Case Report Open Access
Resistance Exercise Combined with Growth Hormone Augments Growth Parameters in a Male Adolescent with Cystic Fibrosis
Lynae J Hanks1, Michael S Stalvey2 and Krista Casazza3*
1Department of Kinesiology, University of Montevallo, Montevallo, Alabama, USA
2Department of Pediatrics/Division of Pediatric Endocrinology and Metabolism, Children's of Alabama (COA), University of Alabama at Birmingham (UAB), CPPII M30 1601 4th Ave S, Birmingham, AL 35233, USA
3Department of Pediatrics, UAB School of Medicine, COA, UAB, CPPI 310, 1601 4th Ave S, University in Birmingham, Alabama, 35233-1711, USA
- *Corresponding Author:
- Krista Casazza
Department of Pediatrics
UAB School of Medicine
COA, UAB, CPPI 310
1601 4th Ave S
University in Birmingham
Alabama, 35233-1711, USA
Tel: +205-638-6856
E-mail: kcasazza@peds.uab.edu
Received: October 02, 2015; Accepted: November 09, 2015; Published: November 15, 2015
Citation: Hanks LJ, Stalvey MS, Casazza K (2015) Resistance Exercise Combined with Growth Hormone Augments Growth Parameters in a Male Adolescent with Cystic Fibrosis. Sports Nutr Ther 1: 102. doi: 10.4172/2473-6449.1000102
Copyright: © 2015 Hanks LJ, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Nutrition Science Research
Abstract
In adolescents with cystic fibrosis (CF) the anabolic benefits of resistance exercise (RT) on skeletal muscle hypertrophy may synergize with recombinant human growth hormone (rhGH) therapy exerting an effect greater than either alone. We present a male adolescent with CF taking rhGH, who began RT. During rhGH alone, weight, height and BMI increased 14, 12 and 10 centiles (21st, 21st, 26th), respectively. When combined with RT, BMI, height and weight rose to the 48%, 47%, and 41%, respectively. While a single experience, combining RT with rhGH treatment may promote overall health improvement beyond that expected in adolescent males with CF on rhGH.
Keywords
Resistance exercise; GH; Cystic fibrosis; Adolescence; Skeletal muscle mass
Introduction
Despite substantial mean life expectancy gains, adolescents with cystic fibrosis (CF) continue to experience delayed growth, short stature and poor musculoskeletal characteristics even when meeting nutritional requirements [1]. Encouraging results from The National Cooperative Growth Study [2], led to evaluation of rhGH treatment in children with CF [3]. Compared to untreated counterparts, rhGH improved pulmonary function, linear growth, and decreased hospitalization frequency although higher circulating insulin levels was observed. Resistance training (RT) can impact several hormones including GH, insulin like growth factor, and testosterone which are requisite for adequate growth, musculoskeletal development as well as maintenance of glucoregulatory control [4]. In addition, following RT protein breakdown is rapidly attenuated and anabolic processes are heightened, speculatively leading to an augmented response combining rhGH with RT on clinical outcomes in CF Further. Despite the many diverse health benefits [5] and the critical importance of the adolescent developmental stage for long term hypertrophic adaptations the potential long-term benefits of RT for patients with CF have not been fully evaluated, particularly in combination with rhGH. We present a 15 year old male with CF who demonstrated significant improvement in growth after combined RT with rhGH treatment.
Patient
At age six months, the patient was admitted to the hospital for FTT and diagnosed with CF, confirmed by sweat test and genetic homozygosity for the F508 del-CFTR mutation. He experienced recurrent hospitalizations for CF-related complications (e.g., bacterial and fungal infections, respiratory complications) thereafter. DXA performed beginning at age 9.5 y revealed a total body bone mineral density (BMD) z-score of −0.7. Subsequently, his BMD z-score gradually increased to −0.5 and 0.1 annually until assessed at 12.75 y, the typical age when pubertal progression accelerates for males, when it dropped to −0.8. Due to decelerated growth, failure to gain weight, HbA1c of 6.0% and elevated glucose concentrations (>140 mg/dL), low dose basal insulin was prescribed, and a gastronomy tube (G-tube) was placed to meet nutritional needs. At 12.9 y, his weight, height and BMI were at the 5th 12th 9th percentile, respectively. After continued poor growth (i.e., velocity of 3.6 cm/year, BMD z-score −1.2) and pubertal delay the patient began rhGH (somatotropin) therapy at age 13.3 y. Prior to starting rhGH, a growth hormone stimulation test revealed a peak response of 7.2 ng/dL (arginine stimulation) and 6.2 ng/dL (L-dopa stimulation).
