Research Article Open Access
Response of Rattus norvegicus to Bitumen Leachate Toxicity
Ayandiran TA1*, Fawole OO1, Dahunsi SO2* and Ogundiran MA11Department of Pure and Applied Biology, Ladoke Akintola University of Technology, Ogbomoso, Nigeria
2Biological Sciences Department, Landmark University, Omu-Aran, Nigeria
- Corresponding Author:
- Dahunsi SO
Biological Sciences Department
Landmark University, Omu-Aran, Nigeria
Tel: +2347032511675
E-mail: dahunsi.olatunde@lmu.edu.ng
Received date: June 13, 2017; Accepted date: June 30, 2017; Published date: July 07, 2017
Citation: Ayandiran TA, Fawole OO, Dahunsi SO, Ogundiran MA (2017) Response of Rattus norvegicus to Bitumen Leachate Toxicity. Biochem Physiol 6:221. doi:10.4172/2168-9652.1000221
Copyright: © 2017 Ayandiran TA, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Biochemistry & Physiology: Open Access
Abstract
This study investigated the response of Rattus norvegicus to bitumen leachate to evaluate its toxicity in a terrestrial animal model following previous aquatic studies on the environmental impacts of Nigerian bitumen exploration. Adult rats were administered different concentrations (20 to 100%) of bitumen leachate for 30 days before analyses. Fourteen blood plasma clinical–chemical parameters (BCCPs), seven hematological parameters as well as histological changes in organs of exposed animals were studied. The analyses showed that all values for the BCCPs and the hematological parameters are significantly different (P<0.05) from the control values. Concentrations of liver enzymes, Alanine aminotransferase (ALAT), Gamma glutamyltransferase (GGT) and Alkaline phosphatase (ALKP) increased with increasing concentrations of bitumen leachate but were not dose-dependent. In the same vein, counts for Packed cell volume (PCV), White blood cell (WBC), Red blood cell (RBC) and hemoglobin (Hb) all decreased with increasing concentration of toxicants but was not so for differential counts (Neutrophils, Lymphocytes and Eosinophils). Results of histological study revealed several changes ranging from mild to severe lesions in organs of exposed rats. The very pronounced changes include irregularly arranged cardiac muscle fibres (Heart), pronounced inflammation (Spleen), hyperchronic nuclei and degenerated flattened squamous epithelial cells lining the Bowman’s capsule (Kidney), pronounced reduction of Graffian follicle (Ovary) and cellular hypertrophy with severe congestion of the central vein (Liver). Based on these results, important organ functions could be negatively affected by continuous exposure to bitumen leachate which reflects health effects having an overall impact on both animal and human populations.
Keywords
Biochemistry; Bitumen; Environment; Hematology; Histology; Rat
Introduction
Toxic/harmful substances may be introduced deliberately or accidentally into the environment, impairing its quality and making it unsuitable for life forms. When the concentration of toxicants exceeds the homeostasis of the organisms, it can lead to death or organ damage [1,2]. Few of the well-known pollutants are herbicides, pesticides, industrial compounds/wastes, etc. [3]. Persistent organic pollutants (POPs) have been widely reported to induce environmental stress due to their inefficient biochemical and transport properties [4,5]. This makes them to be retained within the body of organisms where they biomagnify in food webs especially found in top dwellers. Such induced stress could be the precursor of various health defects, such as neuroendocrine disruption, immune suppression and tissue/organ disruption in animals [4-8].
Blood plasma clinical–chemical parameters (BCCPs) are known to be qualitative biochemical indicators of health disorders such as organ dysfunctions, bone diseases, metabolic/hormonal imbalances, etc. [9]. Series of factors are known to influence BCCPs including infectious diseases, genetic aberrations, starvation, dehydration and pollution [10-12] and they are therefore being used as biomarkers for pollution studies involving different animal models [10-14]. It has equally been enormously reported that biological markers like hematological and biochemical indices are useful tools for monitoring environmental quality and the health conditions of organisms. In fact, previous researches [15-22] have reported that Hematological and Biochemical indices such as hemoglobin (Hb), hematocrit (Ht), red blood cell (RBC) and white blood cell (WBC) counts, glucose, protein, and enzymes activities are currently used as indicators of the general wellbeing and early signals of stress in organisms.
Blood often shows pathological changes before the morphological symptoms usually seen in animal which are exposed to toxicants [23]. Over the decades, many researches have documented changes in blood indices caused by exposures to environmental pollutants or other unfavorable conditions [24-29]. In the same vein, the activities of serum/ plasma enzymes have severally been used as sensitive markers of stress in animals exposed to different pollutants and these are commonly used as pointers of tissue and cell disruption [20,21].
Bitumen was first spotted in Nigeria in 1910 after which two bitumen observatory wells were dug in the Ondo State in the 60s during the early explorative activity of Nigerian natural bitumen. Currently, a large deposit of natural bitumen occurs in the so called bitumen belt of South-western Nigeria. The seepage of the bitumen material exists especially during the dry season when temperature is above 37°C during when it occurs as a free flowing liquid into the environment (aquatic and terrestrial) and serving as the major environmental pollutants in the communities neighboring the bitumen exploration. Previous researches [30,31] have identified bitumen as the major pollutant/toxicant to aquatic life forms in the neighborhoods hence, the need to validate its toxicity level in terrestrial animal in order to evaluate its possible public health effects on humans.
Materials and Methods
All chemicals, reagents and solvents used in the present study were analytical or high-pressure liquid chromatography (HPLC) Grade. Bitumen stock used was obtained from the bitumen observatory well in Agbabu, Ondo State, Nigeria where the bitumen flows out continuously thereby polluting the environment.
Experimental animal
Adult male and female Rattus norvegicus var. albinus, weighing 200-250 g with specific pathogen-free certified status were procured from the animal center, Physiology Department (Faculty of Basic Medical Sciences), Ladoke Akintola University of Technology, Nigeria. Rats were maintained on a 12 h light/dark cycle and at a temperature of 23 ± 2°C with unlimited access to food and water and these conditions persist throughout the experimental period. All experimental animals were given humane treatment according to the “Principles of Laboratory Animal Care” formulated by the National Society for Medical Research and the “Guide for the Care and Use of Laboratory Animals” by the National Academy of Sciences, USA and experiments were carried out in accordance to Ladoke Akintola University of Technology Ethical Committee Acts.
Experimental design
Rats were randomly divided into six groups: A=Control group (Distilled water only); B=20% bitumen leachate treated group; C=40% bitumen leachate treated group; D=60% bitumen leachate treated group; E= 0% bitumen leachate treated group and F=100% bitumen leachate treated group. The toxicants (each bitumen leachate group) were previously prepared by dilution of the bitumen stock with distilled water in appropriate proportions and with serious agitation (for dissolution of soluble portions) for a period of 4 weeks before commencement of administration. Each stock solution was then refrigerated under hygienic condition prior to administration. Each experimental group consisted of six rats (3 each of male and female). 100 mg/kg/day of each bitumen leachate stock composition were administered orally once daily in each group for 30 days. All experimental animals were sacrificed 24 h after the final administration and blood samples were obtained from the femoral arteries by intracardiac puncture.
