Resistance Sources for Fusarium Head Blight Disease of Bread Wheat in Ethiopia
Received: 01-Aug-2023 / Manuscript No. acst-23-110783 / Editor assigned: 03-Aug-2023 / PreQC No. acst-23-110783(PQ) / Reviewed: 21-Aug-2023 / QC No. acst-23-110783 / Revised: 26-Aug-2023 / Manuscript No. acst-23-110783(R) / Published Date: 31-Aug-2023 DOI: 10.4172/2329-8863.1000611
Abstract
Fusarium head blight (FHB); caused by several Fusarium species, is an important disease that causes significant losses in bread wheat yield and quality. This investigation aimed to identify the resistance source(s) against FHB of bread wheat in Ethiopia. Fifty-two genotypes (including two checks) had evaluated against the mixture of dominant FHB pathogens (Fusarium graminearum and Fusarium culmorum). Percent Fusarium damaged kernel (% FDK) varied from 6.9% to 100%. Out of 52 genotypes screened, genotype 31 was showed highly resistant for diseased spikelets per spike (1.0), resistance for %FDK (6.9%), resistance for disease scores (1.0), and lower AUDPC (20.2), followed by genotype 29, which showed similar response except for the %FDK which was moderately resistant (9.3). This investigation concluded that genotypes 31 and 29 can be used as a source of donors to improve the resistance of Ethiopian bread wheat varieties against FHB disease.
Keywords
Triticum aestivum; Fusarium culmorum; Fusarium graminearum; FHB; Type-II resistance; %FDK
Introduction
Globally wheat ranks first among cereal crops in area harvested, second in amount of production, and fifth in productivity. The contribution of Africa to world’s wheat production was 4.55% in area harvested and 3.32% in tonnes. From African countries, Ethiopia ranked third in area allocated for wheat, second in tonnes of wheat production, and seventh in wheat productivity in 2020 [1]. Nowadays, the government of Ethiopia encourages wheat production in all regions for food security. But, wheat production in the country is challenged by Fusarium head blight (FHB) both under rain-fed [2-6]. And irrigated fields [7]. FHB infected wheat fields with 97.5% in Arsi and West-Arsi [41], 93.56% in Southern Nations and Nationalities People (SNNP) and Oromia [5], 93.90% in southwestern Ethiopia [40], and 88.75% in West Shewa [2]. This disease is known for damaging the quality [8, 9]. And quantity of wheat production [10]. FHB is a concern due to the associated secondary metabolites, such as deoxynivalenol and trichothecene mycotoxin [11], which are unsafe for both humans and livestock.
Previous studies in Ethiopia reported that Fusarium graminearum Schwabe and Fusarium culmorum (W.G. Smith) Saccardo were the dominant species responsible for FHB disease of bread wheat in the country [2, 3]. Both of them are capable of causing DON contamination in wheat grains [12-14]. The previous studies in Ethiopia focused on surveys [2, 3, 5], evaluation of released varieties and fungicide trial [4, 6]. But, there is lack of genotype screening efforts in Ethiopia. Therefore, this study was aimed to identify sources of resistance against the dangerous FHB disease of bread wheat in Ethiopia through screening bread wheat genotypes.
Materials and Methods
Description of the study area
This experiment had done in 2020 under Lath-house condition of Assosa Agricultural Research Center (AsARC), Assosa, Benishangul Gumuz region, Ethiopia. Geographically it is situated at 10° 03 N and 34° 59 E (Figure 1). The area has an altitude of 1580 m above sea level. It receives a mean annual rainfall of 1299.2 mm and minimum and maximum temperatures of 13.3-157.7 °C and 24.1-32.8 °C, respectively.
The relative humidity ranged from 26-80% during experimentation (Figure 1).
Planting materials
Fifty-two bread wheat genotypes (including susceptible and resistant checks) were screened to determine resistance sources against FHB of bread wheat in Ethiopia. 50 genotypes had obtained from International Maize and Wheat Improvement Center (CIMMYT), Ethiopia and the two locally adapted bread wheat varieties, Kingbird and Wane, were obtained from Holeta Agricultural Research Center (HARC) (Table 1).
