Review Article Open Access
Research on Nanotoxicity of an Iron Oxide Nanoparticles and Potential Application
Ling Zhang, Hongmei Xie, Yingxun Liu and Jinke Wang*
1School of Biomedical Engineering, Hubei University of Science and Technology, Xianning 437000, China
2State key Laboratory of Bioelectronics, Southeast University, Nanjing 210096, China
- *Corresponding Author:
- Jinke Wang
State key Laboratory of Bioelectronics
Southeast University, Nanjing 210096, China
E-mail: wangjinke@seu.edu.cn
Received Date: July 17, 2017; Accepted Date: August 28, 2017; Published Date: September 01, 2017
Citation: Zhang L, Xie H, Liu Y, Wang J (2017) Research on Nanotoxicity of an Iron Oxide Nanoparticles and Potential Application. Toxicol Open Access 3:130. doi: 10.4172/2476-2067.1000130
Copyright: © 2017 Zhang L, et al. This is an open-access article distributed under the terms of the creative commons attribution license, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Toxicology: Open Access
Abstract
The iron oxide nanoparticles (FeNPs) are widely used in biomedicine for good biocompatibility. To promote its safe application, any potential nanotoxicity should be thoroughly and carefully investigated. This paper systematically summarizes our lab’s research on the nanotoxicity of iron oxide nanoparticles coated with dimercaptosuccinic acid (DMSA), including the effects of FeNPs on viability, apoptosis, cycle, and oxidative stress at cell level. In vitro studies revealed that the FeNPs showed obvious apoptosis of human acute monocyte cells (THP-1) and human hepatoma cells (HepG2) at the highest concentration. FeNPs resulted in common and cell typespecific nanotoxicities of the FeNPs to both human and mouse cells at the gene, disturbed cell’s iron and osmosis homeostasis by the internalization of FeNPs through releasing iron ion in cells, resulted in cytotoxicity of DMSA as coating molecules of FeNPs and the inhibitor of DNA binding/differentiation (Id) related nanotoxicity of FeNPs at gene level. The studies of our lab shed many new insights into the nanotoxicity of the nanoparticle. Furthermore, the toxicity may play more value if it is guided and applied reasonably, such as iron supplement, anti-oxidation, and immunotherapy.
Keywords
Iron oxide nanoparticles; Nanotoxicity; Gene expression profile
Introduction
The iron oxide nanoparticles (FeNPs) are widely applicated in biomedicine, such as magnetic resonance imaging (MRI) contrast agents, drug vectors and hyperthermia [1-3]. FeNPs are well-known unique magnetic properties and good biocompatibility relative to other popular metal nanomaterials [4]. Therefore, FeNPs have been the most intensively studied to commercialize in recent years. However, its potential nanotoxicity has been overlooked by academia and not masked by its bright application [5,6]. To promote its safe application, especially as a clinical agent, any potential nanotoxicity should be thoroughly and carefully evaluated. Some important nanotoxicities of FeNPs have been therefore discovered, such as inductions of cell inflammation [7], mitochondrial injury [8-10], detriment to cell viability [9,11], reactive oxygen species (ROS) [8,9,12,13], apoptosis [9,14,15], oxidative stress [15-17], cell motility impairment [12], autophagy [8,9], and DNA damage [16,17].
As most of these applications are based on the delivery of large numbers of nanoparticles onto or into the cells of interest [18], more and more studies have paid attention to the resulted effects on cells and their degradation. Cells can promptly and moderately regulate its gene expression profile in response to any changes of intra- or extracellular environments. Therefore, detection of gene expression profile is helpful to explore the potential nanotoxicity of nanomaterials [13,19,20]. There are two advanced gene expression profiling techniques, including DNA microarray (genechip) [20,21], and RNA-seq [22]. Finding all genes differently expressed at the cell or tissue levels by a nanomaterial can provide valuable clues to investigate any potential toxicity and analyze the relevant molecular mechanism [13,19,20,23]. Therefore, more and more studies started to evaluate the nanotoxicity of various nanomaterials at the molecular level [13,20,22]. Many previous unknown nanotoxicities of a nanomaterial were thus exposed. For example, Kedziorek et al. studied the gene expression profile of neural stem cell line C 17.2 with DNA microarray and revealed early cellular responses to intracellular magnetic superparamagnetic iron oxide nanoparticles (SPIONs) [24]. The analysis of the gene expression profile found that various nanoparticles produced intracellular ROS and resulted apoptotic cell death, such as silica nanoparticle [19], silver nanoparticles [20], and magnetic nanoparticles [25].
