Research Article Open Access
Reproductive Endocrinology of Female Crustaceans: Perspective and Prospective
CH. Swetha 1, S.B. Sainath 1, P. Ramachandra Reddy 2 and P. Sreenivasula Reddy3*1Department of Biotechnology, Sri Venkateswara University, Tirupati-517 502, AP, India
2Department of Biochemistry, Yogi Vemana University, Kadapa-516 003, AP, India
3Department of Zoology, Sri Venkateswara University, Tirupati-517 502, AP, India
- *Corresponding Author:
- Dr. P. Sreenivasula Reddy
Department of Zoology
Sri Venkateswara University
Tirupati-517 502, India
Tel. +91-877-2249320
Fax. +91-877-2249611
E-mail: reddy_1955@yahoo.co.in
Received date: October 03, 2011; Accepted date: December 23, 2011; Published date: December 26, 2011
Citation: Swetha CH, Sainath SB, Reddy PR, Reddy PS (2011) Reproductive Endocrinology of Female Crustaceans: Perspective and Prospective. J Marine Sci Res Development S3:001. doi: 10.4172/2155-9910.S3-001
Copyright: © 2011 Swetha CH, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Marine Science: Research & Development
Abstract
Reproduction in crustaceans is a highly complex process that requires precise coordination of external and internal factors to be successful. The field of crustacean reproductive endocrinology has moved from the classical approach of endocrinological techniques such as extirpation and additive methods, to the modern era of advanced biochemical, immunological, molecular biology and recombinant DNA technology methods. During last two decades, extensive endeavor has been captivating on the crustacean endocrines regulating reproduction, to authenticate their roles in the regulation of reproduction as well as their biochemical and molecular mechanism of action. In the present chapter, we have tried to recapitulate recent developments in molecular advances taken place in the reproduction regulation of crustacea via hormones, opioids, neurotransmitters and other molecules. In addition to providing a review of the scientific literature, we have also included our perspectives.
Keywords
Reproduction; Eyestalk neuropeptides; Neurotransmitters; Methyl farnesoate; Ecdysteroids
Introduction
Aquaculture is a rapidly growing global industry, comprising cultivation of various freshwater and marine species of finfish, shellfish (crustaceans), molluscs, and ornamental fish. Cultivation of crustacean species play very important role in the world aquaculture industry. It has the capacity to produce large amount of proteinaceous food, to meet the food demand for ever growing human population. Marine shrimps, prawns, lobsters and crabs are valuable sources of food, and thus of considerable economic importance to fishing industries throughout the world. Foods produced in crustacean aquaculture are often more expensive than other sources of animal protein. Perhaps, the most highly priced decapods are the true lobsters (Homarus americanus), regarded as the delicacies both in North America and in Europe. Developing of culture with inferior quality seed (susceptible to bacterial and viral infections) in the pond causes major damage to the aquaculture industry. Limited availability of quality and healthy seed constitutes the largest ultimate cause of destruction of aquaculture industry in recent past. In many respects, aquaculture is still in its infancy in the coastal region, and there is a pressing need to formulate strategies aimed at improving the income and assuring the availability of affordable protein to coastal communities. To accomplish this, it is necessary to improve the costal aquaculture by producing high quality seed from commercially important crustaceans. Fortunately due to a resurgent interest in invertebrate models for comparative studies and their commercial applications in the aquaculture industry, crustacean reproductive physiology is drawing renewed attention.
In the hatcheries, classical eyestalk ablation technique is employed to induce the reproduction in brood stock crustaceans. This method leads to production of inferior quality seed and often causes death in brood stock. To overcome this problem several research groups are working in many directions to induce successful reproduction especially on vitellogenin regulation in crustaceans throughout the world. One of the best ways to induce successful reproduction in crustaceans without damaging the brood stock and with high quality seed production is manipulation of endocrines at molecular level.
The regulation of reproduction in crustaceans is highly diverse [1] and is known to be controlled primarily by eyestalk neuropeptides, biogenic amines, opioids, gonad stimulating hormone, methyl farnesoate, ecdysteroids and vertebrate type steroids. Considerable extension has taken place in the crustacean reproduction with respect to the molecular approaches of endocrine hormones and other factors. In order to provide an overview of the state-of-the-art with regard to crustacean reproductive physiology, this chapter will consist of a brief description of the female reproductive system and its function, and the major advances in our understanding of regulation of reproduction. This chapter is not intended to be comprehensive. The reader is directed to several comprehensive reviews that discuss some aspects of the material covered in this review [2-7].
Description of Female Reproductive System
Most crustaceans have separate sexes, and these can be easily distinguished by observing the appendages on the abdomen called pleopods in shrimps or by observing the brood sac in crabs. The reproductive system of female crustaceans consists of the paired ovaries, oviducts, gonophores and an external sperm reception area. The decapod ovary is a paired and elongated tubular structure, whose transverse sections are identical from one location to other. The anteriolateral extensions lie dorsal to the hepatopancreas and are connected by a commissure located on the posterior edge of the stomach. A seminal receptacle is attached to each of the oviduct leading from the two branches at the anterior end of the pericardium and terminating in a medioventral gonopore.
Identification of Reproductive Stages
The maturation of the ovary can be grossly observed as it undergoes a series of changes in colour and increases in size during development. Different methods were employed by various workers in the identification of reproductive stages. In many studies morphological changes (colour) and gonad index were used as a marker for gonad maturation. In the freshwater crab, Oziotelphusa senex senex, the immature ovaries and adult previtellogenic ovaries are small, translucent to opaque white. With the onset of primary vitellogenesis, the ovary becomes a pale yellow (vitellogenic stage I). During secondary vitellogenesis, the ovary becomes orange (vitellogenic stage II) and it then becomes brown to dark brown prior to spawning (vitellogenic stage III). Maturation of the ovary in the crab Oziotelphusa senex senex also includes an increase in the size of the ovary as the oocytes proliferate and increase in diameter, due to yolk deposition.
In addition to the above, the histological examination of gonad at different stages of reproduction was used as an index for reproduction in crustaceans. Histological examination of ovary helps not only to determine the size of oocytes (oocyte diameter), but also to examine the yolk (vitellogenin) deposition in oocytes. Based on the cytological studies, vitellogenesis or yolk synthesis has been divided into two phases, primary and secondary. The onset of primary or endogenous vitellogenesis begins with the accumulation of proteinaceous and glycoproteinaceous granules on the rough endoplasmic rerticulum, where they are used to form yolk globules. Recently, determination of hemolymph and ovarian vitellogenin levels were used as an index for ovarian maturation [8].
Regulation of Reproduction
The regulation of reproduction in crustaceans is highly diverse, and mediated by both external and internal factors.
External Factors
Reproduction in crustaceans is cyclical in relation to seasonal changes. Environmental factors, such as temperature, photoperiod, food availability and salinity, all influence reproductive activity [9]. Photoperiod regulates the timing of ovarian growth. In crayfish Orconectes virilis, increased light hours accelerated the ovarian cycle of maturation, whereas in freshwater crab, Oziotelphusa senex senex, decreased light hours stimulated ovarian growth. The effects of temperature on ovarian growth and egg hatching have been observed in a number of crustaceans. A warmer water temperature induces egg laying in Orconectes [10] and increases the frequency of egg hatching in Artemia salina [11]. Li C et al., [12] also observed an increase in total fecundity and reproduction frequency in Pseudodiaptomus dubia as the water temperature increases, with the maximum at 30°C. Food abundance is essential for normal oocyte development, providing materials to synthesize yolk proteins and lipids. Starvation induced oocyte resorption and stimulated oocyte lipid conversion to polysaccharides, which are then released into the hemolymph.
Endogenous Factors
Gonad maturation in female crustaceans is regulated by both neuroendocrine and non-neuroendocrine secretions. A number of hormones from neuroendocrine organs play an important role in controlling gonad maturation [13-17]. Gonad maturation in crustaceans appears to be principally regulated by two antagonistic neuropeptides: GIH (also called vitellogenesis inhibiting hormone, VIH, in females) synthesized and secreted from the X-organ–sinus gland (XO-SG) complex of the eyestalk and gonad stimulating hormone (GSH), thought to be produced by the brain and thoracic ganglion. Besides these, methyl farnesoate and ecdysteroids secreted by mandibular organs and Y-organs respectively, are also involved in the regulation of female reproduction.