| Genotype code | Pedigree | Genotype code | Pedigree |
|---|---|---|---|
| 1 | Tacupetof2001/brambling//kiritati/3/francolin#1/blouk#1/4/francolin#1/blouk #1 | 27 | Fret2*2/shama/3/pfau/weaver//brambling*2/4/huw234+lr34/prinia*2//yanac |
| 2 | Bav92//irena/kauz/3/huites*2/4/chil/chum18/5/pbw343*2/kukuna*2//frtl/pifed/6/bav92//irena/kauz/3/huites/4/2*rolf07 | 28 | Tacupetof2001/6/cndo/r143//ente/mexi_2/3/aegilopssquarrosa(taus)/4/weaver/5/pastor/7/rolf07/8/pbw343*2/kukuna*2//frtl/pifed |
| 3 | Kfa//pbw343/pastor/3/pbw343*2/kukuna/4/kachu#1//pi610750/sasia/3/kachu/5/kfa/3/pfau/weaver//brambling/4/pfau/weaver*2//brambling | 29 | Kachu#1/kiritati//kachu/5/bav92//irena/kauz/3/huites/4/2*rolf07 |
| 4 | Neloki*2/4/sokoll//pbw343*2/kukuna/3/attila/pastor | 30 | Ciro16*2/3/muu #1/saual//muu |
| 5 | Baj #1*2/premio | 31 | C80.1/3*batavia//2*wbll1/5/reh/hare//2*bcn/3/croc_1/ae.squarrosa(213)//pgo/4/huites/6/francolin#1/blouk#1/7/c80.1/3*batavia//2*wbll1/5/reh/hare//2*bcn/3/croc_1/ae.squarrosa(213)//pgo/4/huites |
| 6 | Quaiu/yanac//francolin#1/blouk#1/3/francolin #1/blouk #1 | 32 | Trch/huirivis#1/4/kachu#1//pi610750/sasia/3/kachu/5/kachu #1/kiritati//kachu |
| 7 | Pastor/3/ures/jun//kauz/4/wbll1/5/gkaron/agseco7846//2180/4/2*milan/kauz//prinia/3/bav92 | 33 | Kinde*2/4/t.dicocconpi94625/ae.squarrosa(372)//tui/clms/3/2*pastor/5/pbw343*2/kukuna*2//frtl/pifed/6/pbw343*2/kukuna*2//frtl/pifed |
| 8 | Sseri1/chibia/4/bav92//irena/kauz/3/huites/5/kza//wh542/2*pastor/3/baceu#1/6/fret2/kukuna//fret2/3/heilo | 34 | Saual*2/3/wl6718//2*prl/vee#6/4/2*pbw343*2/kukuna*2//frtl/pifed |
| 9 | Baj #1*2/premio | 35 | Prl/2*pastor//parus/5/nac/th.ac//3*pvn/3/mirlo/buc/4/2*pastor/6/kingbird#1//inqalab91*2/tukuru |
| 10 | Norm/wbll1//wbll1/3/tnmu/4/wbll1*2/tukuru/5/pbw343*2/kukuna*2//frtl/pifed/6/pbw343*2/kukuna*2//frtl/pifed | 36 | Kachu#1/yunmai47//kachu/5/saual/3/c80.1/3*batavia//2*wbll1/4/site/mo//pastor/3/tilhi/6/kachu #1/kiritati//kachu |
| 11 | Kfa//pbw343/pastor/3/pbw343*2/kukuna/4/kachu#1//pi610750/sasia/3/kachu/5/kfa/3/pfau/weaver//brambling/4/pfau/weaver*2//brambling | 37 | Quaiu#1/3/kingbird#1//inqalab91*2/tukuru |
| 12 | Wbll1/kukuna//tacupetof2001/3/up2338*2/vivitsi/4/2*pbw343*2/kukuna*2//frtl/pifed | 38 | Cno79//pf70354/mus/3/pastor/4/bav92*2/5/har311/6/trch/huirivis #1 |
| 13 | Pbw343*2/khvaki*2//yanac/4/muu#1//pbw343*2/kukuna/3/muu/5/chipak | 39 | Saual*2/6/cndo/r143//ente/mexi_2/3/aegilopssquarrosa(taus)/4/weaver/5/2*pastor*2/7/pbw343*2/kukuna*2//frtl/ pifed |
| 14 | Pbw343*2/kukuna*2//frtl/pifed*2/3/bokota | 40 | Kachu/sup152 |
| 15 | Babax/lr42//babax/3/er2000/5/w15.92/4/pastor//hxl7573/2*bau/3/wbll1 | 41 | Saual*2/3/wl6718//2*prl/vee#6/4/2*pbw343*2/kukuna*2//frtl/pifed |
| 16 | Kfa/3/pfau/weaver//brambling/4/pfau/weaver*2//brambling*2/5/quelea | 42 | Saual/3/c80.1/3*batavia//2*wbll1/4/site/mo//pastor/3/tilhi*2/5/kingbird#1//inqalab91*2/tukuru |
| 17 | Kachu#1/3/c80.1/3*batavia//2*wbll1/4/kachu/8/tacupetof2001/6/cndo/r143//ente/mexi_2/3/aegilopssquarrosa(taus)/4/weaver/5/pastor/7/rolf07/9/kfa/2*kachu | 43 | Cno79//pf70354/mus/3/pastor/4/bav92*2/5/har311/6/pbw343*2/kukuna*2//frtl/pifed/7/cno79//pf70354/mus/3/pastor/4/bav92*2/5/har311 |