Hamed Arami et al. reported that the effects of size, various additional molecular parameters on the surface of the iron oxide nanoparticles, molecular structure of the coating molecules, administered dose on their degradation rates, and transformation to plasma ferritin still require to be studied systematically in more accurate ways through developed characterization tools with higher mass sensitivities [26]. Our lab continuously and systematically studied the nanotoxicity of iron oxide nanoparticles coated with dimercaptosuccinic acid (DMSA), which possessed a mean particle size of 11 nm through TEM observation. This paper gives a summarily review of our studies on the iron oxide nanoparticle in recent years.
Characteristics and Internalization of FeNPs
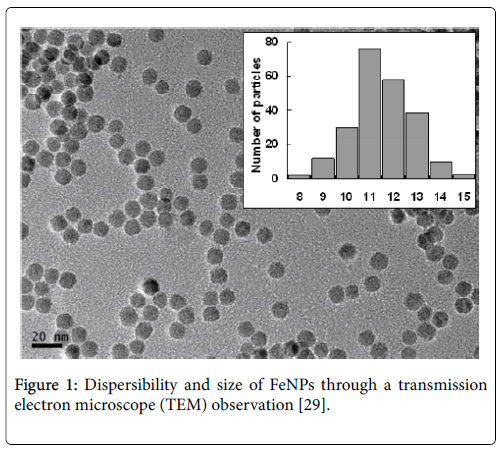
FeNPs showed monodispersed in water solution and had an average size of 11.0 ± 1.25 nm by TEM observation Figure 1. FeNPs were negatively charged and superparamagnetic properties. IR spectral analysis demonstrated that DMSA successfully coated the nanoparticles [27]. The results of Prussian blue staining displayed that FeNPs could be internalized into mouse mononuclear macrophages (RAW264.7), mouse hepatoma cells (Hepa1-6), human acute monocyte cells (THP-1), human hepatoma cells (HepG2), human cervical cancer (HeLa), and human normal liver cells (HL-7702). Thereamong, RAW264.7 was labeled more effectively than other cells at any concentration of the nanoparticles [28]. FeNPs were proved to locate in the cytoplasmic inclusions [29].
Figure 1: Dispersibility and size of FeNPs through a transmission electron microscope (TEM) observation [29].
Evaluation Biocompatibility of FeNPs at Cell Level
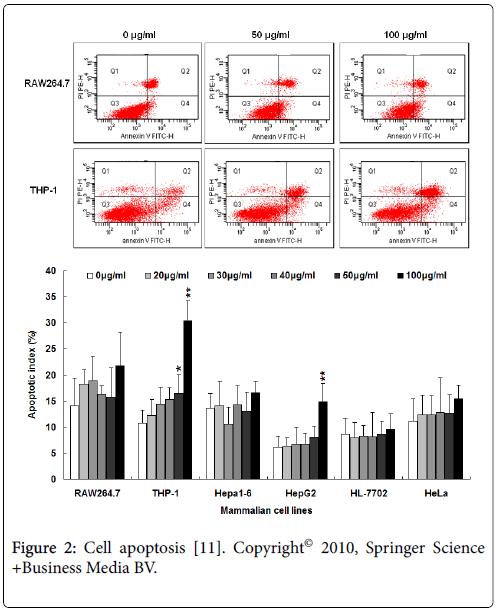
Evaluation biocompatibility of FeNPs at cell level mainly includes effects on viability, apoptosis, cycle, and oxidative stress, which are indispensable assessment of nanotoxicity. We investigated six different mammalian cell lines (RAW264.7, THP-1, Hepa1-6, HepG2, HL-7702, and HeLa) at six different concentrations (0,20,30,40,50 and 100 μg/mL) of FeNPs for 48 h. It is found that the viability of all the cells were not significantly repressed except HepG2 exposed to 100 μg/mL FeNPs. The detection of oxidative stress displayed that the levels of total superoxide dismutase and xanthine oxidase showed no significant changes, while the levels of malonyldialdehyde activity significantly increased. The nanoparticles did not produce any significant influence in cell cycles at any of the doses, but caused obvious apoptosis of THP-1 and HepG2 cells at the highest concentration Figure 2. These results reveal that 30 μg/mL FeNPs used in human studies with an intravascular nanoparticle imaging agent (Combidex) efficiently labeled all the cells studied, but did not produce any significant effects on their viability, oxidative stress, cycle, and apoptosis, indicating better biocompatibility and more useful clinical applications [11].
Figure 2: Cell apoptosis [11]. Copyright© 2010, Springer Science +Business Media BV.