Plasma biochemical parameters evaluation
The biochemical analyses were carried out using the methods of Sonne et al. [10-12,32]. Indices analyzed include the following: Albumin (Alb, gL-1); Glucose (Glu, mmolL-1); Total protein (TP, gL-1); Alkaline phosphatase (ALKP, UL-1); Alanine aminotransferase (ALAT, UL-1); Gamma glutamyltransferase (GGT; UL-1); Total bilirubin (TB, mmolL-1); Total cholesterol (TC, mmolL-1); Bile acids (BA, mmolL-1); Amylase (Amy, UL-1); Urea (Urea, mmolL-1); Calcium (Ca, mmolL-1); Sodium (Na, mmolL-1) and Potassium (K, mmolL-1). All analyses were routinely conducted in the laboratory using an automated spectrophotometric analyzer also containing ion-selective electrodes (ADVIA1800, Siemens) and assays were subjected to daily internal and quarterly external quality control.
Evaluation of hematological parameters
Hematological values were measured following standard methods [33,34]. Packed cell volume (PCV) was evaluated using hematocrit method and hemoglobin (Hb) concentration using (cyanmethaemoglobin method) was analyzed within two hours after collection. Red blood cells (RBC) and White blood cells (WBC) were counted by Neubauer’s improved hematocytometer using Hyem’s and Turk’s solution as a diluting fluid respectively. Differential white cell and thrombocyte counts were done on blood films stained with Giemsa. For every 1,200 erythrocytes counted at random, the number of thrombocytes and the different types of leucocytes was determined on each blood smear and a mean relative percent was calculated. The absolute value was then obtained and was then multiplied by the WBC+thrombocytes from the hemocytometer. Thrombocyte numbers were subtracted from the WBC+thrombocytes count to obtain a total WBC [26]. Replicate counts were made for each blood sample.
Histological analyses
At the end of the toxicant administration, organs (liver, kidney, spleen and heart from both animal sexes and ovaries from female animals) were obtained. Each organ was dissected out, trimmed of excess fat and then fixed in 10% buffered formalin and was processed for paraffin sectioning by dehydration in different concentrations of alcohol, cleared with xylol and embedded in paraffin blocks. Sections of about 5 m thickness were stained with Harris hematoxylin and eosin (H&E) for histological study following the methods of Delafield [35] and Bancroft and Gamble [36].
Statistical analysis
All the results were subjected to analysis of variance (ANOVA). Duncan multiple range test was further used to evaluate the mean differences at 0.05 significant levels.
Results
Blood plasma clinical–chemical parameters (BCCPs)
Fourteen (14) blood plasma clinical–chemical parameters were evaluated in all treatment groups of rats exposed to bitumen leachate plus the control (Table 1). These comprised of three liver enzymes (ALAT, ALKP and GGT), one digestive enzyme (amylase), two protein groups (albumin and total protein), two liver/erythrocyte metabolism products (bile acid and bilirubin), cholesterol, a carbohydrates (glucose), urea (muscle and protein metabolism) and three electrolytes/ minerals (calcium, sodium and potassium). Values obtained from each treatment groups significantly different from the control. In all, values for albumin, total bilirubin, potassium and glucose followed the same pattern of increase with increasing concentration of toxicant while amylase recorded a low value in the 100% bitumen leachate treated group. With regard to ALAT, ALKP, GGT, total protein, bile acids, cholesterol, urea, sodium and calcium, significantly low values were obtained in some of the treatment groups compared to the others.
| Parameter | Control | 20% | 40% | 60% | 80% | 100% | Clinical implications [32] |
|---|---|---|---|---|---|---|---|
| Alanine aminotransferase (ALAT; UL-1) | 59 ± | 65 ± | 64 ± | 73 ± | 100 ± | 115 ± | Increase during liver disease |
| 3.2 | 4.10* | 5.30* | 5.31* | 10.20* | 12.11* | ||
| Gamma glutamyltransferase (GGT; UL-1) | 0.5 ± | 0.6 ± | 0.5 ± | 1.2 ± | 1.4 ± | 1.5 ± | Increase during liver disease |
| 0.1 | 0.01* | 0.01 | 0.01* | 0.11* | 0.20* | ||
| Alkaline phosphatase (ALKP;UL-1) | 120 ± | 117 ± | 131 ± | 140 ± | 156 ± | 148 ± | Increase during liver and bone diseases |
| 10.21 | 9.10* | 12.00* | 12.00* | 14.10* | 13.20* | ||
| Total protein | 7.6 ± | 7.8 ± | 7.9 ± | 7.7 ± | 8.6 ± | 9.1 ± | Increase during liver disease |
| 0.4 | 1.00* | 1.00* | 0.20* | 0.20* | 1.30* | ||
| Amylase | 309 ± | 407 ± | 421 ± | 500 ± | 540 ± | 530 ± | Increase during liver pancreatitis |
| 17.1 | 21.30* | 18.00* | 30.12* | 31.30* | 25.10* | ||
| Albumin | 4.6 ± | 4.7 ± | 4.9 ± | 4.9 ± | 5.6 ± | 8.2 ± | Increase during liver disease |
| 0.2 | 0.21* | 0.10* | 0.10* | 0.40* | 1.00* | and dehydration | |
| Total cholesterol | 77 ± | 86 ± | 82 ± | 92 ± | 93 ± | 89 ± | Increase during liver disease |
| 4.1 | 5.30* | 7.20* | 10.01* | 9.10* | 5.21* | ||
| Urea | 40 ± | 41 ± | 47 ± | 64 ± | 57 ± | 64 ± | Increase during dehydration |
| 2.3 | 2.30* | 3.30* | 5.30* | 6.10* | 5.00* | and kidney disease | |
| Total bilirubin | 17 ± | 19 ± | 22 ± | 23 ± | 23 ± | 25 ± | Increase during liver disease |
| 1.4 | 1.11* | 2.10* | 3.00* | 3.00* | 2.20* | ||
| Bile acids | 89 ± | 93 ± | 100 | 95 ± | 103 ± | 97 ± | Increase during liver disease |
| 5.3 | 5.10* | ± 10.10* | 8.30* | 10.10* | 4.22* | ||
| Sodium | 111 ± | 116 ± | 117 | 123 ± | 120 ± | 124 ± | Increase during dehydration |
| 7.22 | 8.60* | ± 11.20* | 13.10* | 9.11* | 6.10* | ||
| Potassium | 6.7 ± | 9.1 ± | 10.2 | 12.3 ± | 12.8 ± | 13.0 ± | Increase during renal disease |
| 1.5 | 1.21* | ± 1.31* | 1.80* | 1.41* | 1.20* | ||
| Calcium | 6.2 ± | 6.3 ± | 6.8 | 8.1 ± | 7.7 ± | 7.6 ± | Elevated levels largely linked to albumin levels and bone disease |
| 0.2 | 0.20* | ± 0.40* | 0.30* | 0.50* | 0.60* | ||
| Glucose | 143.8 ± | 124 ± | 101 ± | 82.6 ± | 36 ± | 27 | Increase during stress, digestion |
| 11.2 | 10.20* | 10.11* | 6.10* | 3.30* | ± 2.50* | and diabetes mellitus |
Table 1: Statistical summary of 14 blood plasma clinical–biochemical parameters of Rattus norvegicus exposed to bitumen leachate.