| 18 | Babax/lr42//babax/3/er2000/4/nighar | 44 | Saual/mutus/3/pbw343*2/kukuna*2//frtl/pifed/4/pbw343*2/kukuna*2//frtl/pifed |
| 19 | Altar84/ae.squarrosa(221)//3*borl95/3/ures/jun//kauz/4/wbll1/5/mutus/6/kingbird #1//inqalab 91*2/tukuru | 45 | Attila*2/pbw65*2//murga/3/francolin#1//wbll1*2/kiritati |
| 20 | Kfa/2*kachu*2/8/tacupetof2001/6/cndo/r143//ente/mexi_2/3/aegilopssquarrosa(taus)/4/weaver/5/pastor/7/rolf07 | 46 | Bokota/3/2*kingbird #1//inqalab 91*2/tukuru |
| 21 | Trch/huirivis#1/3/pbw343*2/kukuna*2//frtl/pifed | 47 | Kfa/2*kachu*2/8/tacupetof2001/6/cndo/r143//ente/mexi_2/3/aegilopssquarrosa(taus)/4/weaver/5/pastor/7/rolf07 |
| 22 | Kfa/2*kachu/4/wbll1*2/kuruku//kronstad f2004/3/wbll1*2/brambling | 48 | Pbw343*2/kukuna*2//frtl/pifed/5/kachu#1/3/c80.1/3*batavia//2*wbll1/4/kachu |
| 23 | Up2338*2/shama/3/milan/kauz//chil/chum18/4/up2338*2/shama/5/up2338*2/vivitsi/3/fret2/tukuru//fret2/4/misr 1 | 49 | Sumai #3 |
| 24 | Saual/4/croc_1/ae.squarrosa(205)//kauz/3/attila/5/saual/6/kingbird#1//inqalab91*2/tukuru/7/saual/whear//saual | 50 | Gamenya(kenyas117a/2*gabo//mentana/6*gabo) |
| 25 | Bokota/5/up2338*2/vivitsi/3/fret2/tukuru//fret2/4/misr1/6/babax/lr42//babax*2/3/kukuna/4/crosbill #1/5/becard | 51 | Localcheck[kingbird(tam200/tui/6/pvn//car422/ana/5/bow/crow//buc/pvn/3/yr/4/trap#1)] |
| 26 | Otus//wbll1*2/tukuru/3/2*pbw343*2/kukuna*2//frtl/pifed | 52 | Local check [wane (sokoll/excalibur)] |
Table 1: Bread whet genotypes evaluated against Fusarium head blight disease.
Experimental procedures
The experiments were laid on randomized complete block design (RCBD) having two replications. Kernels of each genotype were disinfected and air-dried under laminar flow. An autoclaved sand/peat/ compost: 1:3:1 v/v mix soil had used to fill the pots. Six kernels for each genotype had sown in pot and later thinned to three plants per pot.
Each pot was fertilized, with 5 g NSP at tillering and 5 g urea at booting. Inoculum suspension had prepared from highly virulent F. graminearum and F. culmorum mixtures. The inoculum conidia concentration was 5 x 104 conidia per ml. A single centrally positioned floret was injected with 10 μl conidia suspension at Zadok’s growth stage 65. After inoculation, the spikes were covered with polythene bags for 72 hours (Figure 2) to maintain humidity that facilitates infection.
Data collection
Bleached spikelets were carefully inspected at weekly intervals up to 28 days after inoculation to determine FHB type-II resistance. FHB severities had recorded as described by. Finally, inoculated spikes were harvested and threshed for percent Fusarium damaged kernels (%FDK) determination. The area under the disease progress curve (AUDPC) was determined as follows:
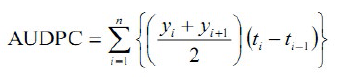
Where: AUDPC is the area under disease progress curve, n is total number of observation days at the ith observation, yi is spikelet infection severity at the ith observation, it is time at the ith observation.
