in the CA3 region of the hippocampus do not innervate any other brain regions except the hippocampus. These neurons exclusively go to primarily CA1 pyramidal cells and CA3 pyramidal cells on the same and adjacent hippocampal levels [38].
One question that arises from the present observations is why an increase in mRNA and protein expression levels of α-synuclein occurred only in the hippocampus and cortex. This will require further investigation. Nonetheless, from the literature it can be seen that α-synuclein mRNA levels are increased in the cerebral cortex, hippocampus and amygdala during the developmental stages in the rat brain [39]. In 2005, Adamczyk et al. [40] also demonstrated marked increases in the α-synuclein mRNA levels in the cortex, hippocampus and additionally the striatum of rats. Additionally, the protein levels were more pronounced in these regions as compared to the other structures in their study. Bate et al. [15] demonstrated α-synuclein associated decreases in synaptophysin in cultured hippocampal and cortical neurons. Liang et al. [41] showed there is an increase in the expression of α-synuclein mRNA in the hippocampus of alcohol preferring rats. Thus, it appears that regionally-selective changes occur in α-synuclein expression.
One reason could be that α-synuclein has been implicated to be involved in dopamine transport via vesicle formation [42]. The cortex and hippocampus are areas with a very large number of dopaminergic and cholinergicsynapses from various different brain structures. Whereas, regions like cerebellum, where there is a paucity of dopaminergic neurons and enrichment of noradrenergic neurons, have marked decrease in the α-synuclein mRNA and protein levels [40]. Thus, it is plausible that α-synuclein plays a role in regulation of dopamine and hence an increase in α-synuclein both at the mRNA and protein level is more prominent in the regions that are rich in dopaminergic connections.
The other prominent question is how does Aβ modulate the α-synuclein expression. The answers could be many-fold. Masliah et al. [13] had proposed that cellular uptake of Aβ42 by cultured neurons leads to the disruption of their lysosomal membrane [43,44]. Synuclein is also known to associate with planer lipid bilayers resulting in extensive bilayer disruption [45]. Synergistic action of both synuclein and Aβ42 plausibly causes endosomal-lysosomal membranes to leak, allowing direct interaction between Aβ42 and synuclein in the cytosol. They also proposed that Aβ could exert oxidative stress, which could cause lead to synuclein cross linkage and subsequent Lewy-body formation [46,47].
Thus, Aβ exacerbates the expression of α-synuclein probably though increased oxidative stress and depletion of dopamine. The increase in the expression may also be a neuroprotective response of α-synuclein.
Conclusion
In conclusion, data from the present study showed that both Aβ40 and Aβ42 (injected intra-hippocampally or intra-amygdalarly) into the rat brain, upregulate the expression of α-synuclein gene and protein in the rat brain. The α-synuclein upregulation is however restricted only to the hippocampus and the cortex. Aβ42 seemed to produce a quantitatively greater effect than Aβ40.
Acknowledgement
The author Neha Mishra is thankful to the University Grants Commission for providing financial assistance through Junior Research Fellowship and Senior Research Fellowship.
References
- Ueda K, Fukushima H, Masliah E, Xia Y, Iwai A, et al. (1993) Molecular cloning of cDNA encoding an unrecognized component of amyloid in Alzheimer disease. Proc Natl Acad Sci U S A 90: 11282-11286.
- Iwai A (2002) Properties of NACP/a-synuclein and its role in Alzheimer’s disease. Biochimica et Biophysica Acta 1502: 95-109.
- Spillantini MG, Crowther RA, Jakes R, Cairns NJ, Lantos PL, et al. (1998) Filamentous alpha-synuclein inclusions link multiple system atrophy with Parkinson's disease and dementia with Lewy bodies. Neurosci Lett 251: 205-208.
- Hope AD, Myhre R, Kachergus J, Lincoln S, Bisceglio G, et al. (2004) Alpha-synuclein missense and multiplication mutations in autosomal dominant Parkinson's disease. Â Neurosci Lett 367: 97-100.
- Lotharius J, Brundin P (2002) Pathogenesis of Parkinson's disease: Dopamine, vesicles and alpha-synuclein. Nat Rev Neurosci 3: 932-942.
- Cheng F, Vivacqua G, Yu S (2011) The role of α-synuclein in neurotransmission and synaptic plasticity. J Chem Neuroanat 42: 242-248.
- Clayton DF, George JM (1998) The synucleins: A family of proteins involved in synaptic function, plasticity, neurodegeneration and disease. Trends Neurosci 21: 249-254.
- Selkoe DJ (2017) Showing transmitters the door: Synucleins accelerate vesicle release. Nat Neurosci 20: 629-631.