Role of eyestalks in regulation of reproduction: Since last century, the classical eyestalk extirpation experiments are in practice and revealed the involvement of eyestalk neuropeptides in the regulation of reproduction in several crustaceans. Panouse [18] was the first to demonstrate the existence of ovarian maturation inhibitory factor in the eyestalks of Leander serratus. This was further confirmed in Orconectes virulis [19] and Uca pugilator [20]. Accelerated ovarian development after eyestalk ablation was reported in the crayfish Procambarus clarkii [21]. Eyestalk ablation (unilateral and bilateral) induced precocious ovarian maturation in the Indian white shrimp Penaeus indicus [22]. Okumura and Aida [23] have studied the effect of bilateral eyestalk ablation on ovarian development in the freshwater prawn Macrobrachium rosenbergii and observed the shortened reproductive cycles in the destalked female prawns. In the kuruma prawn Marsupenaeus japonicus, Okumura and Sakiyama [24] have demonstrated the induced ovarian development (measured gonado somatic index as a reproductive end point) after eyestalk ablation in non-reproductive periods.
In several crustaceans, eyestalk ablation also resulted in premature yolk deposition in the ovary, both during the non-breeding and breeding seasons [25,26]. In the crabs Paratelphusa hydrodromous [25] and Scylla serrata [26] induction of pre-pubertal stages was observed after eyestalk ablation. Bilateral eyestalk ablation caused an increase in gonadosomatic index in immature prawns (M. japonicus) and also increased vitellogenin m-RNA levels in the ovary [27]. In the immature female kuruma prawn M. japonicus bilateral eyestalk ablation induced ovarian development and increased ovarian weight, hemolymph vitellogenin levels and vitellogenin mRNA levels in the ovary significantly [28]. The above studies clearly indicate that eyestalk ablation accelerates the process of ovarian maturation in crustaceans.
The medulla terminalis ganglionic X-organ-sinus gland complex is the major endocrine control center, analogous to vertebrate hypothalamo-neuro-hypophyseal complex, and located bilaterally in the eyestalks of most stalk-eyed crustaceans [3]. The X-organ is made with a cluster of neurosecretory somata. The axons of this X-organ cells form a major nerve in each eyestalk that converges upon a lateral hemolymph sinus where their dilated axon terminals form a discrete storage and releasing site i.e., sinus gland [29]. In crustaceans, hormones produced by the X-organ are stored in the sinus gland, to be secreted under the appropriate stimuli. The major peptide hormones secreted by the sinus gland are the following: MIH (molt inhibiting hormone), VIH (vitellogenesis inhibiting hormone), MOIH (mandibular organ inhibiting hormone), and CHH (crustacean hyperglycemic hormone); collectively called CHH-family peptides. MIH, VIH and MOIH target other endocrine glands, while CHH has somatic tissues as target. All these peptide hormones possess over-lapping biological activities [30- 33]. In addition to CHH-family peptides, the chromatophore regulating peptides viz. pigment dispersing hormone (PDH) and red pigment concentrating hormone (RPCH), and the neurotransmitters like enkephalins, serotonin, melatonin and dopamine were also produced in and released from the crustacean eyestalks. The involvement of eyestalk peptides and other molecules secreted by eyestalk is summarized below:
Vitellogenesis/Gonad-inhibiting hormone (VIH/GIH): The principal reproduction inhibitory principle present in the eyestalk is named as gonad-inhibiting hormone or vitellogenisis-inhibiting hormone (GIH/VIH). GIH was first demonstrated in the female prawn Palaemon (Leander) serratus, where removal of the eyestalk during sexual inactivity led to rapid increase in ovarian size and precocious egg deposition, apparently because of the removal of GIH [18]. Since then, several workers demonstrated the VIH-induced inhibition of ovarian growth in many crustaceans [34-40].
The VIH peptide has been characterized both structurally and functionally in limited species of crustaceans. In H. americanus Soyez et al., [41] elucidated the primary structure of two VIH isoforms. The VIH isolated from the eyestalks of Procambarus bouvieri is characterized by incubating the peptide with ovarian fragments in vitro and observed the reduced levels of vitellogenin transcripts in the ovarian fragments [42]. The gene for VIH has been cloned from several crustacean species including Homarus gammarus [36], the American lobster H. americanus [43], the Norway lobster Nephrops norvegicus [35], the giant river prawn Macrobrachium rosenbergii [44], the shrimp Rimicaris kairei [45] and the woodlouse Armadillidium vulgare [46]. Molecular analysis of VIHs isolated from females of a few crustacean species shows that they consist of signal peptide (20-31 amino acid residues) and mature peptide (77-83 amino acid residues). They also show a considerable degree of sequence similarity with MIH, including the conservation of six cysteine residues at the same virtual locations forming three intra-molecular disulfide bonds (1-5; 2-4; 3-6 fashion), which aid to maintain the VIH tertiary structure [47-49].
Ollivaux et al., [36] cloned and sequenced the cDNA of VIH precursor from X-organ RNA [36]. Edomi et al., [35] synthesized a recombinant protein encoding GIH of the Norway lobster Nephrops norvegicus. Using this recombinant protein, they also localized the VIH synthesizing cells in the eyestalks of the N. norvegicus. Ohira et al., [37], expressed recombinant VIH in bacterial system, then purified and allowed to refold. The refolded VIHs activity was determined using in vitro incubation of rHoa-VIH with M. japonicus ovarian fragments. The rHoa-VIH having amidated c-terminus showed significant reduction in the vitellogenin transcript levels in the ovary.
With the advances taking place in technology, the crustacean molecular endocrine research is also moving forward. The RNAi, is a post-transcription gene-silencing process in which double strand RNA triggers sequence-specific suppression of its cognate m-RNA is a powerful tool to reveal the gene function. In this direction, most recently the silencing of VIH has been reported in Penaeus monodon [39]. In this study, they observed the decreased VIH levels along with increased expression of vitellogenesis in the ovary by the functional knockdown of P. monodon VIH due to the double stranded Pem-VIH m-RNA injections in vivo.
Though the VIH is known to be involved in the regulation of vitellogenesis, the physiological mechanism of action of VIH-induced inhibition requires closed examination. Among the possibilities open to investigation are the following: (i) VIH may act directly on oocytes by inhibiting uptake of Vg or synthesis of yolk protein; this might be studied by in vitro culture of oocytes with or without Vg and a range of concentrations of pure VIH and (ii) by determination of GIH/VIH titer in hemolymph and mRNA levels during different reproductive stages. Another possibility is VIH may inhibit the release of a gonad stimulating hormone (GSH) from thoracic ganglia (TG) or brain of the central nervous system or MF from mandibular organ or ecdysteroid from Y-organ; determination of titers of GSH, MF and ecdysteroid will throw light on site of action of VIH. Clearly, more work is needed in this area.
Crustacean hyperglycemic hormone (CHH): The most abundant neurohormone stored in sinus glands of crustaceans is CHH which is principally involved in the regulation of glycemia [50,51]. Besides regulating carbohydrate metabolism, CHH is also involved in the regulation of onset of vitellogenesis and thereby reproduction. Van Herp [52] in his book chapter explained the involvement of CHH in the regulation of vitellogenesis.
De Kleijn et al. [43,34] have demonstrated the involvement of CHH-A and CHH-B of H. americanus in the regulation of onset of vitellogenesis. They found that HoaCHH-A is responsible for triggering the onset of vitellogenesis and HoaCHH-B is involved in the stimulation of oocyte development before spawning. Recently, Gu et al. [53] also demonstrated the role of CHH-A and CHH-B of Metapenaeus ensis in the regulation of vitellogenesis in the female shrimps and found that high levels of MeCHH-A are needed for the initial gonadal development (vitellogenesis stage I) and a high level of MeCHH-B is required for gonadal maturation during middle (vitellogenesis stage II) and late vitellogenesis (stage III). In contrast, it was also demonstrated that the CHH of Penaeus japonicus induced inhibition of protein and mRNA synthesis in in vitro incubated ovarian fragments of the marine shrimp P. semisulcatus [54].
It is also possible that different isomorphs of the CHH have different receptors in different tissues and exhibit different functions. In support of this, CHH receptors were identified on the Y-organ membrane preparations suggesting the role of CHH in the regulation of ecdysteroid synthesis [55-57]. Webster [58] has also demonstrated the presence of CHH receptors on the oocyte membranes of the crabs Carcinus maenas and Cancer pagurus. Zarubin et al. [57] investigated the effect of recombinant CHH on ecdysteroidogenesis in YOs of European green crab Carcinus maenas and observed 51% reduction in ecdysteroid levels. The above studies confirm that CHH isomorphs may have different functions in different tissues. However, the mechanism of action of CHH in reproduction regulation in crustaceans remains to be elucidated. In addition to CHH family peptides, several additional non-neuroendocrine products also play direct or indirect roles in regulating the reproductive process. For example, MF and ecdysteroids stimulate reproduction in crustaceans and are regulated by mandibular organ inhibiting hormone and molt inhibiting hormone respectively secreted from eyestalks [13-15].