Effects of FeNPs on Global Gene Expression Profile of Five Different Mammalian Cells
To investigate the nanotoxicity of FeNPs, we firstly detected gene expression profile of five different mammalian cells treated with different dose for different time, including two mouse cell lines, the RAW264.7 and Hepa1-6 cells, and three human cell lines, the THP-1, HepG2, and HL7702 cells. Thereamong, RAW264.7 and THP-1 are blood cell and belong to monocyte-macrophage system, while Hepa1-6, HepG2, and HL7702 are liver-derived hepatoma cells. These five cell lines are suitable for evaluating the nanotoxicity of FeNPs because the blood and liver cells are most intensively enriched the nanoparticles in vivo due to the intravenous administration and passive targeting. After data analysis, all differentially expressed genes of five cells under each treatment were thus identified. Thereamong,few genes were differentially expressed in HL7702 because of normal liver cells. We analyzed effects of FeNPs on global gene expression of RAW264.7 cells treated with two different doses (50 and 100 μg/mL) for 4 h, 24 h, and 48 h. The results demonstrated that FeNPs display cytotoxicity in this type of macrophage at high doses [27]. Then, by annotating the functions of the differentially expressed genes of four cancer cells, those common and cell type-specific nanotoxicities of the FeNPs to both human and mouse cells at the gene, biological process and pathway levels affected by the FeNPs were characterized, developing new insights into the nanotoxicity of the FeNPs to these cell lines [30,31].
Effects of FeNPs on Apoptosis Response Genes
Our previous study has revealed that high concentration of FeNPs could increase cell apoptosis and decrease cell viability of some specific mammalian cell types [32]. Apoptosis is an important biological process, which is closely related to evaluate toxicity of nanoparticles. We then investigated the global gene expression profiles of human THP-1 monocytes. It is found that 35 significant up-regulation genes at the concentrations of 100 μg/ml enriched three GO terms related to cell apoptosis, including apoptosis, death and programmed cell death by GO analysis [29]. However, it is only reported a small number of genes correlated with cell apoptosis were determined to be expressed differently via RT-PCR detection in some previous studies restricted by low throughput [33]. A few previous studies have already been performed to understand the mechanism of cell apoptosis induced by nanoparticles. Here, our studies demonstrate that the cell apoptosis was triggered by FeNPs via the caspase-10 mediated apoptosis pathway Figure 3. A large number of gene of cytokine were differentially expressed via NLR signaling pathway and TLR signaling pathway, such as IL-1, PIK3R1, NFKBIA, and TNFSF10. Consequently, the excessive activation of cytokines induced extrinsic apoptosis pathway [29].
Figure 3: KEGG analysis of apoptosis signaling pathway at the concentration of 100 μg/ml FeNPs [29].
Effects of FeNPs on the Transcription of Genes Related to Iron and Osmosis Homeostasis
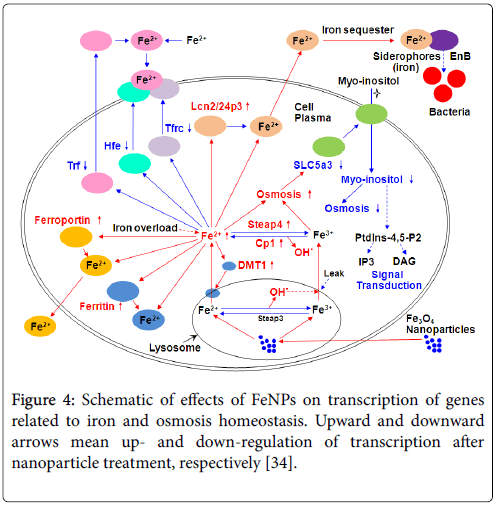
By checking effects of FeNPs on gene expression profile of RAW264.7 cells, we found that several important genes were responsible for intracellular iron homeostasis, which indicate that the iron homeostasis must be disturbed by the internalization of FeNPs through releasing iron ion in cells. Further experiments proved that the transcriptions of Trf , Tfrc , Lcn2 , and Hfe , relevant to iron metabolism, and Slc5a3 , Slc6a12 , related to osmosis were significantly changed by the FeNPs treatment [34]. The subsequent detection of cellular iron content demonstrated that the internalized FeNPs were degraded in lysosomes for its acid environment and thus released iron ions in cells, which damaged the iron and osmosis homeostasis of cells and thus lead to cell’s complementary responses, including repressing the expressions of Trf , Tfrc , and Hfe to prevent the transfer of extracellular iron ions into cells, inducing the expression of Lcn2 to promote the transfer of intracellular iron ions out of cells, and down regulating the expressions of Slc5a3 to prevent the transfer of extracellular myo-inositol (very important organic osmolyte) into cells Figure 4 [34]. The results provided valuable mechanistic insights into various FeNPs-induced toxicities [35].