Hematological parameters
Also, seven (7) different hematological parameters were measured in all the treatment groups plus the control (Table 2). Indices evaluated include four blood parameters (PCV, WBC, RBC and Hb counts) and three differential blood counts (Eosinophil, Neutrophils and Lymphocytes). All values obtained for the treated groups are significantly different from the control. Values obtained for PCV, Hb and Eosinophil count are all concentration dependent, i.e., values increase with increase in toxicity while values for WBC, RBC, Neutrophils and Lymphocytes counts were significantly low in some of the treated groups.
| Parameter | Control | 20% | 40% | 60% | 80% | 100% |
|---|---|---|---|---|---|---|
| PCV (%) | 75 ± 5.22 | 74 ± 4.20* | 63 ± 3.02* | 58 ± 2.12* | 49 ± 2.04* | 46 ± 2.02* |
| WBC (mm3 of blood) | 5000 ± 10.02 | 7800 ± 9.21* | 13600 ± 10.22* | 11000 ± 12.02* | 17600 ± 10.01* | 24000 ± 13.22* |
| RBC (millions/ml) | 6550000 ± 15.20 | 6200000 ± 20.12* | 5050000 ± 19.02* | 5300000 ± 31.02* | 4950000 ± 17.40* | 4500000 ± 23.02* |
| Hb (g/dl) | 23 ± 0.02 | 23 ± 1.03* | 22.6 ± 1.01* | 17.7 ± 1.20* | 16.1 ± 1.22* | 14.8 ± 1.20* |
| Neutrophils (%) | 08 ± 1.01 | 16 ± 1.02* | 18 ± 1.02* | 28 ± 1.01* | 42 ± 3.02* | 41 ± 2.03* |
| Lymphocytes (%) | 54 ± 2.12 | 58 ± 3.32* | 63 ± 2.02* | 84 ± 3.02* | 77 ± 4.02* | 82 ± 4.02* |
| Eosinophils (%) | 01 ± 0.01 | 01 ± 0.01 | 02 ± 0.01* | 04 ± 0.02* | 03 ± 0.02* | 03 ± 0.02* |
Each value is an average of three (3) measurement; **statistically significant
Table 2: Statistical summary of 7 haematological parameters of Rattus norvegicus exposed to bitumen leachate.
Histological analysis
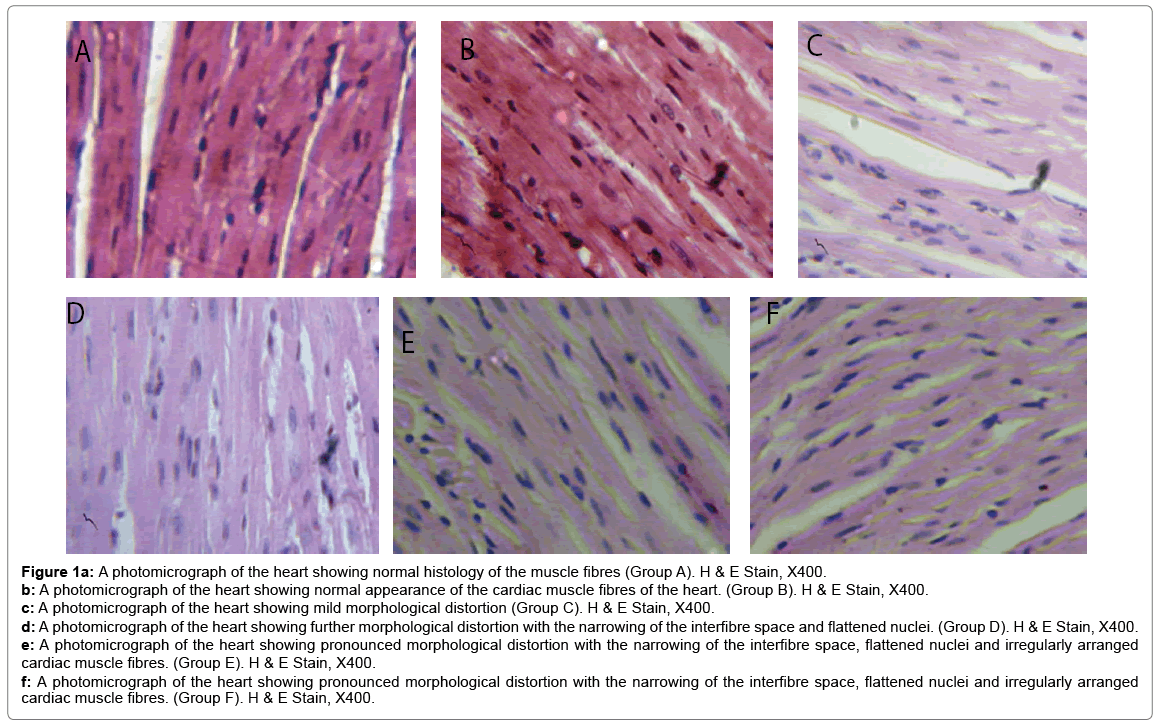
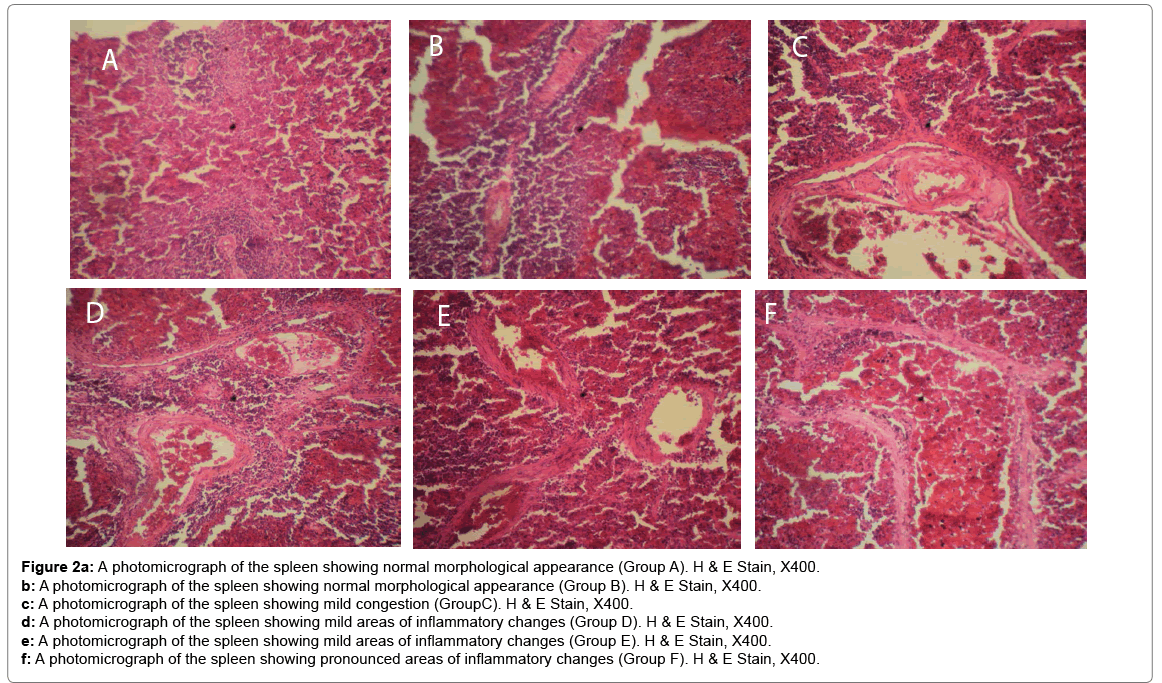
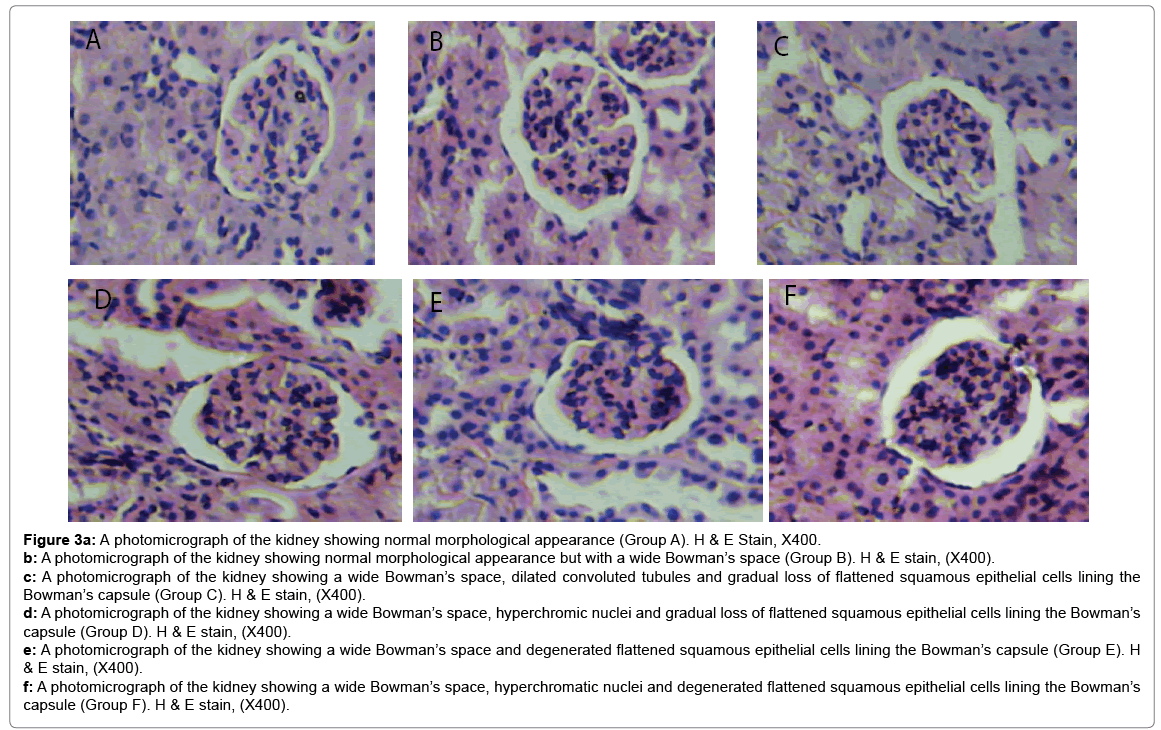
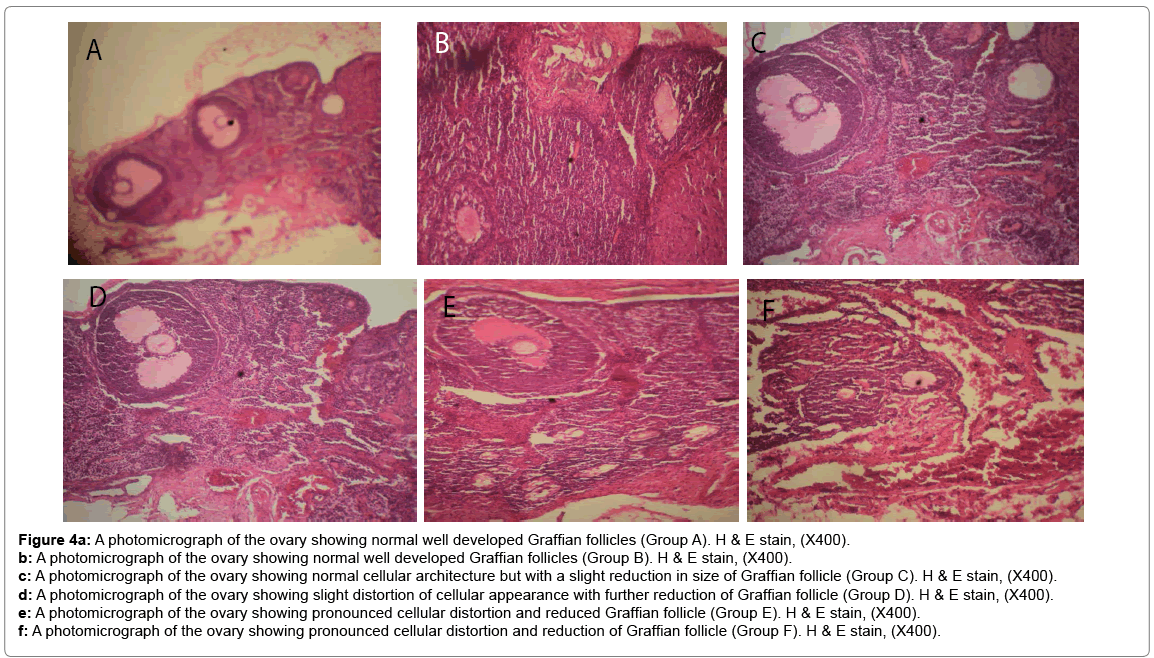
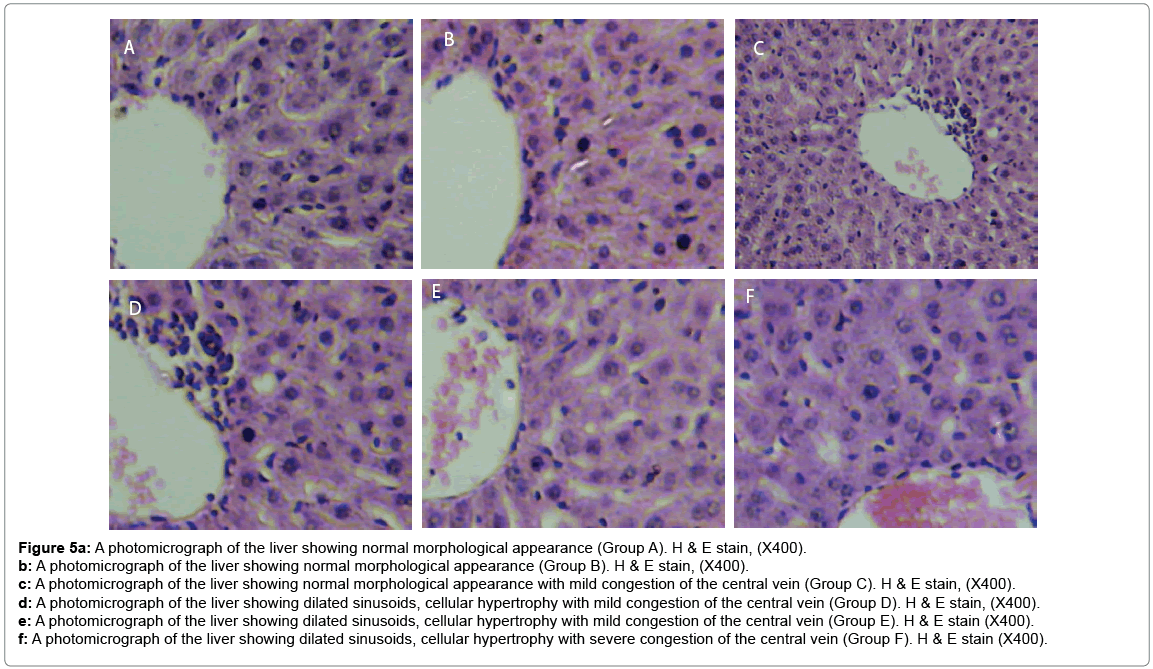
Histological changes in tissues of different organs (heart, spleen, kidney, ovary and liver) from all treated groups and the control are incident and rarely severe as shown in Figures 1 to 5. All histological findings were normal in the organs from control group. Histology of the heart showed normal muscle fibres in the control group, while changes seen in the treated groups are