Mandibular organ-inhibiting hormone (MOIH): It is well established that methyl farnesoate (MF), a sesquiterpenoid secreted from the mandibular organs of crustaceans is involved in the regulation of crustacean reproduction. Byard [59] and Waddy et al., [60] observed an increase in mandibular organ size during ovarian maturation. The implantation of mandibular organ into juvenile crab Libinia emarginata caused pre-mature vitellogenesis [61]. The secretory levels of MF by mandibular organs are high during the oocyte development [62]. Nagaraju et al., [64] observed the hypertrophy of mandibular organ coincides with the rise in the ovarian index during ovarian maturation. The above studies suggest the involvement of mandibular organ and its secretory product (MF) in the induction of ovarian maturation in crustaceans.
It is also well known that mandibular organ is under the negative control of eyestalk neuropeptide mandibular organ-inhibiting hormone (MOIH) [64,65]. In the lobster H. americanus unilateral and bilateral eyestalk ablation elevated MF levels (54.1 and 106.9 ng/ gland respectively) in MO [66]. In the same lobster sinus gland extract injected into the bilateral eyestalk ablated animals caused significant reduction in hemolymph MF levels in a dose-dependent manner [66]. The eyestalk peptide induced inhibition of MF secretion by mandibular organs was demonstrated in vitro in some crustacean species, viz., in the crabs C. pagurus[ 64] and O. senex senex [8,67-69], and in the lobster H. americanus [60]. The effect of MOIH on mandibular organ was evaluated in the crayfish P. clarkii by measuring the activity of farnesoic acid O-methyl transferase, which mediates the final step of methyl farnesoate synthesis [70]. Chaves [70] also observed 20-100 fold increase in methyl transferase activity 8-12 days following eyestalk ablation in P. clarkii.
Using two-step reverse phase HPLC, Liu and Laufer [71] isolated and purified three peptides with MOIH activity from the spider crab L. emarginata. These three peptides having 72-76 amino acid residues are exhibited similarity with each other in terms of amino acid composition. All three peptides significantly inhibited the mandibular organ secretory activity. MOIH peptide and MOIH gene were isolated and characterized from L. emarginata which showed significant sequence similarity with other sinus gland neuropeptides and genes [72,73]. Tang et al., [74] isolated and cloned two isoforms of MOIH cDNAs called MOIH-1 and MOIH-2 (differing by just one amino acid) from sinus glands of the edible crab, C. pagurus. Though the information with reference to MOIH is less, it is clear from the above studies that MOIH inhibits the methyl farnesoate secretion from mandibular organ thereby inhibits reproduction. However, much focus is needed on the role of MOIH in regulating reproduction in crustaceans.
Molt-inhibiting hormone (MIH): The MIH is mainly involved in the inhibition of ecdysteroid synthesis from Y-organs. There are very few reports on MIH regulated reproduction in crustaceans. Tiu and Chan [75] reported the inhibitory action of MIH on vitellogenesis in the shrimp M. ensis. They incubated recombinant MeMIH-B with hepatopancreas and ovary explants and observed the up-regulation of vitellogenin gene in both the explants in a dose-dependent manner. The function of MIH in the regulation of vitellogenesis is predicted to be through tissue specific receptors with different kinetics and signal transduction. Recently Nilli et al., [76] characterized MIH specific binding proteins (50 kDa protein) in the hepatopancreas of vitellogenic female Callinectes sapidus. They also conducted in vitro experiments by incubating hepatopancreas fragments with MIH and observed increased levels of cAMP. Based on the results Nilli et al., [76] suggested that MIH employ cAMP as a secondary messenger in hepatopancreas. Nilli et al., [77] also studied the dynamics of MIH during vitellogenesis in the mature female crab Callinectus sapidus. They found that the MIH titers were four times higher at middle vitellogenesis than at previtellogenesis and suggested that the action of MIH on molting and reproduction in mature female C. sapidus is antagonistic, i.e., inhibition of molting and stimulation of vitellogenesis. Since molting and reproduction are sequential in prawns and shrimps and antagonistic in crabs, studies related to dynamics and physiological effects of MIH in the regulation of molting and reproduction will be very much essential to manipulate crustacean reproduction.
Red pigment concentrating hormone (RPCH): The red pigment concentrating hormone (RPCH), a neuro-endocrine peptide synthesized in and secreted from X-organ-sinus gland complex of eyestalks in crustaceans. The RPCH not only involved in the regulation of pigmentation, also involved in the regulation of ovarian maturation [78,79]. Kulakarni et al, [78] have reported the oocytes that had been incubated with thoracic ganglion and RPCH had entered into the midvitellogenic stage in in vitro. Sarojini et al. [79] demonstrated the in vivo and in vitro effect of RPCH on ovarian maturation in P. clarkii. They observed that the increase in oocyte diameter and mean ovarian indices in an in vivo, and significant ovarian maturation in an in vitro, where the ovaries incubated with thoracic ganglion alone (without eyestalk extract). It is very clear from the above studies that the RPCH induces the ovarian development by releasing the gonad-stimulating hormone from thoracic ganglia.
Other eyestalk factors involved in the regulation of reproduction: In addition to eyestalk neuropeptide hormones, some other factors present in the sinus gland have been reported to be stimulating the vitellogenesis [80-86].
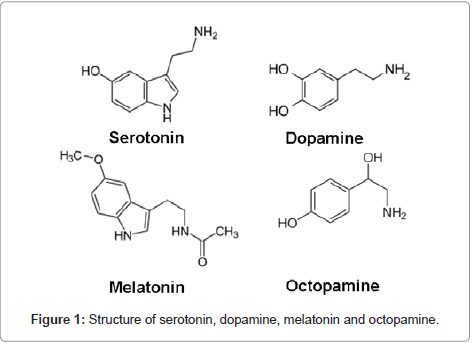
Neurotransmitters: The role of neurotransmitters in the regulation of crustacean reproduction is well documented by Fingerman [87]. The major neurotransmitters identified in crustaceans are serotonin, dopamine, melatonin and octopamine (Figure 1). The neurotransmitter serotonin (5-hydroxytryptamine; 5-HT) is produced and released from eyestalks is involved in the induction of reproduction in crustaceans. 5-HT stimulates the release of gonad-stimulating hormone (GSH) from the brain and thoracic ganglion, where the GSH synthesized [81,88]. Fingerman et al., [88] have measured the levels of 5-HT in the eyestalks and brain tissues of the fiddler crab U. pugilator. They also observed, 5-HT triggers the release of RPCH from eyestalks. 5-HT induced ovarian maturation was observed in P. clarkii [89]. The treatment of 5-HT caused significant increase in the ovarian index in the eyestalk-ablated crab Paratelphusa hydrodromus when compared with the eyestalk ablated saline injected crabs [90]. Recently Vaca and Alfaro [91] and Kumlu [92] have demonstrated 5-HT-induced ovarian maturation in the penaeid shrimp P. semisulcatus and found that the action of serotonin on ovarian maturation and spawning is significant but less effective than eyestalk ablation. The above results suggest that 5-HT induced reproduction is mediated either by release of GSH from brain and thoracic ganglion or by stimulating the release of RPCH from eyestalk neural tissue, there by GSH in crustaceans. It remains for future study to determine which model is the correct one.
Dopamine was identified in the eyestalks of several crustaceans employing the fluorescence method of Falck et al. [93]. Dopamine when injected into female Procambarus clarkii inhibited ovarian maturation. Cahansky et al. [94] studied the role of spiperone (dopaminergic antagonist) on ovaries of Cherax quadricarinatus. They also observed significant stimulation in the ovarian development in the presence of thoracic ganglia in vitro. The inhibitory action of dopamine could have been due to (a) Inhibition of GSH release;
(b) Stimulation of release of GSH antagonist, GIH; (or)
(c) Both (a) and (b).
Octopamine, another biogenic amine was identified in central nervous system of H. americanus using thin layer chromatography. The role of octopamine in reproduction appears to be atleast in part due to stimulation of contraction of the ovarian walls, liberating oocytes.