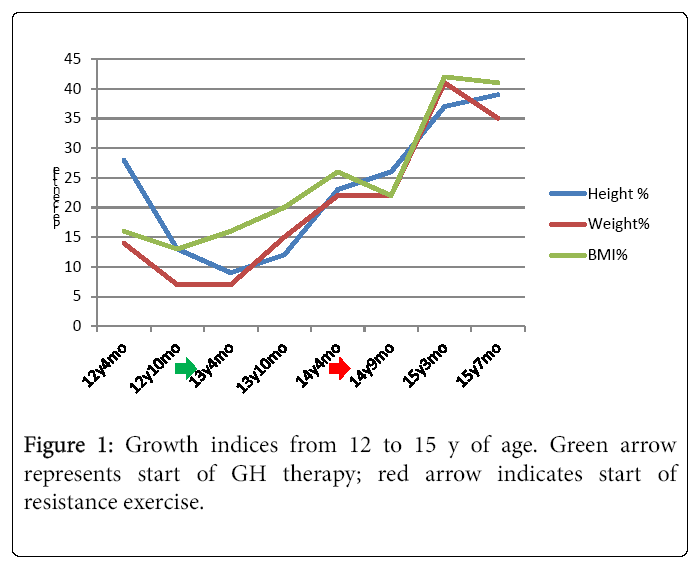
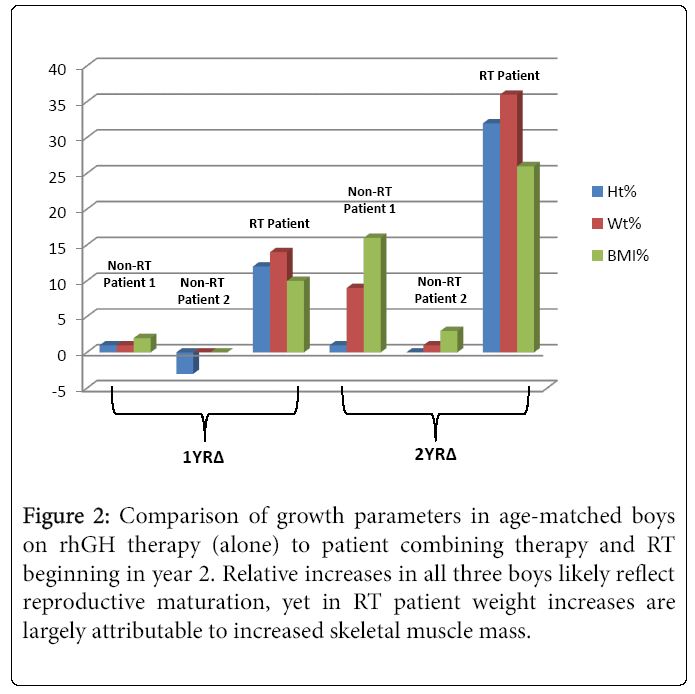
Over the course of one year of rhGH treatment, his weight, height and BMI increased by 9.4 kg, 11.9 cm, and 10 centiles, respectively, yet he continued to plot in the “nutrition failure” category. He received 50% of his caloric needs and 100% of this protein needs via G-tube. “Feeling better” and desiring more weight and muscle development, he began a supervised RT program. Over four months, he participated in approximately two 40 minute sessions/month with a personal trainer targeting different muscle groups using free weights. RT was initially supervised, 50% 1 RM, 10-12 reps, 3 sets three days/week. This was progressively increased and eventually, after 6 sessions conducted unsupervised. Muscle groups were divided into chest and arms on Day 1, Back and shoulders on day 2 and legs on Day 3. Warm-up included a treadmill walking with progressive increase in speed from 4 mph initially to 6 mph by 4 months. With increased appetite and tolerance to food, he reduced his complementary G-tube-feeding to ~ three days/week. At age 15.2 y, his heretofore declining BMD z-score reconciled 0.3 points. He then began participating regularly in RT three days/week and meeting 100% caloric requirements orally. Several weeks there after he began a supervised cardiovascular exercise component to his workout sessions. This included a 20 minute treadmill run, in which he has steadily increased his speed from 3.2-4.8 miles/hour. In two months, his height, weight and BMI rose 13 (47th percentile), 4 (41st percentile), and 10 (48th percentile) centiles, respectively. Moreover, his HbA1c decreased to 5.6%. Figure 1 illustrates the height, weight, and BMI percentiles from age 12.3-15.4 y. Notably, he far exceeded the two year change for the other two rhGH treated patients who did not participate in RT but were treated with rhGH (Figure 2). His DXA demonstrated a two centile BMD z-score increase, all absolute measures of body composition compartments increased, and percent lean mass rose approximately 3.5% offsetting an approximate 3.5% decrease in percent fat mass.