Data analysis
Analysis of variance for spikelet bleaching severity and AUDPC had done with the general linear model procedure of the SAS 9.3 version. LSD was used to separate treatment means at a probability level of 0.05 (Figures 1 & 2).
Results and Discussions
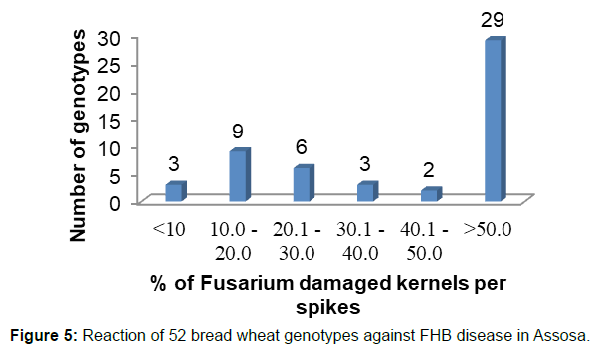
The resistant (Sumai#3) and susceptible (Gamenya) checks showed FHB severities of 34.2% and 100%, indicating the isolates were virulent. The susceptible Gamenya had the highest (100%) FHB severity and %FDK per spike (Figure 3, Tables 3 & 4). Of the genotypes evaluated, 22 (42.31%) showed resistance levels better than Sumai#3 (Table 3). This study found that three (5.8%) genotypes were exhibited less than 10% FDK (Table 4) (Table 2).
| Sources | Df | DH | SS | %ISS | AUDPC | %FDK |
|---|---|---|---|---|---|---|
| Genotypes | 51 | 30.58*** | 23.19*** | 3381.17*** | 236884.01*** | 3710.01*** |
| Error | 103 | 5.63 | 3.58 | 1394.40 | 176825.24 | 1289.20 |
| Means | 71.16 | 16.23 | 39.36 | 474.88 | 50.86 | |
| CV | 3.33 | 11.66 | 94.86 | 88.55 | 28.55 |
Table 2: Analysis of variance for days to heading, number of spikelets per spike, area under disease progress curve, and percent of Fusarium damaged kernels.
| Genotypes | Days to heading | Plant height (cm) | FHB severity at 28 DAI | AUDPC | ||
|---|---|---|---|---|---|---|
| Disease scores | Resistance reaction | % infected spikelets | ||||
| 16 | 66.5g-m | 65.6c-j | 1.0b | R | 3.1b | 10.9g |
| 21 | 66.5g-m | 68.5b-j | 1.0b | R | 2.8i | 9.7g |
| 29 | 65.0i-n | 72.0a-g | 1.0b | R | 6.2b | 21.7g |
| 31 | 65.0i-n | 79.4ab | 1.0b | R | 5.8b | 20.2g |
| 4 | 69.0c-m | 68.5b-j | 1.0b | R | 3.9b | 13.5g |
| 39 | 63.5k-n | 65.3c-j | 2.0ab | R | 10.3b | 49.6fg |
| 41 | 72.0c-j | 60.3hij | 2.0ab | R | 8.0b | 56.3fg |
| 6 | 72.0c-j | 66.3c-j | 2.0ab | R | 7.5b | 35.0fg |
| 12 | 65.0i-n | 67.8c-j | 2.5ab | MR | 12.8b | 68.1e-g |
| 18 | 62.0mn | 71.4a-h | 2.5ab | MR | 29.3ab | 194.4b-g |
| 25 | 67.0f-m | 72.3a-g | 2.5ab | MR | 17.8b | 78.2e-g |
| 3 | 70.5c-k | 69.4b-i | 2.5ab | MR | 15.4b | 98.8d-g |
| 34 | 62.5lmn | 70.0lmn | 2.5ab | MR | 10.0b | 46.7fg |
| 35 | 71.0c-j | 68.5b-j | 2.5ab | MR | 9.2b | 48.9fg |
| 40 | 75.5a-d | 57.9j | 2.5ab | MR | 10.1b | 57.1fg |
| 52 | 68.0e-m | 76.5abc | 2.5ab | MR | 11.7b | 55.4fg |
| 13 | 68.0e-m | 73.5a-e | 3.0ab | MR | 53.9ab | 289.4a-g |
| 15 | 66.5g-m | 70.5a-h | 3.0ab | MR | 20.0b | 105.0d-g |
| 17 | 72.5c-h | 67.5c-j | 3.0ab | MR | 53.1ab | 360.9a-g |