Melatonin (N-acetyl-5-methoxy trypatamine) is an evolutionary conserved molecule involved in the transduction of photoperiodic information from environment to organisms and popularly known as ‘chemical of darkness’ [95]. The presence of melatonin in invertebrates including crustaceans is well documented but its role in regulating their physiology is not clear [96]. Melatonin in crustaceans was first discovered in the visual system of crayfish, P. clarkii using spectrophotoflurometry [97]. The role of melatonin in the regulation of crustacean reproductive physiology is poorly understood. Recently, it was reported that injection of melatonin into female intact crabs, O. senex senex induced ovarian maturation [98] as evidenced by increase in the weight of the ovary, oocyte size and increase in ovarian vitellogenin levels over controls. From the results it is clear that melatonin is also involved in the stimulation of ovarian growth and vitellogenesis and regulation of melatonin synthesis can be used to manipulate crustacean reproduction. The relative roles and interaction of these biogenic amines in the regulation of reproduction remain to be elucidated.
Opioids: Opioids are the small peptides containing five amino acids and their significance is well known in vertebrates [82]. The search for the presence of endogenous opioids in invertebrates initially met with some uncertainty. However, using techniques like immunohistochemistry, radioimmunoassay (RIA) and high performance liquid chromatography (HPLC), opioids were discovered in several invertebrates, including crustaceans.
The potential role of opioids in the regulation of crustacean reproduction was well-documented [85]. The putative involvement of endogenous opioid system in the regulation of reproduction in the crab U. pugilator was investigated in vivo [99]. Injection of methionineenkephalin caused significant dose dependent inhibition of ovarian maturation, but the naloxone-injected crabs produced dose-dependent ovarian maturation. Sarojini et al. [100] incubated ovarian explants of P. clarkii with methionine-enkephalin and thoracic ganglia and found reduced ovarian development in a dose dependent manner as compared with explants incubated with thoracic ganglia alone. Reddy [84] reported the possible involvement of endogenous opioids in the regulation of reproduction in the prawn P. indicus, where injection of leucine-enkephalin caused significant increase in ovarian index and oocyte diameter in a dose-dependent manner. Increased ovarian growth has been observed in the ovarian explants of C. quadricarinatus incubated with naloxone, an enkephalonergic angonist [94]. It is clear from the literature that the opioid peptides are involved in the regulation of crustacean reproduction. Additional research is needed to clarify the molecular mechanism of action of opioid peptides in regulating crustacean reproduction.
Fushi tarazu-factor (FTZ-F1): The recently discovered another eyestalk factor which is responsible for the promotion of reproduction in crustaceans is fushi tarazu-factor (FTZ-F1). FTZ-F1 titer is more in late vitellogenic ovary as well as in early nauplius larvae of the shrimp M. ensis [83]. Since the levels of FTZ-F1 is more during vitellogenic stage and larval stage it was concluded that FTZ-F1 play a crucial role in embryogenesis and larval development of the shrimp.
Extra-eyestalk hormones and their role in the regulation of reproduction
Gonad-stimulating hormone (GSH): The brain and thoracic ganglia have been identified as sources of a neurohormone called gonad stimulating hormone (GSH). Although GSH has not yet been isolated and purified to electrophoretic homogeneity, several in vivo and in vitro studies demonstrated the stimulating effects of the brain and thoracic ganglia extract on ovarian growth [13].
The involvement of GSH in the regulation of reproduction was demonstrated in different crustacean species. Gomez [25] implanted the brain tissues into the crab P. hydrodromus and observed accelerated oocyte development. The brain tissue induced ovarian growth was also observed in Parapenaeopsis hardwickii, Macrobrachium kistnesis, Paratya compressa and Penaeus vannamei [101-104]. Otsu [105] observed the factors that are present in thoracic ganglion are involved in the stimulation or acceleration of oocytes of sexually quiescent female crab P. dehaani. Brain and thoracic ganglia induced ovarian maturation were demonstrated in the red swamp crayfish P. clarkii in in vitro [89,100]. Thoracic ganglion induced reproduction is also reported in C. quadricarinatus [11].
GSH is a peptide regulates the onset of vitellogenesis by direct action on vitellogenin synthesizing sites [89,101]. Recently Medasani et al. [106] studied the role of heavy metals such as copper and cadmium on GSH thereby gonad maturation. They incubated ovarian explants along with thoracic ganglia and heavy metals, and found no change in the ovarian growth. Based on the results, they hypothesized that the copper and cadmium are having inhibitory effect on GSH release thereby on reproduction. Though there are reports stating the presence of GSH, but up to now it has not been characterized and it should be one important area of crustacean endocrinology for future research.
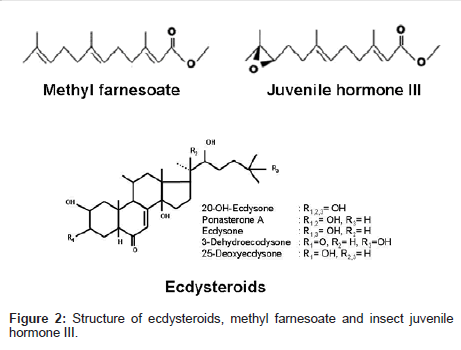
Methyl farnesoate (MF): Recently, the role of MF, a sesquiterpene secreted by the mandibular organs in the regulation of reproduction is well acknowledged [3,6,15,107]. MF is structurally similar to the insect JH III (Figure 2), only differing in the presence of an epoxide moiety at the terminal end. MF is negatively regulated by mandibular organ inhibiting hormone produced by the eyestalk [66,68,108-110]. The levels of MF were determined in the hemolymph of the mud crab S. serrata [111], American lobster H. americanus [62], crayfish P. clarkii [112] and giant fresh water prawn M. rosenbergii [113,114]. MF binding proteins which transport MF from synthesizing site to the target site was also characterized in H. americanus [115] and L. emarginata [116]. The highest MF levels in the individuals with an active vitellogenic stages, indicating the role of MF in the regulation of reproduction. Whether these alterations in the MF titers are due to changes in the rate of MF secretion by the MO or to some other mechanism, such as differential degradation, remains to be elucidated.
The ecdysiotropic nature of MF was described in the crab Cancer magister [117]. Tamone and Chang [117] have demonstrated the effect of methyl farnesoate on ecdysteroid secretion from crab C. magister Y-organ in in vitro. They found that Y-organs when incubated in the presence of MF secreted significantly more ecdysteroids into the medium after 24 hours incubation and also find the magnitude of this stimulation increased with higher concentrations of MF and increasing incubation times.
There is evidence that MF may have functions in addition to acting as an ecdysiotropin. It may influence reproduction [118-121] and larval development [62]. Tsukimura and Kamemoto [122] have studied the effect of juvenile hormone III and MF on ovarian development in the shrimp P. vannamei. Laufer et al. [121] observed accelerated ovarian maturation in the red swamp crayfish P. clarkii after the administration of MF and concluded that exogenous MF can stimulate ovarian maturation in P. clarkii. Ovarian index and oocyte diameter were significantly increased in MF injected P. indicus and the increase of the ovary was 4 fold than those of controls [123]. They also observed that the ovaries of MF received animals, entered into the late vitellogenic stage (dark brown colour ovary). Reddy and Ramamurthi [124] observed that the ovaries of the crab, O. senex senex injected with MF had entered into the late vitellogenic stage (bright orange colour ovary) but the ovaries of initial control and concurrent control crabs were in the early vitellogenic stage (white colour ovary). Administration of MF, incorporated in food, resulted in the stimulation of ovarian growth in crayfish [121]. Similarly, pellets enriched with JHIII stimulated ovarian growth in crabs [125]. In vitro stimulation of ovarian growth by MF has also been reported for both penaeid shrimp [122] and crayfish [126].
In order to know more about the MF mediated reproductive regulation in crustaceans, the researchers are trying to exploit the intermediates of MF biosynthetic pathway. In the female crab Charybdis feriatus farnesoic acid, an intermediate of MF biosynthetic pathway induced vitellogenin gene expression [127,128]. Mak et al. [127] incubated hepatopancreas explants with farnesoic acid, MF and JH III and observed upregulation of Vg gene expression in hepatopancreas of farnesoic acid incubated explants. Whereas incubation of hepatopancreas explants with MF and JH caused insignificant change in Vg gene expression. The data suggests farnesoic acid may be the potential candidate to induce reproduction in crustaceans, which needs more focus in future.