- Di Rosa G, Puzzo D, Sant'Angelo A, Trinchese F, Arancio O (2003) Alpha-synuclein: Between synaptic function and dysfunction. Histol Histopathol 18: 1257-1266.
- Li W, Lesuisse C, Xu Y, Troncoso JC, Price DL, et al. (2004) Stabilization of alpha-synuclein protein with aging and familial Parkinson’s disease-linked A53T mutation. J Neurosci 24: 7400-7409.
- Miller DW, Hague SM, Clarimon J, Baptista M, Gwinn-Hardy K, et al. (2004) Alpha-synuclein in blood and brain from familial Parkinson disease with SNCA locus triplication. Neurology 62: 1835-1838.
- Masliah E, Rockenstein E, Veinbergs I, Sagara Y, Mallory M, et al. (2001) Beta-amyloid peptides enhance alpha-synuclein accumulation and neuronal deficits in a transgenic mouse model linking Alzheimer's disease and Parkinson's disease. Proc Natl Acad Sci U S A 98: 12245-12250.
- Pletnikova O, West N, Lee MK, Rudow GL, Skolasky RL, et al. (2005) Abeta deposition is associated with enhanced cortical alpha-synuclein lesions in Lewy body diseases. Neurobiol Aging 26: 1183-1192.
- Bate C, Gentleman S, Williams A (2010) α-synuclein induced synapse damage is enhanced by amyloid-β1-42. Mol Neurodegener 5: 55.
- Soto C, Sigurdsson EM, Morelli L, Kumar AR, Castaño EM, et al. (1998) ß-sheet breaker peptides inhibit fibrillogenesis in a rat brain model of amyloidosis: Implications for Alzheimer's therapy. Nat Med 4: 822-826.
- Zheng MQ, Yin DZ, Zhang L, Lei B, Cheng DF, et al. (2008) Biological characters of [18F]O-FEt-PIB in a rat model of Alzheimer’s disease using micro-PET imaging. Acta Pharmacol Sin 29: 548-554.
- Sharma D, Maurya AK, Singh R (1993) Age-related decline in multiple unit action potentials of ca3 region of rat hippocampus: Correlation with lipid peroxidation and lipofuscin concentration and the effect of centrophenoxine. Neurobiol Aging 14: 319-330.
- Paxinos G, Watson C (2010) The rat brain in stereotaxic coordinates. (6th Edtn), Academic Press/Elsevier, Amsterdam, Boston.
- Sandhir R, Onyszchuk G, Berman NE (2008) Exacerbated glial response in the aged mouse hippocampus following controlled cortical impact injury. Exp Neurol 2: 372-380.
- Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72: 248-254.
- Chen SG, Stribinskis V, Rane MJ, Demuth DR, Gozal E, et al. (2016) Exposure to the functional bacterial amyloid protein curli enhances alpha-synuclein aggregation in aged Fischer 344 Rats and Caenorhabditis elegans. Sci Rep 6: 34477.
- Flores-Cuadrado A, Ubeda-Bañon I, Saiz-Sanchez D, de la Rosa-Prieto C, Martinez-Marcos A (2015) α-synuclein staging in the amygdala of a Parkinson's disease model: Cell types involved. Eur J Neurosci 41: 137-146.
- Ziolkowska B, Gieryk A, Wawrzczak-Bargiela A, Krowka T, Kaminska D (2008) Alpha-Synuclein expression in the brain and blood during abstinence from chronic alcohol drinking in mice. Neuropharmacology 54: 1239-1246.
- Ziolkowska B, Gieryk A, Bilecki W, Wawrzczak-Bargiela A, Wedzony K, et al. (2005) Regulation of alpha-synuclein expression in limbic and motor brain regions of morphine-treated mice. J Neurosci 25: 4996-5003.
- Majd S, Chegini F, Chataway T, Zhou XF, Gai W (2013) Reciprocal induction between a-synuclein and ß-amyloid in adult rat neurons. Neurotox Res 23: 69-78.
- Bachhuber T, Katzmarski N, McCarter JF, Loreth D, Tahirovic S, et al. (2015) Inhibition of amyloid-β plaque formation by α-synuclein. Nat Med 21: 802-807.
- Manning-Bog AB, McCormack AL, Purisai MG, Bolin LM, Di Monte DA (2003) Alpha-synuclein overexpression protects against paraquat-induced neurodegeneration. J Neurosci 23: 3095-3099.
- Masliah E, Rockenstein E, Veinbergs I, Mallory M, Hashimoto M, et al. (2000) Dopaminergic loss and inclusion body formation in alpha-synuclein mice: Implications for neurodegenerative disorders. Science 287: 1265-1269.
- Matsuoka Y, Vila M, Lincoln S, McCormack A, Picciano M, et al. (2001) Lack of nigral pathology in trans-genic mice expressing human alpha-synuclein driven by the tyrosine hydroxylase promoter. Neurobiol Dis 8: 535-539.