Figure 4: Schematic of effects of FeNPs on transcription of genes related to iron and osmosis homeostasis. Upward and downward arrows mean up- and down-regulation of transcription after nanoparticle treatment, respectively [34].
Iron is essential to virtually all living organisms and involves in multiple metabolic functions, including oxygen transport in hemoglobin [36]. Iron deficiency anemia is prevalent. Furthermore, it is reported that 90% of patients older than 65 years with iron deficiency anemia suffer from a gastrointestinal cancer [35]. Iron oxide nanoparticles are attracting attention to be developed as iron supplement. For example, feraheme is approved by FDA for the treatment of iron deficiency anemia in adult patients with chronic kidney disease (CKD) [37]. FeNPs may be improved as another iron supplement in future.
Effects of DMSA Coating on the Expression of Genes Coding Cysteine-Rich Proteins
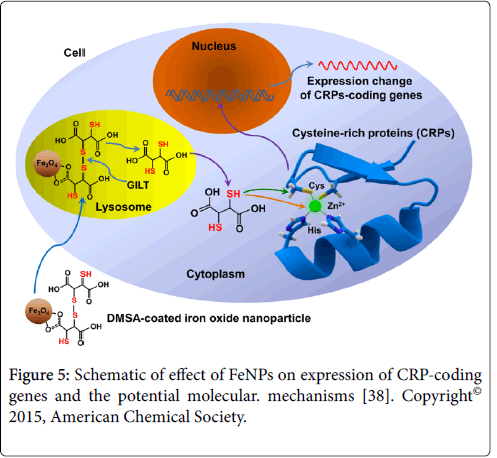
We found that about one fourth of the differentially expressed genes (DEGs) coded cysteine-rich proteins (CRPs) in all four mammalian cells under each treatment through analysis of DEGs [38]. DMSA contains abundant thiol groups. The results indicate that FeNPs greatly affected the expressions of CRP-coding genes. Furthermore, about 26% of CRP-coding DEGs were enzyme genes in all cells. Our further experiments revealed that the effect mainly resulted from DMSA carried into cells by the nanoparticles [38]. FeNPs were internalized into cells by endocytosis and positioned in lysosomes [39]. The lysosomal thiol reductase (GILT) catalyzes disulfide bond reduction under acidic conditions of lysosome [40]. The produced abundant thiol groups can directly bind with both cysteines and metal ions in cysteine-rich proteins, which are nearly transcription factors. Therefore, a large number of target genes were regulated differently Figure 5. The bare FeNPs have hydrophobic surfaces and a propensity to agglomerate [41]. It has been proved that the uncoated Fe3O4 nanoparticles are toxic to cells [42]. Thus, FeNPs are often produced with various coating molecules, such as 2,3-dimercaptosuccinic acid (DMSA) [43]. It was reported that DMSA coating could improve stability, internalization, biodistribution, and biocompatibility of FeNPs in vivo and in vitro [44]. DMSA is used as orally administered metal chelating agent receiving the approval of Food and Drug Administration of USA [45]. DMSA alone showed low toxicity in various biological systems due to its extracellular distribution [46]. Our study thus firstly reported the cytotoxicity of DMSA as coating molecules of FeNPs at the gene transcription level [38].
It is well known that free radicals cause the oxidation of biomolecules, such as protein, lipid, and DNA, which results cell injury and death [47]. Free radicals is deleterious to mammalian cells and involves in the pathogenesis of many chronic diseases [48]. FeNPs with rich thiol groups may be available antioxidants to remove excess free radicals by redox reactions.