| 2 | 64.0j-n | 69.3b-j | 3.0ab | MR | 50.0ab | 175.0c-g |
| 20 | 66.0h-n | 65.0d-j | 3.0ab | MR | 53.9ab | 340.1a-g |
| 23 | 68.0e-m | 69.4b-i | 3.0ab | MR | 50.0ab | 229.7b-g |
| 49 | 81.0a | 81.3a | 3.0ab | MR | 34.2ab | 175c-g |
| 24 | 72.0c-i | 71.4a-h | 3.0ab | MR | 25.0ab | 165.3c-g |
| 27 | 72.0c-i | 67.3c-j | 3.0ab | MR | 53.1ab | 360.9a-g |
| 30 | 68.5d-m | 69.8b-i | 3.0ab | MR | 34.8ab | 232.8a-g |
| 36 | 74.0a-f | 71.0a-h | 3.0ab | MR | 20.2b | 109.7d-g |
| 37 | 65.0i-n | 71.6a-h | 3.0ab | MR | 32.5ab | 196.9b-g |
| 42 | 66.0h-n | 60.3hij | 3.0ab | MR | 53.3ab | 250.3a-g |
| 43 | 68.0e-m | 68.8b-j | 3.0ab | MR | 16.7b | 87.5d-g |
| 45 | 65.0i-n | 62.3e-j | 3.0ab | MR | 50.0ab | 262.5a-g |
| 46 | 75.0a-e | 71.5a-h | 3.0ab | MR | 19.4b | 106.9d-g |
| 47 | 67.5f-m | 67.3c-j | 3.0ab | MR | 12.2b | 64.2e-g |
| 48 | 65.5h-n | 67.1c-j | 3.0ab | MR | 53.9ab | 366.0a-g |
| 5 | 67.0f-m | 71.8a-g | 3.0ab | MR | 34.3ab | 214.4b-g |
| 10 | 68.5d-m | 76.0a-d | 4.0ab | MS | 42.2ab | 287.3a-g |
| 11 | 62.5lmn | 68.9b-j | 4.0ab | MS | 55.9ab | 240.9a-g |
| 22 | 59.0n | 68.5b-j | 4.0ab | MS | 55.9ab | 264.8a-g |
| 51 | 73.5b-g | 72.5a-f | 4.0ab | MS | 24.4b | 85.6d-g |
| 9 | 66.5g-m | 70.8a-h | 4.0ab | MS | 36.7ab | 186.7b-g |
| 14 | 68.0e-m | 72.0a-g | 4.5ab | S | 60.0ab | 309.2a-g |
| 32 | 71.5c-i | 64.0e-j | 4.5ab | S | 34.4ab | 200.3b-g |
| 7 | 71.0c-j | 58.5ij | 4.5ab | S | 72.2ab | 454.2a-f |
| 1 | 69.5c-l | 67.4c-j | 5.0a | S | 100.0a | 615.3ab |
| 19 | 68.5d-m | 72.4a-f | 5.0a | S | 100.0a | 517.0a-d |
| 26 | 63.5k-n | 75.5a-d | 5.0a | S | 71.9ab | 389.3a-g |
| 28 | 70.5c-k | 73.5a-e | 5.0a | S | 57.0ab | 308.3a-g |
| 33 | 68.5d-m | 62.0f-j | 5.0a | S | 100.0a | 411.8a-g |
| 38 | 65.0i-n | 69.3b-j | 5.0a | S | 100.0y | 584.1a-c |
| 44 | 69.5c-l | 72.3a-g | 5.0a | S | 73.8ab | 489.7a-e |
| 50 | 80.5ab | 60.9g-j | 5.0a | S | 100.0a | 662.1a |
| 8 | 76.0abc | 73.3a-f | 5.0a | S | 76.2ab | 371.9a-g |
| Mean | 71.16 | 3.20 | 39.33 | 217.98 | ||
| LSD | 7.5 | 11.65 | 3.94 | 75.6 | 431.52 | |
| CV | 5.4 | 8.32 | 61.06 | 95.5 | 88.9 | |
Table 3: Reaction of bread wheat genotypes against the mixture of dominant Fusarium head blight pathogens in Ethiopia.
Df degree of freedom, DH days to heading, SS number of spikelets per spike, %ISS percent of infected spikelets per spike, AUDPC area under disease progress curve, %FDK percent Fusarium damaged kernels, *** highly significant (p < 0.0001).