Ecdysteroids: The Y-organs are paired non-neural endocrine organs, homologous to prothoracic glands of insects are the sole source of molting hormones, secreted as a precursor, ecdysone, into the hemolymph, to be converted into the physiologically active hormone, 20-hydroxyecdysone by a 20-hydroxylase activity present in the epidermis and other organs and tissues of crustaceans [4]. The structure of 20-hydroxyecdysone (although originally called crustecdysone or ecdysterone) was elucidated as 2ß, 3ß, 14α, 20R, 22R, 25-hexahydroxy- 5ß-cholest-7-en-6-one; Figure 2). Ecdysteroids stimulates ovarian growth, especially in those crustaceans where reproduction occurs just after molting. In other crustaceans also, ecdysteroids are involved in the regulation of reproduction, but it is restricted to the first, i.e., proliferative stage of ovarian growth [129]. The primary function of ecdysteroid in crustaceans is to induce precocious molting. Recent studies indicate ecdysteroids also play an important role in the regulation of crustacean reproduction [130,131].
Charniaux-Cotton and Touir [132] observed the failure of folliculogenesis which is fundamental prerequisite for vitellogenin uptake by oocytes during secondary vitellogenesis by Y-organectomy in the shrimp Lysmata seticaudata. Removal of Y-organ also resulted in decreased vitellogenin synthesis and ovarian growth in the amphipod Orchestia gammarella [133]. A close correlation between the hemolymph ecdysteroid levels and ovarian maturation stages was observed in the prawn Macrobrachium nipponense [134] High concentrations of hemolymph ecdysteroids were also observed in reproducing females of terrestrial isopod Oniscus asellus [135]. However, situation is different in Brachyuran crab species where the entire reproductive cycle is completed in the intermolt period, when the ecdysteroid levels at very low [136]. The occurrence of different types of ecdysteroids in large amounts within the ovary is another evidence, to confirm the role of ecdysteroids in the regulation of reproduction in many crustacean species [130,131]. Despite the fact that extensive work has been carried out to establish the relationship between ecdysteroids and reproduction, the functional role of ecdysteroids in the control of reproduction is controversial and the mechanism of action on vitellogenesis is yet to be determined.
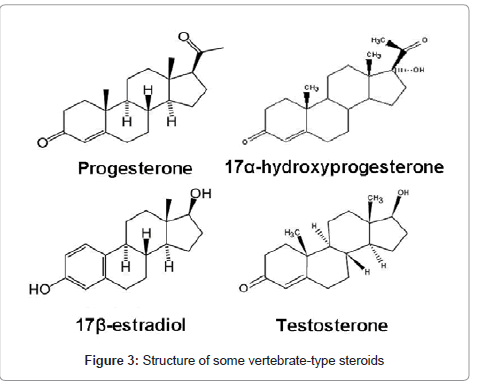
Vertebrate-type steroids: Crustacean gonads have been shown to possess steroids more typically acknowledged with vertebrates and the enzymatic capacity to synthesize vertebrate sex steroids [137-139]. Progesterone, 17α-hydroxyprogesterone, estradiol (17β-estradiol), and testosterone are the vertebrate-type steroids discovered from several crustaceans (Figure 3). Progesterone and pregnenolone have been identified in the gonads and hemolymph of Astacus leptodactylus and H. americanus [140,141]. Fairs and colleagues [142] found high titers of estrogens during vitellogenic stages, suggesting a possible role in the stimulation of vitellogenesis.
A positive relationship between Vg levels in hemolymph and circulatory levels of both progesterone and 17β-estradiol has been observed in shrimps [143], prawns [144] and crabs [145]. Fluctuating levels of estradiol and progesterone in the ovary and hemolymph at different vitellogenic stages of the crab Scylla serrata were also reported [146]. In contrast, Okumura and Sakiyama [24] observed a negative relationship between ovarian maturation and hemolymph levels of steroids in M. japonicus. Injection of progesterone induced ovarian development in the shrimp P. hardwickii [147]. Progesterone and estradiol apparently stimulated Vg gene expression in both hepatopancreas and ovary explants of M. ensis [148]. Administration of 17α-hydroxyprogesterone stimulated ovarian growth and vitellogenesis in the kuruma prawn M. japonicus [149]. 17β-Estradiol stimulated vitellogenesis in ovary explants in vitro [144] and in vivo in crayfish [150]. It is also known that injection of progesterone and 17α-hydroxyprogesterone are able to induce ovarian maturation in the shrimp M. ensis [151] and in the crab O. senex senex [8] respectively. 17α-hydroxyprogesterone administered both by injection and by incorporation into the food, increased gonadosomatic index in prereproductive and post-reproductive periods of female estuarine crab Chasmagnathus granulate [125]. They also observed increased uptake of the amino acid L-Leucine by the ovaries incubated with 17α-hydroxyprogesterone in vitro.
Vertebrate-type steroids are also found in the shrimp P. monodon and the levels of some of these steroids (progesterone and 17β-estradiol) are high in the ovaries at the mature stage of the animal [143], suggesting the role of steroids in crustacean reproduction. In the red mud crab S. serrata the levels of 17β-estradiol and progesterone increased steeply in the tissues at the onset of vitellogenesis (vitellogenesis stage I) [146]. Rodriguez et al. [126] also reported the stimulatory role of steroids in the regulation of reproduction in the crayfish P. clarkii.
Some studies also showed circulating levels of progesterone and estradiol-like steroids that correlated with the vitellogenesis cycles of several crustaceans [143,145]. Both in vivo [125,152] and in vitro [122,125] stimulation of ovarian growth by 17α-hydroxyprogesterone have been reported. The precise role of steroid hormones in crustaceans is still unknown; however, stimulation of vitellogenin production within the ovary itself or in extra-ovarian sites has been suggested [144,152,153].
At present, it is not known whether crustaceans obtain the vertebrate type steroids from the environment i.e., via feed or synthesize internally. Additional studies are necessary to examine the mode and action of steroid hormones. Steroid hormones have to bind to nuclear receptors in order to generate physiological action. Progesterone and estradiol receptors have been detected in the hepatopancreas and gonad of the freshwater crayfish Austropotamobius pallipes using immunodiagnostic kits [154]. Though classical vertebrate steroid hormones and their receptors were identified from limited number of crustaceans, the manner by which they exert their effect on crustacean reproduction is less clear than steroid-induced responses in mammals. However, the general scheme seems remarkably similar in all organisms.
Involvement of other vertebrate hormones: Besides the presence and effects of vertebrate steroid hormones, the effects of other classical vertebrate gonadotropic hormones (follicle stimulating hormone, FSH, and luteinizing hormone, LH) were also studied in crustaceans. Zukowska-Arendarczyk [155] reported that administration of hypophysis gonadotropins induced ovarian maturation in Crangon crangon. However, to date no structurally similar peptides have been isolated from any invertebrate. Injection of human chorionic gonadotropin (hCG) stimulated maturation and spawning in shrimp and prawn [156,157]. Laufer and Landau [158] also observed ovarian maturation in P. indicus fed with food containing hCG. hCG administration also stimulated vitellogenin synthesis in the isopod, Idotea balthica [159], in the prawn Crangon crangon [160], and in the sand shrimp, C. crangon [158]. A molecule very similar to hCG has been identified in the prawn Penaeus serratus with the aid of a radioimmunoassay using hCG antibodies [161]. Further studies are required to fully understand the effects of mammalian gonadotropins on regulation of reproduction in crustaceans.
Interaction of Environmental and Internal Factors in the Regulation of Reproduction
Crustacean reproduction is controlled by hormones of diversified chemical nature (peptide, steroid, terpenoid, biogenic amines, prostaglandins etc.) which aid in evaluating environmental conditions to determine the best time to reproduce. Environmental variables such as salinity, temperature, photoperiod and availability of food play vital roles in regulating crustacean physiology, including reproduction [13,16,162,163]. Lovett et al. [164] observed 5- to 10 fold increase in hemolymph MF level in the crab C. maenas when the crabs transferred from isosmotic sea water to 5 ppt seawater. Similar results were also observed in crabs when they were treated with low saline water at 11°C [165]. In contrast, exposure of another euryhaline crab, C. sapidus, to low salinity sea water (15 ppt) did not increase its hemolymph levels of MF [166]. Significant increase in hemolymph MF level was observed in the crab C. maenas after exposure to elevated temperature (32°C) and anoxia (0.25 ppm O2). Olmstead and LeBlanc [167] observed that environmental factors can cause aberrant sex determination via perturbations in MF signaling in D. magna. Neuroendocrine coordination of the appropriate environmental factor is essential to ensure that seasonal reproductive events takes place in the appropriate temporal sequence. Information about the environmental and endogenous factors that control crustacean reproduction is required to enhance profitable aquaculture programs.