Figure 2: Comparison of growth parameters in age-matched boys on rhGH therapy (alone) to patient combining therapy and RT beginning in year 2. Relative increases in all three boys likely reflect reproductive maturation, yet in RT patient weight increases are largely attributable to increased skeletal muscle mass.
Discussion
The characteristic decrease in myofiber size has been noted in skeletal muscle lacking functional GH receptors as well as a potential effect on testosterone mediated protein synthesis at least in part contributes to the poor musculoskeletal growth and function in adolescents with CF. Interestingly, although whole body protein synthesis increased with rhGH treatment, increased skeletal muscle protein synthesis is limited, leading to the supposition that the anabolic effects of rhGH therapy may be limited to synthesis of noncontractile tissue (i.e., collagen). While growth related parameters would be expected to increase, in the disease state, overall health gains would be most substantial with increased lung function. The patient response to GH was primarily observed in linear growth aspects. There were no significant changes in pulmonary function with initiation of GH treatment alone. However, resistance training in combination with GH promoted FVC increase. Furthermore, rhGH administration does not necessarily mimic the in vivo response to exercise-induced GH secretions or receptor interactions either temporally or in magnitude. Considering that the anabolic milieu is primed during the early puberty it is conceivable that hormonal changes related to RT may facilitate muscular interactions requisite in optimizing development. Across populations and disease states, the capacity to maintain musculoskeletal function is among the strongest determinants of healthy aging. For adolescents with CF, participation in RT may enhance the capacity to optimize musculoskeletal mass and function during the critical developmental period with the greatest impact. Skeletal muscle, the largest glucose-utilizing organ, is an obvious target for improved metabolic health. In response to targeted progressive musculoskeletal overload, growth-inducing hormones which peak during puberty (e.g., insulin, testosterone and GH) are elevated post- RT, enabling utilization of available glucose and other nutrients for muscle protein synthesis. We speculate the progressive muscle activation via RT led to recruitment of greater muscle fibers, which in turn enabled greater hormone-tissue interaction. In this male, two years of GH therapy and one year of RT contributed to a rise in height, weight and BMI by 28, 34 and 26 centiles, respectively. Concomitantly, his BMD z-score nearly doubled, HbA1c normalized, and he was able to meet nutrient needs orally.
Although the patient’s linear growth is in part attributable to pubertal age and rhGH treatment, a salutary effect of RT cannot be discounted, particularly when compared to two other maturity matched males who also received rhGH therapy without RT. Herein we demonstrate weight increases reflective of lean mass gains, increased glucoregulatory control, and overall improved health with combined RT. Although a single patient experience, combining RT and rhGH treatment is viable strategy for optimizing growth parameters in adolescents with CF.
Acknowledgments
We would like to acknowledge the patient, his family, his clinical care providers, Valerie Tarn, MS, RDN, his dietitian, and Hector Gutierrez, MD, his primary care provider, in particular, for their valuable contributions.
References
- Orenstein DM, Winnie GB, Altman H (2002) Cystic fibrosis: a 2002 update. The Journal of Pediatrics 140:156-164.
- Root AW, Kemp SF, Rundle AC, Dana K, Attie KM (1998) Effect of long-term recombinant growth hormone therapy in children-the National Cooperative Growth Study, USA, 1985-1994. Journal of pediatric endocrinology & metabolism 11: 403-412.
- Hardin DS, Ellis KJ, Dyson M, Rice J, McConnell R, et al. (2001) Growth hormone improves clinical status in prepubertal children with cystic fibrosis: results of a randomized controlled trial. The Journal of paediatrics 139: 636-642.
- Stalvey MS, Anbar RD, Konstan MW, Jacobs JR, Bakker B,et al. (2012) A multi-center controlled trial of growth hormone treatment in children with cystic fibrosis.Pediatric Pulmonology 47: 252-263.
- Orenstein DM, Hovell MF, Mulvihill M, Keating KK, Hofstetter CR, et al. (2004) Strength vs. aerobic training in children with cystic fibrosis: A randomized controlled trial. Chest 126: 1204-1214.
Relevant Topics
- Aminoacid Suppliments
- Bodybuilding Nutrition
- Clinical Sports Nutrition
- Creatine Sports Nutrition
- Diet
- Fitness Nutrition
- Food and Nutrition
- Gym Suppliments
- Herbal Suppliments
- Micronutrients
- Natural Suppliments
- Nutrition Sport Fitness
- Nutritional Health
- Protein Diet
- Protein Suppliments
- Sports Nutrition Suppliments
- Vitamin Supplement
Recommended Journals
Article Tools
Article Usage
- Total views: 10516
- [From(publication date):
March-2016 - Jul 04, 2025] - Breakdown by view type
- HTML page views : 9667
- PDF downloads : 849