According to FHB disease scores, 15.38% of the genotypes had shown R reaction (Figure 4), but most of the genotypes (51.92%) had shown MR reaction to the mixture of dominant F. graminearum and F. culmorum (Table 3). In the same way, based on diseased or bleached spikelets per spike, 9.62%, 21.15%, and 7.69% of the genotypes showed HR, R, and MR reactions, respectively (Table 4) (Figures 3 & 4).
| Genotypes | Number of spikelets per spike | Diseased spikelets per spike | Resistance level | % of Fusarium damaged kernels per spike | Response |
|---|---|---|---|---|---|
| 4 | 11.5k | 0.5g | HR | 20.6de | S |
| 16 | 17.0c-i | 0.5g | HR | 37.5a-e | S |
| 21 | 16.5d-j | 0.5g | HR | 14.9de | MS |
| 29 | 16.5d-j | 1.0g | HR | 9.3e | MR |
| 31 | 17.5b-h | 1.0g | HR | 6.9e | R |
| 6 | 20.0a-d | 1.5fg | R | 19.0de | MS |
| 34 | 14.5g-k | 1.5fg | R | 21.3de | S |
| 39 | 16.0e-j | 1.5fg | R | 16.6de | MS |
| 40 | 17.0c-i | 1.5fg | R | 20.0de | MS |
| 41 | 18.5a-f | 1.5fg | R | 26.1cde | S |
| 52 | 13.5i-k | 1.5fg | R | 9.2e | MR |
| 12 | 16.5d-j | 2.0fg | R | 13.7de | MS |
| 25 | 11.5k | 2.0fg | R | 30.6b-e | S |
| 35 | 21.0ab | 2.0fg | R | 12.9de | MS |
| 43 | 13.5i-k | 2.0fg | R | 72.0a-e | VS |
| 47 | 16.5d-j | 2.0fg | R | 14.0de | MS |
| 3 | 20.5a-c | 3.0efg | MR | 92.6abc | VS |
| 15 | 13.5i-k | 3.0efg | MR | 21.4de | S |
| 36 | 16.0e-j | 3.5d-g | MR | 26.0cde | S |
| 46 | 19.0a-e | 3.5d-g | MR | 35.1a-e | S |
| 5 | 13.5i-k | 4.5c-g | MS | 40.8a-e | S |
| 24 | 18.0b-g | 4.5c-g | MS | 91.7abc | VS |
| 51 | 19.0a-e | 4.5c-g | MS | 17.1de | MS |
| 18 | 17.5b-h | 5.0b-g | MS | 29.4cde | S |
| 9 | 15.0f-k | 5.5b-g | MS | 47.2a-e | S |
| 30 | 15.0f-k | 5.5b-g | MS | 56.0a-e | VS |
| 32 | 16.5d-j | 5.5b-g | MS | 73.8a-e | VS |
| 37 | 16.5d-j | 5.5b-g | MS | 60.9a-e | VS |
| 42 | 13.0jk | 6.0b-g | MS | 53.2a-e | VS |
| 2 | 13.0jk | 6.5b-g | S | 36.6a-e | S |
| 49 | 22.0a | 6.5b-g | S | 15.6de | MS |
| 10 | 15.5e-j | 7.0a-g | S | 58.8a-e | VS |
| 20 | 14.0h-k | 8.0a-g | S | 63.5a-e | VS |
| 23 | 14.0h-k | 8.0a-g | S | 60.6a-e | VS |
| 45 | 14.0h-k | 8.0a-g | S | 53.6a-e | VS |
| 11 | 16.0e-j | 8.5a-g | S | 54.8a-e | VS |
| 27 | 16.0e-j | 8.5a-g | S | 75.0a-e | VS |
| 48 | 14.5g-k | 8.5a-g | S | 56.4a-e | VS |
| 7 | 11.5k | 9.0a-g | S | 100.0a | VS |
| 13 | 15.5e-j | 9.5a-g | S | 62.5a-e | VS |
| 14 | 15.5e-j | 9.5a-g | S | 56.6a-e | VS |
| 17 | 17.0c-i | 9.5a-g | S | 80.4a-d | VS |
| 22 | 17.5b-h | 10.0a-g | S | 60.6a-e | VS |
| 28 | 17.5b-h | 10.0a-g | S | 92.3abc | VS |
| 26 | 16.5d-j | 12.0a-g | S | 79.0a-d | VS |
| 44 | 19.0a-e | 13.5a-f | S | 98.8ab | VS |
| 8 | 20.0a-d | 15.0a-e | S | 100.0a | VS |
| 38 | 15.5e-j | 15.5a-d | HS | 100.0a | VS |
| 1 | 16.0e-j | 16.0abc | HS | 94.4abc | VS |
| 19 | 17.0c-i | 17.0ab | HS | 100.0a | VS |
| 33 | 17.0c-i | 17.0ab | HS | 55.6a-e | VS |
| 50 | 19.0a-e | 19.0a | HS | 100.0a | VS |
| LSD | 2.008 | 12.5 | 69.4 |
Table 4: Response of bread wheat genotypes to the mixture of F graminearum and F culmorum at Assosa.