mild and pronounced morphological distortion, narrowing of the interfibre space and flattened nuclei and irregularly arranged cardiac muscle fibres. Histology of the spleen in all treated animal groups reveal mild congestion and pronounced inflammatory changes. In the kidney, histological changes seen after the exposure to different concentrations of bitumen leachate includes wide Bowman’s space, dilated convoluted tubules and gradual loss of flattened squamous epithelial cells lining the Bowman’s capsule, hyperchronic nuclei and degenerated flattened squamous epithelial cells lining the Bowman’s capsule. In the ovary, histological changes in the treated groups includes slight reduction in size of Graffian follicle, slight distortion of cellular appearance with further reduction of Graffian follicle, pronounced cellular distortion and reduced Graffian follicles. In the liver tissues, the histological changes seen are mild congestion of the central vein, dilated sinusoids, cellular hypertrophy and severe congestion of the central vein.
Figure 1a: A photomicrograph of the heart showing normal histology of the muscle fibres (Group A). H & E Stain, X400.
b: A photomicrograph of the heart showing normal appearance of the cardiac muscle fibres of the heart. (Group B). H & E Stain, X400.
c: A photomicrograph of the heart showing mild morphological distortion (Group C). H & E Stain, X400.
d: A photomicrograph of the heart showing further morphological distortion with the narrowing of the interfibre space and flattened nuclei. (Group D). H & E Stain, X400.
e: A photomicrograph of the heart showing pronounced morphological distortion with the narrowing of the interfibre space, flattened nuclei and irregularly arranged cardiac muscle fibres. (Group E). H & E Stain, X400.
f: A photomicrograph of the heart showing pronounced morphological distortion with the narrowing of the interfibre space, flattened nuclei and irregularly arranged cardiac muscle fibres. (Group F). H & E Stain, X400.
Figure 2a: A photomicrograph of the spleen showing normal morphological appearance (Group A). H & E Stain, X400.
b: A photomicrograph of the spleen showing normal morphological appearance (Group B). H & E Stain, X400.