Effects of FeNPs on the Expression of Id Genes
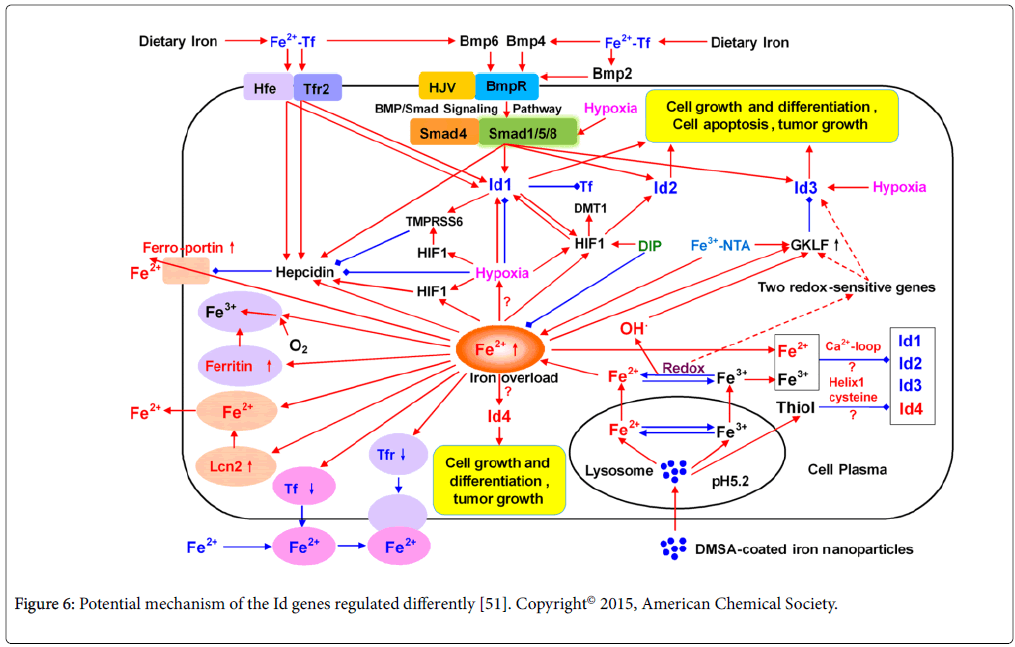
In our DNA microarray studies, it is worth to note that the transcription of the Id3 gene was significantly down-regulated in five cell lines (RAW264.7, Hepa1-6, THP-1, HepG2, and HL7702) treated with FeNPs at two doses. Id genes of the inhibitor of DNA binding/ differentiation code for a class of well-known helix-loop-helix (HLH) transcription factors, which are related to cell growth, proliferation, differentiation, lineage commitment, tumor cell aggressiveness and metastatic behavior [49,50]. We then did more investigations on the effect of FeNPs upon the Id genes. We detected the expression of Id genes in six cell lines (the above cell lines plus HeLa) at the same conditions through quantitative PCR. Under each treatment, the Id1, Id2, and Id3 gene was significantly down-regulated in both cell lines and the liver tissues of mice, while Id4 gene was obviously upregulated. These results reveal that the nanoparticle exerts a significant effect on the in vitro and in vivo expression of Id genes [51]. FeNPs may regulate the Id genes via hypoxia, iron ions or redox and hydroxyl radicals Figure 6. To our knowledge, it is the first report of this cellular effect of FeNPs. Our study thus provides new insights into the Idrelated nanotoxicity of FeNPs and the close relationship between the regulation of Id genes and iron [51].
Figure 6: Potential mechanism of the Id genes regulated differently [51]. Copyright© 2015, American Chemical Society.
Since Id3 gene was significantly down-regulated in six cell lines, which can be identified as a nanotoxicity biomarker of the FeNPs. It is reported that overexpressed Id3 inhibited cell proliferation and induced apoptosis and may be a potential target for tumor suppression [52]. Here, downregulated Id3 may activate cell proliferation, such as T cell, macrophages and may be a potential drug for immunodeficiency therapy.
Effects of FeNPs on the Immune System
Our previous study revealed that intravenously-injected FeNPs were passive targeted to the liver and spleen [51], which are known classically part of the important immune system termed mononuclear phagocytic system (MPS) [53,54]. The MPS system includes macrophages located in different organs and monocytes circulating in the blood [55], clearing pathogens or foreign bodies such as viruses [56]. The previous data demonstrated that FeNPs induced response to virus and hepatitis C pathway in the THP-1 cells, TLR signaling pathway and JAK-STAT signaling pathway in the RAW264.7 cells, indicating that the nanoparticles may influence in immune responses of MPS like virus [30,31]. Immune system plays the most critical role in immunotherapy [57]. We are systematically investigating the effect of FeNPs on immune cells and explore the application of FeNPs in immunotherapy.
In summary, we investigate systematically the nanotoxicity of FeNPs at cell level and gene level Figure 7. The results show that FeNPs did not produce any significant influence in cell level at any of the doses, except that obvious apoptosis of THP-1 and HepG2 cells at the highest concentration. At gene level, we analyzed gene expression profile in depth. It is found that many genes were differently expressed, which were involved in cell growth, cell apoptosis, iron homeostasis, cysteinerich proteins, and immune response. The data analysis is helpful for extracting the useful biological information, finding new biological phenomena, postulating the possible underlying molecular mechanism and conceiving new hypothesis. More importantly, it can provide key clues and instructions for designing and performing new experiments for verify the conceived hypothesis, which shed many new insights into the nanotoxicity of the nanoparticle. The iron oxide nanoparticles show great application potential in biomedical field. The research on their nanotoxicity is most significant. At the same time, the toxicity may play more value if it is guided and applied reasonably.