A significant difference in the mean values for the area under the disease progress curve (AUDPC) had observed between different genotypes (Table 2). The current study found that the AUDPC of the number of Fusarium-infected spikelets for the genotypes 21, 16, 4, 31, and 29 are the lowest AUDPC of 9.7, 10.9, 13.5, 20.2, and 21.7 (Table 3), while the susceptible Gamenya recorded very high AUDPC value (662.1). In this study, 42.3% genotypes recorded lower AUDPC values than the resistant check Sumai#3 (175). It is, therefore, clearly established that genotypes 21, 16, 4, 31, and 29 behave resistant to the progression of bleaching spikelets after inoculation (Table 3).
The analysis of variance showed that there is a significant difference (p < 0.001) between genotypes for %FDK (Table 2). The mean %FDK values showed a variable response, with a minimum value of 6.9% recorded on genotype 31 and a maximum of 100% recorded on genotypes 7, 8, 19, 38, and Gamenya (susceptible check), meaning a 90- fold difference (Table 4; Figure 5). According to Agostinelli et al, %FDK reveals kernel damage and is more linked to a decrease in test weight and, somehow drops in yield. Also, kernels with less %FDK exhibited fewer toxins. Similarly, Bai et al. and Lehoczki-Krsjak et al. reported a direct correlation among %FDK and DON. Based on this, %FDK was reported as another parameter for determining FHB type-II resistance in wheat. FHB type-II resistance is ascribed to cell wall thickening of rachis nodes and mycotoxin decomposition. Based on this evidence, the present study found that genotype 31 was the most FHB type-II resistant, followed by genotypes 59 and 29 with 9.2% and 9.3% percent FDK, respectively (Table 4, Figure 5). Out of 52 genotypes of bread wheat evaluated in this study, genotype 31 had chosen as putative FHB resistant sources based on disease score, diseased spikelets per spike, and %FDK (Table 3, Table 4). In addition, genotype 29 showed good resistance to FHB under controlled condition in Ethiopia (Table 3, Table 4) (Figure 5).
As illustrated in Table 2, bread wheat genotypes had shown significant differences for days to heading (DH). Sumai#3 (81 DH) and Gamenya (80.5 DH) showed the highest number of DH, respectively. In addition, the promising FHB-resistant genotypes 31 and 29 recorded 65 DH, which is almost similar to well adapted and released Kingbird (73.5 DH) and Wane (68 DH) varieties (Table 5). Thus, these promising FHB-resistant genotypes were acceptable height in Ethiopia (Table 4).
This study also found significant differences among bread wheat genotypes in plant height (Table 2). The mean values of bread wheat genotypes for plant height (PH) were shorter at Assosa than at Holeta. These could be related to higher altitude, higher rain fall, and relatively lower temperature at Holeta site, which might have increased the height of bread wheat plants at this location. Likewise, Muhder et al. reported that temperature, altitude, and precipitation meaningfully and evenly influenced the plant height of bread wheat genotypes.
According to Buerstmayr et al. and Khanizadeh et al. taller plants have less FHB disease than shorter ones, which indicates that plant height is a passive mechanism for FHB resistance? The promising genotypes 31 and 29 had almost the same plant height as the well-adapted and released varieties in the country (Table 5).
Conclusions
Five genotypes (4, 16, 21, 29, and 31) were highly resistant based on diseased spikelets of 0.5–1.0 (disease severity 2.8%–6.2%), eleven genotypes (6, 39, 41, 34, 40, 52, 12, 25, G35, 43, and 47) were resistant with diseased spikelets of 1.5–2.0 (disease severity 7.5%–17.8%). However, based on %FDK, genotype 31 was founded to be resistant and only produced 6.9%FDK. Next, to genotype 31, cultivar wane (with 9.2%FDK) and genotype 29 (with 9.3%FDK) showed moderate resistance against FHB disease. Undeniably, genotype 31 was founded to be highly resistant based on diseased spikelets per spike and resistant based on disease scores. Therefore, the bread wheat breeding program may introduce genotypes 31 and 29 as a source of resistance to improve the resistance of bread wheat against FHB disease in Ethiopia. Many genes control FHB disease resistance in bread wheat. Therefore, it requests a continuous screening of bread wheat genotypes to identify resistance sources and using them for evolving resistant cultivars. These can exist when there are motivated researchers and strong support from the government to have FHB-resistant cultivars that ensure safe foods.