c: A photomicrograph of the spleen showing mild congestion (GroupC). H & E Stain, X400.
d: A photomicrograph of the spleen showing mild areas of inflammatory changes (Group D). H & E Stain, X400.
e: A photomicrograph of the spleen showing mild areas of inflammatory changes (Group E). H & E Stain, X400.
f: A photomicrograph of the spleen showing pronounced areas of inflammatory changes (Group F). H & E Stain, X400.
Figure 3a: A photomicrograph of the kidney showing normal morphological appearance (Group A). H & E Stain, X400.
b: A photomicrograph of the kidney showing normal morphological appearance but with a wide Bowman’s space (Group B). H & E stain, (X400).
c: A photomicrograph of the kidney showing a wide Bowman’s space, dilated convoluted tubules and gradual loss of flattened squamous epithelial cells lining the Bowman’s capsule (Group C). H & E stain, (X400).
d: A photomicrograph of the kidney showing a wide Bowman’s space, hyperchromic nuclei and gradual loss of flattened squamous epithelial cells lining the Bowman’s capsule (Group D). H & E stain, (X400).
e: A photomicrograph of the kidney showing a wide Bowman’s space and degenerated flattened squamous epithelial cells lining the Bowman’s capsule (Group E). H & E stain, (X400).
f: A photomicrograph of the kidney showing a wide Bowman’s space, hyperchromatic nuclei and degenerated flattened squamous epithelial cells lining the Bowman’s capsule (Group F). H & E stain, (X400).
Figure 4a: A photomicrograph of the ovary showing normal well developed Graffian follicles (Group A). H & E stain, (X400).
b: A photomicrograph of the ovary showing normal well developed Graffian follicles (Group B). H & E stain, (X400).
c: A photomicrograph of the ovary showing normal cellular architecture but with a slight reduction in size of Graffian follicle (Group C). H & E stain, (X400).
d: A photomicrograph of the ovary showing slight distortion of cellular appearance with further reduction of Graffian follicle (Group D). H & E stain, (X400).
e: A photomicrograph of the ovary showing pronounced cellular distortion and reduced Graffian follicle (Group E). H & E stain, (X400).
f: A photomicrograph of the ovary showing pronounced cellular distortion and reduction of Graffian follicle (Group F). H & E stain, (X400).