Acknowledgements
This work was supported by the National Key Research and Development Program of China (2017YFA0205502) and the National Natural Science Foundation of China (61571119). We greatly thank LICOR Biosciences (Shanghai) for providing a free Pearl Imager machine for using in our lab in animal research.
References
- Gupta AK, Gupta M (2005) Synthesis and surface engineering of iron oxide nanoparticles for biomedical applications. Biomaterials 26: 3995-4021.
- Zhao Y, Song W, Wang D, Ran H, Wang R, et al. (2015) Phase-Shifted PFH@PLGA/Fe3O4 Nanocapsules for MRI/US Imaging and Photothermal Therapy with near-Infrared Irradiation. ACS Appl Mater Interfaces 7: 14231-14242.
- Blasiak B, Van Veggel FCJM, Tomanek B (2013) Applications of Nanoparticles for MRI Cancer Diagnosis and Therapy. J Nanomater 148578.
- Karlsson HL, Cronholm P, Gustafsson J, Möller L (2008) Copper oxide nanoparticles are highly toxic: a comparison between metal oxide nanoparticles and carbon nanotubes. Chem Res Toxicol 21: 1726-1732.
- Soenen SJ, De Cuyper M (2010) Assessing iron oxide nanoparticle toxicity in vitro: current status and future prospects. Nanomedicine (Lond) 5: 1261-1275.
- Singh N, Jenkins GJS, Asadi R, Doaka SH (2010) Potential toxicity of superparamagnetic iron oxide nanoparticles (SPION). Nano Rev 1.
- Liu Y, Chen Z, Gu N, Wang J (2011) Effects of DMSA-coated Fe3O4 magnetic nanoparticles on global gene expression of mouse macrophage RAW264.7 cells. Toxicol Lett 205: 130-139.
- Khan MI, Mohammad A, Patil G, Naqvi SA, Chauhan LK, et al. (2012) Induction of ROS, mitochondrial damage and autophagy in lung epithelial cancer cells by iron oxide nanoparticles. Biomaterials 33: 1477-1488.
- Yang FY, Yu MX, Zhou Q, Chen WL, Gao P, et al. (2012) Effects of iron oxide nanoparticle labeling on human endothelial cells. Cell Transplant 21: 1805-1820.
- Baratli Y, Charles AL, Wolff V, Ben Tahar L, Smiri L, et al. (2014) Age Modulates Fe3O4 Nanoparticles Liver Toxicity: Dose-Dependent Decrease in Mitochondrial Respiratory Chain Complexes Activities and Coupling in Middle-Aged as Compared to Young Rats. Biomed Res Int 474081.
- Liu Y, Chen Z, Wang J (2011) Systematic evaluation of biocompatibility of magnetic Fe3O4 nanoparticles with six different mammalian cell lines. J of Nanoparti Res 13: 199-212.
- Diana V, Bossolasco P, Moscatelli D, Silani V, Cova L (2013) Dose dependent side effect of superparamagnetic iron oxide nanoparticle labeling on cell motility in two fetal stem cell populations. PLoS One 8: e78435.
- Kedziorek DA, Muja N, Walczak P, Ruiz-Cabello J, Gilad AA, et al. (2010) Gene expression profiling reveals early cellular responses to intracellular magnetic labeling with superparamagnetic iron oxide nanoparticles. Magn Reson Med 63: 1031-1043.
- Liu YX, Huang JY, Wang DL, Wang JK (2013) Identification of DMSA-Coated Fe3O4 Nanoparticles Induced-Apoptosis Response Genes in Human Monocytes by cDNA Microarrays. Adv Mater Res 749: 377-383.
- Zhu MT, Wang Y, Feng WY, Wang B, Wang M, et al. (2010) Oxidative stress and apoptosis induced by iron oxide nanoparticles in cultured human umbilical endothelial cells. J Nanosci Nanotechnol 10: 8584-8590.
- Alarifi S, Ali D, Alkahtani S, Alhader MS (2014) Iron oxide nanoparticles induce oxidative stress, DNA damage, and caspase activation in the human breast cancer cell line. Biol Trace Elem Res 159: 416-424.
- Ahamed M, Alhadlaq HA, Alam J, Khan MA, Ali D, et al. (2013) Iron oxide nanoparticle-induced oxidative stress and genotoxicity in human skin epithelial and lung epithelial cell lines. Curr Pharm Des 19: 6681-6690.