Conclusions and Prospectives
The neuroendocrine and non-neuroendocrine regulation of reproduction in decapods has been the basis of study and a topic of debate for the past six decades because of interest in aquacultural activities. Various X-organ borne neurohormones are suggested to affect several physiological events, including the activity of peripheral endocrine glands, production of vitellogenin, the precursor of the main yolk protein, and gonad development. Due to multitropic nature and existence of different isomorphs of eyestalk peptides, it is also possible that other members of CHH-family (excluding VIH) may have influence on the reproduction. CHH receptors were detected on the oocyte membranes of C. maenas and C. pagurus [58]. CHH mediated reproduction was studied in H. americanus [34,43], P. semisulcatus [54] and M. ensis [53]. The reproductive inhibition was also observed by the eyestalk peptide MOIH in C. pagurus [64]. It affects the reproduction by inhibiting the synthesis and secretion of MF by mandibular organs in the crab O. senex senex [8]. The possible explanation for isoforms with multiple activities in CHH-family peptides is that they may be originated from single ancestral gene during evolution. This is in support with the highest % of structural similarity and amino acid homologies between CHH-family peptides isolated from intra and inter species. Furthermore, detection of specific target structures by means of identification and colocalization of hormone receptors has not been carried out. In addition to CHH-family peptides, RPCH belongs to chromatophores of eyestalk has stimulatory activity on ovary by releasing the GSH from the brain and thoracic ganglion [79]. The other eyestalk factors, like 5-HT [89], DA [168], Opioids [84,85] and FTZ-F1 [83] also influences the reproduction in crustaceans.
GSH which possesses antagonistic activity to VIH is involved in the stimulation of reproduciton [100,104,105] and it is not yet characterized. In addition to the above hormones, the terpenoid, methyl farnesoate [118,169,170] and the steroid molting hormone, 20-hydroxyecdysone [130,131,171], are suggested to be involved in the expression of vitellogenin. More recently the farnesoic acid [127] and vertebrate-type steroids [8,125,146] have attracted the attention of crustacean reproductive endocrinologists. Though the hormones and neuromodulators involved in the regulation of vitellogenesis are well recognized, the cross-talk between them as well as overlapping and multiple functions of hormones, remain unclear.
Little is known about the role of eicosanoids in the regulation of reproduction in crustaceans, although considerable evidence has been collected during the last three decades pertaining to their synthesis and mode of action. In many cases, the evidence for an eicosanoid function is based on treatment of animals with a single compound and observation of the response. At this level of observation, it remains to be established that eicosanoids are physiologically involved. Eicosanoids are derived from fatty acids acquired through diet. It is well known that eicosanoids are produced to act at local, tissue, or cellular level in mammals. They play a very important role in the regulation of reproduction throughout animal kingdom. Martin-Creuzburg et al. [172] reported that population growth of D. magna is improved more by the addition of sterols and eicosapentaenoic acid (EPA) together than by adding either nutrient separately, which implies a synergistic effect of sterol and EPA on reproduction. Further, they also found in D. magna that somatic growth is primarily constrained by the availability of sterols, and reproduction is primarily constrained by the availability of EPA. Current evidence suggests that the effect of nonsteroidal antiinflammatory drug ibuprofen in D. magna is interruption of eicosanoid biosynthesis, thereby reduces fecundity [173], where both prostanoids and lipoxygenase products appear to be important agents in oogenesis and embryogenesis [174]. One can assume that as details of eicosanoid action in crustaceans become known they will contribute greatly to our understanding of crustacean reproduction. Our earlier work suggested that administration of PGE2 induced ovarian growth and ovarian vitellogenin levels in fresh water crab, Oziotelphusa [67]. Studies on decapod eicosanoids may generate information on fundamental aspects of eicosanoid biosynthesis and mechanisms of action, and may yield information of practical importance in hatchery industry. Young scientists are entering this emerging field, and these investigators will surely make truly exciting discoveries in the future.
The field of crustacean reproductive endocrinology is entering an exciting period with the introduction of modern techniques which permit detailed studies on the complexity of endocrine control of reproduction. Modern methodologies such as immunohistochemistry and molecular and biochemical methods have to be extended to crustaceans to elucidate the cellular dynamics of the neuroendocrine cell systems synthesizing VIH, MIH, MOIH and CHH. Using polyclonal/monoclonal antibodies, some studies have already been described the quantification methods for GIH [175] and CHH [176]. Based on the titers of circulatory hormones it might be possible to develop simple immunodiognostic kits which can be used in hatcheries for determining the conditions of the cultured females and even to manipulate their reproductive cycle by controlling external conditions such as temperature and photoperiod.
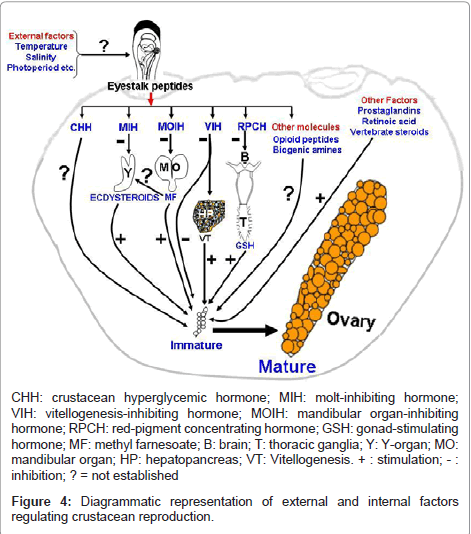
Several crustacean hormones exhibit multifunctional activities. Members of CHH family of neuropeptides not only present from embryos to adults and a single peptide can have multiple biological functions. Ecdysteroids may serve as morphogens during embryonic development, function as molting hormones during larval to adult life and also act as gonadotropins in adults. Methyl farnesoate also function as developmental hormone in larvae and as a gonadotropin in adults. These examples illustrate the complexity of crustacean endocrine system that several chemically diverse hormones can mediate different functions at different life stages. Understanding the cross-talk of these molecules in the internal milieu may provide rewarding insights into the regulation of crustacean reproduction. However, as crustacean endocrinologists probe deeper and deeper into the hormonal regulation of reproduction, they eventually will have to return to the whole animal to examine the ultimate stimulus to reproduction, namely, how the animal translates environmental cues viz., temperature, salinity, photoperiod, availability of food etc. into information that is utilized in the regulation of endocrine axis? Several workers acknowledged the importance of temperature, photoperiod and availability of food for breeding. This and other hypothetical and epithetical aspects of crosstalk between different internal molecules are summarized in Figure 4.
Acknowledgements
This review manuscript is dedicated to Prof. R. Ramamurthi, a committed teacher and advisor, and a brilliant scientist who introduced us to crustacean endocrinology. His suggestions helped us a lot to improve the manuscript. The work related to the crab Oziotelphusa and described in this paper was funded in part by grants from the Department of Science and Technology and Department of Biotechnology, New Delhi to PSR. Valuable contributions of colleagues and the students and postdoctoral researchers from PSR’s laboratory are specially acknowledged.
References
- Chang E, Sagi A (2008) Male reproductive hormones. Reproductive Biology of Crustaceans. In: Mente E, (Ed.), Science Publishers, Enfield, NH. Pg: 299-318.
- Adiyodi KG (1985) Reproduction and its control. In: Liss DE, Mantel LH, (Eds.) The Biology of crustacean. Academi Press, New York. 9: 146-215.
- Reddy PS, Ramamurthi R (1999) Recent Trends in Crustacean Endocrine Research. PINSA 65: 15-32.
- Huberman A (2000) Shrimp endocrinology: A review. Aquaculture 191: 191-208
- Tsukimura B (2001) Crustacean vitellogenesis: Its role in oocyte development. Amer Zool 41: 465-476.
- Wilder MN, Subramoniam T, Aida K (2002) Yolk proteins of crustacea In: Raikhel AS, Sappington TW (Eds.) Reproductive Biology of Invertebrates. Vol XII, Part A: Progress in Vitellogenesis. Science Publishers, Inc., Plymouth, UK. Pg: 131-173.
- Diwan A (2005) Current progress in shrimp endocrinology-A review. Indian J Exp Biol 43: 209-223.