Figure 5a: A photomicrograph of the liver showing normal morphological appearance (Group A). H & E stain, (X400).
b: A photomicrograph of the liver showing normal morphological appearance (Group B). H & E stain, (X400).
c: A photomicrograph of the liver showing normal morphological appearance with mild congestion of the central vein (Group C). H & E stain, (X400).
d: A photomicrograph of the liver showing dilated sinusoids, cellular hypertrophy with mild congestion of the central vein (Group D). H & E stain, (X400).
e: A photomicrograph of the liver showing dilated sinusoids, cellular hypertrophy with mild congestion of the central vein (Group E). H & E stain (X400).
f: A photomicrograph of the liver showing dilated sinusoids, cellular hypertrophy with severe congestion of the central vein (Group F). H & E stain (X400).
Discussion
Evaluation of BCCPs is a common practice in the clinical diagnosis of animal’s physiology and this gives an indication of the general health status [18,37]. The significant decrease in blood glucose during exposure can be attributed to high utilization of glucose for oxidation or hypoxic conditions leading to an excess utilization of stored carbohydrates which are the main source of energy in organisms. Alterations of enzyme activity, e.g. the transaminase enzymes in animals under exposed conditions have been documented as a strong biochemical parameter most useful in diagnosis [38,39]. The transaminase enzymes in this study were found to increase significantly as the concentration of toxicant increased and when this is found in blood plasma; it can be linked to organ dysfunction in organisms during stress condition as earlier reported by Gabriel and George [40]. Several other researchers have reported elevated levels of plasma enzymes in animals exposed to different toxicants [39,41,42]. All the fourteen BCCPs were significantly different between the various treatment groups. Such differences could be linked to the variation in toxicant concentration which they were exposed to and may not be dietary since all animals were fed the same diet all through the experimental period. Another important factor to note here is the individual physiology of animals which usually shows different response to toxicants. Most of the indices evaluated in the experimental groups recorded increase values as toxicant concentration increased, indicating that the toxicant load is a major factor in the resultant responses (damages and lesions) found in the animal. This is supported by many studies who have reported on several organochlorines inducing toxicity which was evident in the increased values for blood plasma parameters especially those of ALAT, GGT, bile acid, total bilirubin, albumin, total protein and cholesterol [14,43].
Different substances have been found to induce various toxicological responses in animals in which blood parameters followed a regular pattern of increase with concentration of toxicant or variation in location of animal samples but with significantly reduced values at some points during the exposure [9-13,44]. For the electrolytes calcium, potassium and sodium, the relationships may be dietary and/ or bitumen related, which also have a strong linkage to renal disorders or bone metabolism [9,45,46].
In this study, the decreased level of RBC, hemoglobin and PCV counts in rats treated with bitumen leachate might have resulted from hemolysis caused by this toxicant. Previous studies, [47,48] have reported similar observations in experimental animals exposed to different toxicants while other reported a decrease in the same parameters on exposure to other toxicants [49]. The observed increase in WBC during treatment may be due to stimulated lymphopoiesis and/or enhanced release of lymphocytes from lymphomyeloid tissue as a defense mechanism of the rats to withstand the toxic effect of the bitumen leachate and this agrees with the submission of Kavitha et al. [20]. The increase in leucocyte count indicates the stimulatory effect of the toxicant on immune system and also depends on the toxicant stress and this is confirmatory to the findings of Ates et al. [50].
Several histological responses were shown in organs of exposed animal specimens with the most prevalent one being pronounced distortion of cardiac muscle, degeneration of epithelial lining of kidneys Bowman capsule, congestion of blood vessels, and pronounced cellular swelling of ovarian Graffian follicle. All these could be associated to the response of organs to bitumen leachate toxicity. Previous studies [51,52] have reported similar submissions. Cellular swelling is known to occur either directly by denaturation of volume-regulating ATPases or indirectly by disruption of the cellular energy transfer pathway required for ionic regulation [51]. The present study showed that induced morphological damages are associated with increased oxidative stress and apoptosis in tissues. It has been proved that chronic exposure to toxicants inhibits cholinesterase enzyme and introduces oxidative stress damage as well as free radicals production in different organs such as cardiovascular system [53-58]. Such histological findings also indicated inducement of congestion, infiltration of inflammatory cells and multifocal necrosis in cardiac tissue and there has been evidence that oxidative stress is a major apoptotic stimulus in various diseases [59]. Several studies also revealed that apoptosis could be a possible mechanism of toxicity in low-dose exposure to some toxicants [60-62].
Conclusion
Judging by the level of blood and histological alterations found in this study, our conclusion is that the indiscriminate release of bitumen into the environment should be discouraged. The exploration activities in the bitumen belt of Nigeria are causing lots of environmental pollution with the continuous discharge of bitumen runoff into rivers and land. Though, the effects are chronic as seen here, the continuous exposure of animals (aquatic and terrestrial) to bitumen leachate could in the long run pose serious health dangers to humans who are at the peak of the food chain. Moreover, fishes from bitumen polluted rivers in Nigeria constitute good sources of food and income for the inhabiting human population and they alongside the water from the river has been implicated to be polluted [31,63].
Conflict of Interest
Authors declare that there has been no conflict of any sort throughout the period of this research till now. Both of us approves of this submission.
Highlights
1. The toxicological effect of bitumen leachate was established.
2. Biochemical analyses showed multiple abnormalities.
3. Hematological analysis also revealed multiple abnormalities.
4. Histological changes were pronounced in exposed rat.
5. Observed abberations were all concentration dependent.
References
- Dahunsi SO, Oranusi SU, Ishola RO (2012) Differential bioaccumulation of heavy metals in selected biomarkers of Clarias gariepinus exposed to chemical additives effluent. J Res Environ Sci Toxicol 1: 100-106.
- Dahunsi SO, Oranusi SU (2012) Acute toxicity of synthetic resin effluent to African catfish, Clarias gariepinus. Am J Food Nutr 2: 42-46.
- Agrawal SP, Ravi S, Bechan S (2010) Water pollution with special reference to pesticide contamination in India. J Water Res Prot2: 432-448.
- Letcher RJ, Bustnes JO, Dietz R, Jenssen BM, Jørgensen EH, et al. (2010) Exposure and effects assessment of persistent organohalogen contaminants in arctic wildlife and fish. Sci Total Environ 408: 2995-3043.
- Sonne C (2010) Health effects from long-range transported contaminants in Arctic top predators: An integrated review based on studies of polar bears and relevant model species. Environ Int 36: 461-491.
- Rocca CL, Mantovani A (2006) From environment to food: The case of PCB. Ann Ist Super Sanita 42: 410-416.
- Richards MP, Proszkowiec-Weglarz M (2007) Mechanisms regulating feed intake, energy expenditure and body weight in poultry. Poult Sci 86: 1478-1490.
- Verreault J, Gabrielsen GW, Bustnes JO (2010) The Svalbard glaucous gull as bioindicator species in the European Arctic: Insight from 35 years of contaminants research. Rev Environ Contam Toxicol 205: 77-116.
- Thrall MA, Baker DC, Campbell TW, DeNicola DB, Fettman MJ, et al. (2006) Veterinary hematology and clinical chemistry: Text and clinical case presentations set. Blackwell Publishing, Iowa, USA.