- Rathke-Hartlieb S, Kahle PJ, Neumann M, Ozmen L, Haid S, et al. (2001) Sensitivity to MPTP is not increased in Parkinson's disease-associated mutant alpha-synuclein transgenic mice. J Neurochem 77: 1181-1184.
- da Costa CA, Ancolio K, Checler F (2000) Wild-type but not Parkinson's disease-related ala-53 --> Thr mutant alpha-synuclein protects neuronal cells from apoptotic stimuli. J Biol Chem 275: 24065-24069.
- Hashimoto M, Hsu LJ, Rockenstein E, Takenouchi T, Mallory M, et al. (2002) Alpha-synuclein protects against oxidative stress via inactivation of the c-Jun N-terminal kinase stress-signaling pathway in neuronal cells. J Biol Chem 277: 11465-11472.
- Dauer W, Kholodilov N, Vila M, Trillat AC, Goodchild R, et al. (2002) Resistance of alpha -synuclein null mice to the Parkinsonian neurotoxin MPTP. Proc Natl Acad Sci U S A 99: 14524-14529.
- Hsu LJ, Mallory M, Xia Y, Veinbergs I, Hashimoto M, et al. (1998) Expression pattern of synucleins (non-Abeta component of Alzheimer's disease amyloid precursor protein/alpha-synuclein) during murine brain development. J Neurochem 71: 338-344.
- Kholodilov NG, Neystat M, Oo TF, Lo SE, Larsen KE, et al. (1999) Increased expression of rat synuclein in the substantia nigra pars compacta identified by mRNA differential display in a model of developmental target injury. J Neurochem 73: 2586-2599.
- Kholodilov NG, Oo TF, Burke RE (1999) Synuclein expression is decreased in rat substantia nigra following induction of apoptosis by intrastriatal 6-hydroxydopamine. Neurosci Lett 275:105-108.
- Amaral DG, Witter MP (1995) Hippocampal formation. In The Rat Nervous System (3rd Edtn), Paxinos Academic, New York.
- Petersen K, Olesen OF, Mikkelsen JD (1999) Developmental expression of alpha-synuclein in rat hippocampus and cerebral cortex. Neuroscience 91: 651-659.
- Adamczyk A, Solecka J, Strosznajder JB (2005) Expression of alpha-synuclein in different brain parts of adult and aged rats. J Physiol Pharmacol 56: 29-37.
- Liang T, Spence J, Liu L, Strother WN, Chang HW, et al. (2003) Alpha-Synuclein maps to a quantitative trait locus for alcohol preference and is differentially expressed in alcohol-preferring and non-preferring rats. Proc Natl Acad Sci U S A 100: 4690-4695.
- Lee FJ, Liu F, Pristupa ZB, Niznik HB (2001) Direct binding and functional coupling of alpha-synuclein to the dopamine transporters accelerate dopamine-induced apoptosis. FASEB J 15: 916-926.
- Yang AJ, Chandswangbhuvana D, Margol L, Glabe CG (1998) Loss of endosomal/lysosomal membrane impermeability is an early event in amyloid Abeta1-42 pathogenesis. J Neurosci Res 52: 691-698.
- Yang AJ, Chandswangbhuvana D, Shu T, Henschen A, Glabe CG (1999) Intracellular accumulation of insoluble, newly synthesized abetan-42 in amyloid precursor protein-transfected cells that have been treated with Abeta1-42. J Biol Chem 275: 20650-20656.
- Jo E, McLaurin J, Yip CM, St George-Hyslop P, Fraser PE (2000) Alpha-Synuclein membrane interactions and lipid specificity. J Biol Chem 275: 34328-34334.
- Giasson B, Duda JE, Murray IV, Chen Q, Souza JM, et al. (2000) Oxidative damage linked to neurodegeneration by selective alpha-synuclein nitration in synucleinopathy lesions. Science 290: 985-989.
- Hashimoto M, Takeda A, Hsu LJ, Takenouchi T, Masliah E (1999) Role of cytochrome c as a stimulator of alpha-synuclein aggregation in Lewy body disease. J Biol Chem 274: 28849-28852.
Citation: Mishra N, Kumar P, Singh R, Sharma D (2017) Response of α-Synuclein Expression to Amyloid Β40 and Amyloid β42 administration into Rat Brain. J Alzheimers Dis Parkinsonism 7: 376. DOI: 10.4172/2161-0460.1000376
Copyright: © 2017 Mishra N, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 5566
- [From(publication date): 0-2017 - Jan 30, 2025]
- Breakdown by view type
- HTML page views: 4844
- PDF downloads: 722