- Reddy PR, Reddy PS (2006) Isolation of peptide hormones with pleiotropic activities in the freshwater crab, Oziotelphusa senex senex. Aquaculture 259: 424-431.
- Adiyodi KG, Adiyodi RG (1970) Endocrine control of reproduction in decapod Crustacea. Biol Rev Camb Philos Soc 45: 121-165.
- Aiken DE (1969) Ovarian maturation and egg laying in the crayfish Orconectes virilis: influence of temperature and photoperiod. Can J Zool 47: 931-935.
- Von Hentig R (1971) Influence of Salinity and Temperature on Development, Growth, Reproduction and Energy Budget of Artemia Salina. Marine Biology 9: 145-182.
- Li C, Luo X, Huang X, Gu B (2009) Influences of temperature on development and survival, reproduction and growth of a calanoid copepod (Pseudodiaptomus dubia). ScientificWorldJournal 9: 866-879.
- Fingerman M (1997) Crustacean endocrinology: a retrospective, prospective, and introspective analysis. Physiol Zool 70: 257-269.
- Chang E, Chang S, Mulder E (2001) Hormones in the lives of crustaceans: an overview. Amer Zool 41: 1090-1097.
- Nagaraju GPC (2007) Is methyl farnesoate a crustacean hormone? Aquaculture 272: 39-54.
- Mazurov√?¬° E, Hilscherov√?¬° K, Triebskorn R, K√?¬∂hler H, Mars√?¬°lek B, et al. (2008) Endocrine regulation of the reproduction in crustaceans: identification of potential targets for toxicants and environmental contaminants. Biologia 63: 139-150.
- Raviv S, Parnes S, Sagi A (2008) Coordination of reproduction and molt in decapods. In: Mente E, (Ed.) Reproductive Biology of Crustaceans. Science Publishers, Enfield, NH. Pg: 365-390.
- Panouse JM (1943) Influence de l√ʬ?¬?ablation de pedoncule ocularie sur la croissance de √ʬ?¬?ovarie chez la crevette, Leander serratus. C R Acad Sci Paris 217: 553-555.
- Gorell TA, Gilbert LI (1971) Protein and RNA synthesis in the premolt crayfish, Orconectes virilis. J Comp Physiol A Neuroethol Sens Neural Behav Physiol 73: 345-356.
- Eastman-Reks S, Fingerman M (1984) Effects of neuroendocrine tissue and cyclic AMP on ovarian growth in vivo and in vitro in the fiddler crab, Uca pugilator. Comp Biochem Physiol 79: 679-684.
- Chaves AR (2000) Effect of x-organ sinus gland extract on (35S) methionine incorporation to the ovary of the red swamp cray fish, Procambarus clarkii. Comp Biochem Physiol A Mol Integr Physiol 126: 407-413.
- Mohamed KS, Diwan AD (1991) Neuroendocrine regulation of ovarian maturation in the Indian white prawn Penaeus indicus H. Milne Edwards. Aquaculture 98: 381-393.
- Okumura T, Aida K, (2001) Effects of bilateral eyestalk ablation on molting and ovarian development in the giant freshwater prawn Macrobrachium rosenbergii. Fish Sci 67: 1125-1135.
- Okumura T, Sakiyama K (2004) Hemolymph levels of vertebrate-type steroid hormones in female kuruma prawn Marsupenaeus japonicus (Crustacea: Decapoda: Penaeida) during natural reproductive cycle and induced ovarian development by eyestalk ablation. Fish Sci 70: 372-380.
- Gomez R (1965) Acceleration of development of gonads by implantations of brain in the crab Paratelphusa hydrodromous. Naturwissenschaften 52: 216.
- Ragneker PV, Deshmukh RD (1968) Effect of eyestalk removal on the ovarian growth of the marine crab, Scylla serrata (Forskal). J Anim Morph Physiol 17: 84-88.
- Tsutsui N, Kim YK, Jasmani S, Ohira T, Wilder MN, et al. (2005) The dynamics of vitellogenin gene expression differs between intact and eyestalk ablated kuruma prawn Penaeus (Marsupeaneus) japonicus. Fish Sci 71: 249-256.
- Okumura T (2007) Effects of bilateral and unilateral eyestalk ablation on vitellogenin synthesis in immature female kuruma prawns, Marsupenaeus japonicus. Zool Sci 24: 233-240.
- Spaziani E, Mattson MP, Rudolph PH, (1994) Regulation of crustacean molt-inhibiting hormone. In: Davey KG, Peter RE, Tobe SS (Eds.) Perspect Comp Endocrinol 243-250.
- Keller R (1992) Crustacean neuropeptides: structures, functions and comparative aspects. Experientia 48: 439-448.
- Webster SG (1998) Neuropeptides inhibiting growth and reproduction in crustaceans. In: Cost GM, Webster SG (Eds.) Recent advances in arthropod endocrinology. Cambridge University Press, Cambridge. Pg: 33-52.
- Chan SM, Gu PL, Chu KH, Tobe SS (2003) Crustacean neuropeptide genes of the CHH/MIH/GIH family: implications from molecular studies. Gen Comp Endocrinol 134: 214-219.
- Nagaraju GP (2011) Reproductive regulators in decapod crustaceans: an overview. J Exp Bio 214: 3-16.
- De Kleijn DP, Janssen KP, Waddy SL, Hegeman R, Lie WY, et al. (1998) Expression of the crustacean hyperglycemic hormones and the gonad-inhibiting hormone during the reproductive cycle of the female American lobster Homarus americanus. J Endocrinol 156: 291-298.
- Edomi P, Azzoni E, Mettulio R, Pandolfelli N, Ferrero EA, et al. (2002) Gonad inhibiting hormone (GIH) of the Norway lobster (Nephrops norvegicus): cDNA cloning, expression, recombinant protein production, and immunolocalization. Gene 284: 93-102
- Ollivaux C, Vinh J, Soyez D, Toullec JY (2006) Crustacean hyperglycemic and vitellogenesis-inhibiting hormones in the lobster Homarus gammarus. FEBS J 273: 2151-2160.
- Ohira T, Okumura T, Suzuki M, Yajima Y, Tsutsui N, et al. (2006) Production and characterization of recombinant vitellogenesis0inhibiting hormone from the American lobster Homarus americanus. Peptides 27: 1251-1258
- Tsutsui N, Ohira T, Kawazoe I, Takahashi A, Wilder MN (2007) Purification of sinus gland peptides having vitellogenesis-inhibiting activity from the whiteleg shrimp Litopenaeus vannamei. Mar Biotechnol 9: 360-369.
- Treerattrakool S, Panyim S, Chan SM, Withyachumnarnkul B, Udomkit A (2008) Molecular characterization of gonad-inhibiting hormone of Penaeus monodon and elucidation of its inhibitory role in vitellogenin expression by RNA interference. FEBS J 275: 970-980.
- Treerattrakool S, Panyim S, Udomkit A (2011) Induction of ovarian maturation and spawning in Penaeus monodon broodstock by double-stranded RNA. Mar Biotechnol 13: 163-169.
- Soyez D, le Caer JP, Noel PY, Rossier J (1991) Primary structure of two isoforms of the vitellogenesis inhibiting hormone from the lobster Homarus americanus. Neuropeptides 20: 25-32.
- Augilar MB, Quackenbush LS, Hunt DT, Shabanowitz J, Huberman A (2002) Identification, purification and initial characterization of the vetellogenesis-inhibiting hormone from the Mexican crayfish Procambarus bouvieri (Ortmann). Comp Biochem Physiol B 102: 491-498.
- De Kleijn DP, De Leeuw EP, Van Den Burg BC, Martens GJ, Van Herp F (1995) Cloning and expression of two mRNAs encoding structurally different crustacean hyperglycemic hormone precursors in the lobster Homarus americanus. Biochem Biophys Acta 1260: 62-66.
- Yang WJ, Ranga Rao K (2001) Cloning of precursors for two MIH/VIH-related peptides in the prawn, Macrobrachium rosenbergii. Biochem Biophys Res Commun 289: 407-413.
- Qian YQ, Dai L, Yang JS, Yang F, Chen DF, et al. (2009) CHH family peptides from an √ʬ?¬?eyeless√ʬ?¬? deep-sea hydrothermal vent shrimp, Rimicaris kairei: characterization and sequence analysis. Comp Biochem Physiol B Biochem Mol Bio154: 37-47.
- Gr√?¬®ve P, Sorokine O, Berges T, Lacombe C, Van Dorsselaer A, et al. (1999) Isolation and amino acide sequence of a peptide with vitellogenesis inhibiting activity from the terrestrial isopod Armadillidium vulgare (Crustacea). Gen Comp Endocrinol 115: 406-414.