- Sonne C, Dietz R, Kirkegaard M, Letcher RJ, Shahmiri S, et al. (2008) Effects of organohalogen pollutants on haematological and urine clinical–chemical parameters in Greenland sledge dogs (Canis familiaris). Ecotoxicol Environ Saf 69: 381-390.
- Sonne C, Bustnes JO, Herzke D, Jaspers VLB, Covaci A, et al. (2010) Relationships between organohalogen contaminants and blood plasma clinical-chemical parameters in chicks of three raptor species from Northern Norway. Ecotoxicol Environ Saf 73: 7-17.
- Sonne C, Bustnes JO, Herzke D, Jaspers VLB, Covaci A, et al. (2012) Blood plasma clinical-chemical parameters as biomarker endpoints for organohalogen contaminant exposure in Norwegian raptor nestlings. Ecotoxicol Environ Saf 80: 76-83.
- Fox GA, Jeffrey DA, Williams KS, Kennedy SW, Grasman KA (2007) Health of Herring Gulls (Larus argentatus) in relation to breeding location in the early 1990s. I. biochemical measures. J Toxicol Environ Health A 70: 1443-1470.
- Kutlu S, Colakoglu N, Halifeoglu I, Sandal S, Seyran AD, et al. (2007) Comparative evaluation of hepatotoxic and nephrotoxic effects of aroclors 1221 and 1254 in female rats. Cell Biochem Funct 25: 167-172.
- Kohler HR, Sandu C, Scheil V, Nagy-Petrica EM, Segner H, et al. (2007) Monitoring pollution in River Mures, Romania, Part III: Biochemical effect markers in fish and integrative reflection. Environ Monit Assess 127: 47-54.
- Kori-Siakpere O, Ubogu EO (2008) Sublethal haematological effects of zinc on the freshwater fish, Heteroclarias sp. (Osteichthyes: Clariidae). Afr J Biotechnol 7: 2068-2073.
- Olufayo MO (2009) Hematological characteristics of Clarias gariepinus (Burchell 1822) juveniles exposed to Derris elliptica root powder. Afr J Food Agric Nutr Sci 9: 920-933.
- Osman AGM, Koutb M, Sayed Ael-D (2010) Use of hematological parameters to assess the efficiency of quince (Cydonia oblonga Miller) leaf extract in alleviation of the effect of ultraviolet-A radiation on African catfish Clarias gariepinus (Burchell, 1822). J Photochem Photobiol B 99: 1-8
- Dahunsi SO, Oranusi SU, Ishola RO (2011) Biochemical profile of Clarias gariepinus exposed to sub-lethal concentrations of chemical additives effluent. Int’l J Res Environ Sci Technol 1: 52-58
- Kavitha C, Malarvizhi A, Kumaran SS, Ramesh M (2010) Toxicological effects of arsenate exposure on hematological, biochemical and liver transaminases activity in an Indian major carp, Catla catla. Food Chem Toxicol 48: 2848-2854.
- Saravanan M, Karthika S, Malarvizhi A, Ramesh M (2011) Ecotoxicological impacts of clofibric acid and diclofenac in common carp (Cyprinus carpio) fingerlings: Hematological, biochemical, ionoregulatory and enzymological responses. J Hazard Mat 195: 188-194.
- Dahunsi SO, Oranusi US (2013) Haematological response of Clarias gariepinus to rubber processing effluent. Ann Rev Res Biol 3: 624-635.
- Li ZH, Velisek J, Zlabek V, Grabic R, Machova J, et al (2011) Chronic toxicity of verapamil on juvenile rainbow trout (Oncorhynchus mykiss): Effects on morphological indices, hematological parameters and antioxidant responses. J Hazard Mat 185: 870-880.
- Borges A, Scotti LV, Siqueira DR, Zanini R, Amaral F, et al. (2007) Changes in hematological and serum biochemical values in jundia Rhamdia quelen due to sub-lethal toxicity of cypermethrin. Chemosphere 69: 920-926.
- Sudova E, Piackova V, Kroupova H, Pijacek M, Svobodova Z (2009) The effect of praziquantel applied per os on selected haematological and biochemical indices in common carp (Cyprinus carpio L). Fish Physiol Biochem 35: 599-605.
- Vasylkiv OY, Kubrak OI, Storey KB, Lushchak VI (2010) Cytotoxicity of chromium ions may be connected with induction of oxidative stress. Chemosphere 80: 1044-1049.
- Vasylkiv OY, Kubrak OI, Storey KB, Lushchak VI (2011) Catalase activity as a potential vital biomarker of fish intoxication by the herbicide aminotriazole. Pestic Biochem Physiol 101: 1-5.
- Li ZH, Velisek J, Zlabek V, Grabic R, Machova J (2010) Hepatic antioxidant status and hematological parameters in rainbow trout, Oncorhynchus mykiss, after chronic exposure to carbamazepine. Chem-Biol Inter 183: 98-104.
- Li ZH, Velisek J, Grabic R, Li P, Kolarova J, et al. (2011) Use of hematological and plasma biochemical parameters to assess the chronic effects of a fungicide propiconazole on a freshwater teleost. Chemosphere 83: 572-578.
- Olajire AA, Alade AO, Adeniyi AA, Olabemiwo OMJ (2007) Distribution of polycyclic aromatic hydrocarbons in surface soils and water from the vicinity of Agbabu bitumen field of South Western Nigeria.Environ Sci Health 42: 1043-1049.
- Ayandiran TA, Ayandele AA, Dahunsi SO, Ajala OO (2014) Microbial assessment and prevalence of antibiotic resistance in polluted Oluwa River, Nigeria. Egyp J Aqua Res 40: 291-299.
- Sonne C, Rige´t FF, Leat EHK, Bourgeon S, Borg K, et al. (2013) Organohalogen contaminants and Blood plasma clinical-chemical parameters in three colonies of North Atlantic Great skua (Stercorarius skua). Ecotoxicol Environ Saf 92: 245-251.
- Ptashynski MD, Pedlar RM, Evans RE, Baron CL, Klaverkamp JF (2002) Toxicological effects of dietary arsenic exposure in lake whitefish (Coregonus clupeaformis). Aquat Toxicol 57: 167-189.
- Kubrak OI, Atamaniuk TM, Storey KB, Lushchak VI (2013) Goldfish can recover after short-term exposure to 2,4-dichlorophenoxyacetate: Use of blood parameters as vital biomarkers. Comp Biochem Physiol C Toxicol Pharmacol 157: 259-265.
- Delafield F (1984) Haematoxylin and eosin for general staining. Staining of the animal tissues practical and theoretical. Oxford University Press, London.
- Bancroft JD, Gamble M (2002) Theory and practice of histological techniques, 5th Edn. Churchill Livingstone, Edinburgh, London.