- Pisanic TR 2nd, Blackwell JD, Shubayev VI, Fiñones RR, Jin S (2007) Nanotoxicity of iron oxide nanoparticle internalization in growing neurons. Biomaterials 28: 2572-2581.
- Li X, He Q, Shi J (2014) Global gene expression analysis of cellular death mechanisms induced by mesoporous silica nanoparticle-based drug delivery system. ACS Nano 8: 1309-1320.
- Foldbjerg R, Irving ES, Hayashi Y, Sutherland DS, Thorsen K, et al. (2012) Global gene expression profiling of human lung epithelial cells after exposure to nanosilver. Toxicol Sci 130: 145-157.
- Waters KM, Masiello LM, Zangar RC, Tarasevich BJ, Karin NJ, et al. (2009) Macrophage responses to silica nanoparticles are highly conserved across particle sizes. Toxicol Sci 107: 553-569.
- Simon DF, Domingos RF, Hauser C, Hutchins CM, Zerges W, et al. (2013) Transcriptome sequencing (RNA-seq) analysis of the effects of metal nanoparticle exposure on the transcriptome of Chlamydomonas reinhardtii. Appl Environ Microbiol 79: 4774-4785.
- Van Aerle R, Lange A, Moorhouse A, Paszkiewicz K, Ball K, et al. (2013) Molecular mechanisms of toxicity of silver nanoparticles in zebrafish embryos. Environ Sci Technol 47: 8005-8014.
- Kedziorek DA, Muja N, Walczak P, Ruiz-Cabello J, Gilad AA, et al. (2010) Gene expression profiling reveals early cellular responses to intracellular magnetic labeling with superparamagnetic iron oxide nanoparticles. Magn Reson Med 63: 1031-1043.
- Shim W, Paik MJ, Nguyen DT, Lee JK, Lee Y, et al. (2012) Analysis of changes in gene expression and metabolic profiles induced by silica-coated magnetic nanoparticles. ACS Nano 6: 7665-7680.
- Arami H, Khandhar A, Liggitt CD, Krishnan KM (2015) In vivo delivery, pharmacokinetics, biodistribution and toxicity of iron oxide nanoparticles. Chem Soc Rev 44: 8576-8607.
- Liu Y, Chen Z, Gu N, Wang J (2011) Effects of DMSA-coated Fe3O4 magnetic nanoparticles on global gene expression of mouse macrophage RAW264. 7 cells. Toxicol lett 205: 130-139.
- Liu Y, Wang J (2011) Comparative and quantitative investigation of cell labeling of a 12-nm DMSA-coated Fe3O4 magnetic nanoparticle with multiple mammalian cell lines. J Mat Res 26: 822-831.
- Liu YX, Huang JY, Wang DJ, Wang JK (2013) Identification of DMSA-coated Fe3O4 nanoparticles induced-apoptosis response genes in human monocytes by cDNA microarrays. Adv Mater Res Trans Tech 749: 377-383.
- Liu Y, Zou J, Wang X, Wang T, Wang J (2014) Effects of 2,3-dimercaptosuccinic acid-coated Fe3O4 nanoparticles on genes in two mouse cell lines. J Biomed Nanotechnol 10: 1574-1587.
- Zhang L, Wang X, Zou J, Liu Y, Wang J (2015) Effects of an 11-nm DMSA-coated iron nanoparticle on the gene expression profile of two human cell lines, THP-1 and HepG2. J Nanobiotechnology 13: 3.
- Liu Y, Wang J (2012) Effects of DMSA-coated Fe3O4 nanoparticles on the transcription of genes related to iron and osmosis homeostasis. Toxicol sci 131: 521-536.
- Orrenius S, Nicotera P, Zhivotovsky B (2010) Cell death mechanisms and their implications in toxicology. Toxicol Sci 119: 3-19.
- Liu Y, Wang J (2013) Effects of DMSA-coated Fe3O4 nanoparticles on the transcription of genes related to iron and osmosis homeostasis. Toxicol Sci 131: 521-536.
- Killip S, Bennett JM, Chambers MD (2007) Iron deficiency anemia. Am Fam Physician 75: 671-678.
- Naigamwalla DZ, Webb JA, Giger U (2012) Iron deficiency anemia. Can Vet J 53: 250-256.
- Castaneda RT, Khurana A, Khan R, Daldrup-Link HE (2011) Labeling stem cells with ferumoxytol, an FDA-approved iron oxide nanoparticle. J Vis Exp: e3482.
- Zhang L, Wang X, Zou J, Liu Y, Wang J (2015) DMSA-Coated Iron Oxide Nanoparticles Greatly Affect the Expression of Genes Coding Cysteine-Rich Proteins by Their DMSA Coating. Chem res toxicol 28: 1961-1974.