Acknowledgment
The Author presents apparitions for the Plant Protection Research Directorate of the Ethiopian Institute of Agricultural Research and Assosa Agricultural Research Centre for financial and facility support. Also, I thank the Holeta Agricultural Research Center for providing bread wheat genotypes. I thank Mr. Yeneneh Chane for his help in soil preparation and watering the experiment.
References
- Abdissa T, Bekele B (2020) Assessment of wheat Fusarium head blight and associated Fusarium species in west Shewa, Ethiopia. International Journal of Agriculture and Biosciences 9(6): 299-304.
- Kebede M, Adugna G, Hundie B (2021) Identification of Fusarium species responsible to cause wheat head blight in Southwestern Ethiopia. International Journal of Plant Pathology12 (1): 21-31.
- Mengesha GG, Abebe SM, Lera ZT, Shertore MM, Fedilu KB, et al. (2021) Integration of host resistance, fungicides, and spray frequencies for managing Fusarium head blight of bread wheat under field conditions in southern Ethiopia. Heliyon 7(9): 1-13.
- Getahun M, Fininsa C, Bekeko Z, Mohammed A (2022) Analysis of the spatial distribution and association of wheat Fusarium head blight with biophysical factors in Ethiopia. European Journal of Plant Pathology 164(3): 391-410.
- Mengesha GG, Abebe SM, Fedilu KB, Tadesse YB, Mekonnen AA, et al. (2022) Fusarium head blight progression and yield response of bread wheat as affected by fungicides and spray regimes under field condition in southern Ethiopia. Journal of Crop Science and Biotechnology 25(5): 565-582.
- Hundie B, Badebo A, Hodson D, Bacha N, Yirga F, et al (2020) Enhancement of rust surveillance, early warnings and phenotyping of wheat genotypes in Ethiopia. Achievements in Fast-Track Variety Testing, Seed Multiplication and Scaling of Rust Resistant Varieties: Lessons from the Wheat Seed Scaling Project, Ethiopia 9-24.
- Capouchová I, Papoušková L, Kostelanská M, Prokinová E, Škeříková A, et al. (2012) Effect of different intensities of Fusarium infestation on grain yield, deoxynivalenol content and baking quality of winter wheat. Romanian Agricultural Research 29: 297-306.
- McMullen M, Bergstrom G, De Wolf E, Dill-Macky R, Hershman D, et al. (2012) A Unified Effort to Fight an Enemy of Wheat and Barley Fusarium Head Blight. Plant Disease 96: 1712-1728.
- Salgado JD, Madden LV, Paul PA (2015) Quantifying the effects of Fusarium head blight on grain yield and test weight in soft red winter wheat. Phytopathology 105(3): 295-306.
- Khaneghah AM, Martins LM, von Hertwig AM, Bertoldo R, Sant’Ana AS (2018) Deoxynivalenol and its masked forms: Characteristics, incidence, control and fate during wheat and wheat based products processing-A review. Trends in Food Science & Technology 71: 13-24.
- Matthäus K, Dänicke S, Vahjen W, Simon O, Wang J, et al. (2004) Progression of mycotoxin and nutrient concentrations in wheat after inoculation with Fusarium culmorum. Archives of Animal Nutrition 58(1): 19-35.
- Christ DS, Gödecke R, von Tiedemann A, Varrelmann M (2011) Pathogenicity symptom development, and mycotoxin formation in wheat by Fusarium species frequently isolated from sugar beet. Phytopathology 101(11): 1338-1345.
- Cowger C, Arellano C (2013) Fusarium graminearum infection and deoxynivalenol concentrations during development of wheat spikes. Phytopathology 103(5): 460-471.
- Abebele GM, Zerihun AA, Negash T (2021) Survey of Major Wheat Diseases in South Eastern Ethiopia. Science Research 9(4): 46-50.
- Abdissa T, Bekele B, Selvaraj T (2022) Evaluation of Response of Wheat (Triticum spp) Genotypes against Wheat Fusarium Head Blight in Ethiopia. Advances in Crop Science and Technology 10 (4): 1-5.
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Citation: Earecho MK (2023) Resistance Sources for Fusarium Head Blight Disease of Bread Wheat in Ethiopia. Adv Crop Sci Tech 11: 611. DOI: 10.4172/2329-8863.1000611
Copyright: © 2023 Earecho MK. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 2344
- [From(publication date): 0-2023 - Nov 05, 2025]
- Breakdown by view type
- HTML page views: 1993
- PDF downloads: 351