- Ferreira JG, Hawkins AJS, Bricker SB (2007) Management of productivity, environmental effects and profitability of shellfish aquaculture-the farm aquaculture resource management (FARM) model. Aqua 264: 160-174.
- Vutukuru SS, Pauleena JS, Rao JV, Anjaneyulu Y (2007) Architectural changes in the gill morphology of the freshwater fish, Esomus danricus as potential biomarkers of copper toxicity using automated video tracking system. Environ Bioind 2: 3-14.
- Adamu KM (2009) Sublethal effects of tobacco (Nicotiana tobaccum) leaf dust on enzymatic activities of Heteroclarias (a hybrid of Heterobranchus bidorsalis and Clarias gariepinus). Jor J Biol Sci 2: 151-158.
- Gabriel UU, George ADI (2005) Plasma enzymes in Clarias gariepinus exposed to chronic levels of round up (glyphosate). Environ Ecol 23: 271-276.
- Tiwari S, Singh A (2003) Control of common freshwater predatory fish, Channa punctatus, through Nerium indicum leaf extracts. Chemosphere 53: 865-875.
- Gabriel UU, Obomanu FG, Edori OS (2009) Haematology, plasma enzymes and organ indices of Clarias gariepinus after intramuscular injection with aqueous leaves extracts of Lepidagathis alopecuroides. Afr J Biochem Res 3: 312-316.
- Edqvist LE, Madej A, Forsberg M (1992) Biochemical blood parameters in pregnant mink fed PCB and fractions of PCB. Ambio 21: 577-581.
- Klaassen CD, Amdur MO, Doull J (2007) Casarett and Doulls Toxicology: The Basic Science of Poisons, sixth ed. McGraw-Hill, New York 1280.
- Harr KE (2002) Clinical chemistry of companion avian species: A review. Vet Clin Pathol 31: 140-151.
- Musso CG (2009) Magnesium metabolism in health and disease. Int Urol Nephrol 41: 357-362.
- Omoniyi I, Agbon AO, Sodunke SA (2002) Effect of lethal and sublethal concentrations of tobacco (Nicotiana tobaccum) leaf dust extract on weight and hematological changes in Clarias gariepinus (Burch). J Appl Sci Environ Mgt 6: 37-41.
- Adeyemo OK (2005) Haematological and histopathological effects of cassava mill effluent in Clarias gariepinus. Afr J Biomed Res 8: 179-183.
- Ayotunde EO, Fagbenro OA, Adebayo OT, Amoo AI (2004) Toxicity of aqueous extracts of drumstick Moringa oleifera seeds to Nile tilapia Oreochromis niloticus, fingerlings and adults. In: Proceedings of 6th international symposium on tilapia.
- Ates B, Orun I, Talas ZS, Durmaz G, Yilmaz I (2008) Effects of sodium selenite on some bio-chemical and hematological parameters of rainbow trout (Oncorhynchus mykiss Walbaum, 1792) exposed to Pb2+ and Cu2+. Fish Physiol Biochem 34: 53-59.
- Hinton DE, Laure´n DJ (1990) Integrative histopathological effects of environmental stressors on fishes. American Fisheries Society Symposium 8: 51-66.
- van Dyk JC, Pieterse GM, van Vuren JHJ (2007) Histological changes in the liver of Oreochromis mossambicus (Cichlidae) after exposure to cadmium and zinc. Ecotoxicol Environ Saf 66: 432-440.
- Calore E, Perez N, Herman M (2007) Morphometric studies of cardiac miocytes of dietary nickel in Lake Whitefish (Coregonus clupeaformis). Aqua Toxicol 58: 229-247.
- Cetin N, Cetin E, Eraslan G, Bilgili A (2007) Chlorpyrifos induces cardiac dysfunction in rabbits. Res Vet Sci 82: 405-408.
- Manna P, Sinha M, Sil PC (2008) Amelioration of cadmium induced cardiac impairment by taurine. Chem-Biol Interact 74: 88-97.
- Hariri A, Moallem S, Mahmoudi M, Memar B, Hosseinzadeh H (2010) Sub-acute effects of diazinon on biochemical indices and specific biomarkers in rats: Protective effects of crocin and safranal. Food Chem Toxicol 48: 2803-2808.
- El-Shenawy NS, El-Salmy F, Al-Eisa RA, El-Ahmary B (2010) Amelioratory effect of vitamin E on organophosphorus insecticide diazinon-induced oxidative stress in mice liver. Pestic Biochem Physiol 96: 101-107.
- Jafari M, Salehi M, Ahmadi S, Asgari A, Abasnezhad M, et al. (2012) The role of oxidative stress in diazinon-induced tissues toxicity in Wistar and Norway rats. Toxicol Mech Methods 22: 638-647.
- Lukaszewicz-Hussain A (2008) Subchronic intoxication with chlorfenvinphos, an organophosphate insecticide, affects rat brain antioxidative enzymes and glutathione level. Food Chem Toxicol 46: 82-86.
- Oral B, Guney M, Demirin H, Ozguner M, Giray SG, et al. (2006) Endometrial damage and apoptosis in rats induced by dichlorvos and ameliorating effect of antioxidant vitamins E and C. Reprod Toxicol 22: 783-790.
- Guney M, Oral B, Demirin H, Ozguner M, Take G, et al. (2007) Evaluation of caspase-dependent apoptosis during methyl parathion-induced endometrial damage in rats: Ameliorating effect of Vitamins E and C, Environ Toxicol Pharmacol 23: 221-227.
- Aluigi MG, Guida C, Falugi, C (2010) Apoptosis as a specific biomarker of diazinon toxicity in NTera2-D1 cells. Chem Biol Interact 187: 299-303.
- Ayandiran TA, Dahunsi SO (2017) Microbial evaluation and occurrence of antidrug multi-resistant organisms among the indigenous Clarias species in River Oluwa, Nigeria. J King Saud Univ Sci 29: 96-105.
Relevant Topics
- Analytical Biochemistry
- Applied Biochemistry
- Carbohydrate Biochemistry
- Cellular Biochemistry
- Clinical_Biochemistry
- Comparative Biochemistry
- Environmental Biochemistry
- Forensic Biochemistry
- Lipid Biochemistry
- Medical_Biochemistry
- Metabolomics
- Nutritional Biochemistry
- Pesticide Biochemistry
- Process Biochemistry
- Protein_Biochemistry
- Single-Cell Biochemistry
- Soil_Biochemistry
Recommended Journals
- Biosensor Journals
- Cellular Biology Journal
- Journal of Biochemistry and Microbial Toxicology
- Journal of Biochemistry and Cell Biology
- Journal of Biological and Medical Sciences
- Journal of Cell Biology & Immunology
- Journal of Cellular and Molecular Pharmacology
- Journal of Chemical Biology & Therapeutics
- Journal of Phytochemicistry And Biochemistry
Article Tools
Article Usage
- Total views: 3344
- [From(publication date):
June-2017 - Nov 21, 2024] - Breakdown by view type
- HTML page views : 2656
- PDF downloads : 688