- Villanueva A, Cañete M, Roca AG, Calero M, Veintemillas-Verdaguer S, et al. (2009) The influence of surface functionalization on the enhanced internalization of magnetic nanoparticles in cancer cells. Nanotechnology 20: 115103.
- Phan UT, Maric M, Cresswell P (2002) Disulfide reduction in major histocompatibility complex class II-restricted antigen processing by interferon-gamma-inducible lysosomal thiol reductase. Meth Enzymol 348: 43-48.
- Cheng FY, Su CH, Yang YS, Yeh CS, Tsai CY, et al. (2005) Characterization of aqueous dispersions of Fe3O4 nanoparticles and their biomedical applications. Biomaterials 26: 729-738.
- Häfeli UO, Pauer GJ (1999) In vitro and in vivo toxicity of magnetic microspheres. J of mag magnet mater 194: 76-82.
- Valois CR, Braz JM, Nunes ES, Vinolo MA, Lima EC, et al. (2010) The effect of DMSA-functionalized magnetic nanoparticles on transendothelial migration of monocytes in the murine lung via a ß 2 integrin-dependent pathway. Biomaterials 31: 366-374.
- Chen Z, Zhang Y, Zhang S, Xia JG, Liu JW, et al. (2008) Preparation and characterization of water-soluble monodisperse magnetic iron oxide nanoparticles via surface double-exchange with DMSA. Colloid Surface A 316: 210-216.
- Ercal N, Treeratphan P, Hammond TC, Matthews RH, Grannemann NH, et al. (1996) In vivo indices of oxidative stress in lead-exposed C57BL/6 mice are reduced by treatment with meso-2, 3-Dimercaptosuccinic Acid or N-acetylcysteine. Free Radic Biol Med 21: 157-161.
- Aposhian HV, Aposhian MM (1990) meso-2,3-Dimercaptosuccinic acid: chemical, pharmacological and toxicological properties of an orally effective metal chelating agent. Annu Rev Pharmacol Toxicol 30: 279-306.
- Fridovich I (1999) Fundamental aspects of reactive oxygen species, or what's the matter with oxygen? Ann N Y Acad Sci 893: 13-18.
- McCord JM (2000) The evolution of free radicals and oxidative stress. Am J Med 108: 652-659.
- Norton JD (2000) ID helix-loop-helix proteins in cell growth, differentiation and tumorigenesis. J cell sci 113: 3897-3905.
- Benezra R (2001) Role of Id proteins in embryonic and tumor angiogenesis. Trends Cardiovasc Med 11: 237-241.
- Zou J, Wang X, Zhang L, Wang J (2015) Iron nanoparticles significantly affect the in vitro and in vivo expression of Id genes. Chem res toxicol 28: 373-383.
- Chen FF, Liu Y, Wang F, Pang XJ, Zhu CD, et al. (2015) Effects of upregulation of Id3 in human lung adenocarcinoma cells on proliferation, apoptosis, mobility and tumorigenicity. Cancer gene ther 22: 431-437.
- Ferrari M (2005) Cancer nanotechnology: opportunities and challenges. Nat Rev Cancer 5: 161-171
- Peer D, Karp JM, Hong S, Farokhzad OC, Margalit R, et al. (2007) Nanocarriers as an emerging platform for cancer therapy. Nat Nanotechnol 2: 751-760.
- Singh I (2006) Textbook of human neuroanatomy. Jaypee Brothers Publishers.
- Mashimo Y, Mie M, Suzuki S, Kobatake E (2011) Detection of small RNA molecules by a combination of branched rolling circle amplification and bioluminescent pyrophosphate assay. Anal Bioanal chem 401: 221-227.
- Masihi KN (2001) Fighting infection using immunomodulatory agents. Expert Opin Biol Ther 1: 641-653.
Relevant Topics
- Aflatoxins
- Cardiac Toxicity
- Chemical Toxicology
- Developmental Toxicology
- Drug Toxicity
- Heavy Metal Toxicity
- Heavy Metal Toxins
- Industrial Hygiene Toxicology
- Insecticides Toxicology
- Metal Toxicology
- Nano Toxicology
- Pesticidal Toxicology
- Renal Toxicity
- Reproductive Toxicology
- Skin Toxicology
- Tetanus Toxin
- Toxicogenomics
- Toxicology Reports
- Toxicology Testing
Recommended Journals
Article Tools
Article Usage
- Total views: 5009
- [From(publication date):
September-2017 - Apr 04, 2025] - Breakdown by view type
- HTML page views : 3925
- PDF downloads : 1084