- Udomkit A, Chooluck S, Sonthayanon B, Panyim S (2000) Molecular cloning of a cDNA encoding a member of CHH/MIH/GIH family from Penaeus monodon and analysis of its gene structure. J Exp Mar Biol Ecol 224: 145-156.
- Soyez D, Van Deijnen J, Martin M (2005) Isolation and characterization of a vitellogenesis-inhibiting factor from sinus glands of the lobster, Homarus americanus. J Exp Zool 244: 479-484.
- Nagaraju GP, Kumari NS, Prasad GL, Rajitha B, Meenu M, et al. (2009) Structural prediction and analysis of VIH-related peptides from selected crustacean species. Bioinformation 4: 6-11.
- Keller R, Sedlmeier D, (1988) A metabolic hormone in crustaceans: The hyperglycemic neuropeptide. In: Laufer H, Downer RGH (Eds.), Invertebrate endocrinology. Endocrinology of selected invertebrate types. Alan R. Liss., Inc., New York. 2: 315-326.
- Lugo JM, Morera Y, Rodr√?¬≠guez T, Huberman A, Ramos L, et al. (2006) Molecular cloning and characterzation of the crustacean hyperglycemic hormone cDNA from Litopenaeus schmitti; functional analysis by double-stranded RNA interference technique. FEBS J 273: 5669-5677.
- Van Herp, F., 1998. Molecular, cytological and physiological aspects of the crustacean hyperglycemic hormone family. In: Cost, E.M., Webster, S.G. (Eds.), Recent Advances in Arthropod Endocrinology. Cambridge University Press, Cambridge, UK 65: 53-70.
- Gu PL, Yu KL, Chan SM (2000) Molecular characterization of an additional shrimp hyperglycemic hormone: cDNA cloning, gene organization, expression and biological assay of recombinant proteins. FEBS Lett 472: 122-128.
- Khayat M, Yang W, Aida K, Nagasawa H, Tietz A, et al. (1998) Hyperglycemic hormone inhibits protein and m-RNA synthesis in vitro - incubated ovarian fragments of the marine shrimp, Penaeus semisulcatus. Gen Comp Endocrinol 110: 307-318
- Webster SG, Keller R (1986) Purification, characterisation and amino acid composition of the putative moult-inhibiting hormone (MIH) of Carcinus maenas (Crustacea, Decapoda). J Comp Physiol 156: 617- 624.
- Chung JS, Webster SG (2003) Moult cycle-related changes in biological activity of moult-inhibiting hormone (MIH) and crustacean hyperglycaemic hormone (CHH) in the crab, Carcinus maenas. From target to transcript. Eur J Biochem 270: 3280-3288.
- Zarubin TP, Chang ES, Mykles DL (2009) Expression of recombinant eyestalk crustacean hyperglycemic hormone from the tropical land crab, Gecarcinus lateralis, that inhibits Y-organ ecdysteroidogenesis in vitro. Mol Biol Rep 36: 1231-1237
- Webster SG (1993) High-affinity binding of putative moult-inhibiting hormone MIH and crustacean hyperglycaemic hormone CHH to membrane bound receptors on the Y-organ of the shore crab Carcinus maenas. Proc R Soc London 251: 53-59.
- Byard EH (1975) The female specific protein and reproduction in the lobster, Homarus americanus. PhD thesis, University of Western Ontario, London, Ontario.
- Waddy SL, Aiken DE, De Kleijn DPV (1995) Control of reproduction. In: Factor, J.R. (Ed.), Biology of the lobster, Homarus americanus. Academic press, San Diego, USA. 217-266.
- Hinsch GW (1980) Effect of mandibular organ implants upon the spider crab ovary. Trans Amer Micros Soc 99: 317-322
- Borst DW, Laufer H, Landau M, Change ES, Hertz WA, et al. (1987) Methyl farnesoate and its role in crustacean reproduction and development. Insect Biochem 17: 1123-1127.
- Nagaraju GP, Reddy PR, Reddy PS (2004) Mandibular organ: it√ʬ?¬?s relation to body weight, sex, molt and reproduction in the crab, Oziotelphusa senex senex Fabricius (1791). Aquaculture 232: 603-612.
- Wainwright G, Webster SG, Wilkinson MC, Chung JS, Rees HH (1996) Structure and significance of mandibular organ-inhibiting hormone in the crab, Cancer pagurus. Involvement in multihormonal regulation of growth and reproduction. J Biol Chem 271: 12749-12754.
- Reddy PR, Kiranmayi P, Thanuja Kumari K, Reddy PS (2006) 17√?¬Ī-Hydroxyprogesterone induced ovarian growth and vitellogenesis in the freshwater rice field crab Oziotelphusa senex senex. Aquaculture 254: 768-775.
- Tsukimura B, Borst DW (1992) Regulation of methyl farnesoate in the hemolymph and mandibular organ of the lobster, Homarus americanus. Gen Com Endocrinol 2: 296-303.
- Reddy PS, Reddy PR, Nagaraju GP (2004) The synthesis and effects of prostaglandins on the ovary of the crab Oziotelphusa senex senex. Gen Comp Endocrinol 135: 35-41.
- Nagaraju GP, Prasad GL, Reddy PS (2005) Isolation and characterization of mandibular organ-inhibiting hormone from the eyestalks of freshwater crab Oziotelphusa senex senex. Int J Appl Sci Engineering 3: 61-68.
- Nagaraju GPC, Reddy PR, Reddy PS (2006) In vitro methyl farnesoate secretion by mandibular organs isolated from different molt and reproductive stages of the crab Oziotelphusa senex senex. Fish Sci 72: 410-414.
- Chaves AR (2001) Effects of sinus gland extract on mandibular organ size and methyl farnesoate synthesis in the crawfish. Comp Biochem Physiol A Mol Integr Physiol 128: 327-333.
- Liu L, Laufer H (1996) Isolation and characterization of sinus gland neuropeptides with both MOIH and hyperglycemic effects from the spider crab Libinia emarginata. Arch Insect Biochem Physiol 32: 375-385.
- Liu L, Laufer H, Wang Y, Hayes T (1997) A neurohormone regulating both methyl farnesoate synthesis and glucose metabolism in a crustacean. Biochem Biophys Res Commun 237: 694-701.
- Liu L, Laufer H, Gogarten PJ, Wang M (1997) cDNA cloning of a mandibular organ inhibiting hormone from the spider crab Libinia emarginata. Invert Neurosci 3: 199-204.
- Tang C, Weiqun LU, Wainwright G, Webster SG, Rees HH, et al. (1999) Molecular characterization and expression of mandibular organ inhibiting hormone a recently discovered neuropeptide involved in the regulation of growth and reproduction in the crab Cancer pagurus. Biochem J 343: 355-360.
- Tiu SH, Chan SM (2007) The use of recombinant protein and RNA interference approaches to study the reproductive functions of a gonad-stimulating hormone from the shrimp Metapenaeus ensis. FEBS J 274: 4385-4395.
- Zmora N, Sagi A, Zohar Y, Chung JS (2009) Molt-inhibiting hormone stimulates vitellogenesis at advanced ovarian developmental stages in the female blue crab, Callinectes sapidus 2: novel specific binding sites in hepatopancreas and cAMP as a second messenger. Saline Systems5: 6.
- Zmora N, Trant J, Zohar Y, Chung JS (2009) Molt-inhibiting hormone stimulates vitellogenesis at advanced ovarian developmental stages in the female blue crab, Callinectes sapidus 1: an ovarian stage dependent involvement.Saline Systems 5: 7.
- Kulkarni GK, Glade L, Fingerman M (1991) Oogenesis and effects of neuroendocrine tissues on in vitro synthesis of protein by the ovary of the red swamp crayfish Procambarus clarkii (Girard). J Crust Biol 11: 513-522.
- Sarojini R, Nagabhushanam R, Fingerman M (1995) A neurotransmitter role for red-pigment-concentrating hormone in ovarian maturation in the red swamp crayfish Procambarus clarkii. J Exp Biol 198: 1253-1257.
- Charniaux-Cotton H (1985) Vitellogenesis and Its Control in Malacostracan Crustace a. Amer Zool 25: 197-206.
- Kulkarni GK, Nagabhushanam R, Amaldoss G, Jaiswal RG, Fingerman M (1991) 5-hydroxytryptamine stimulation of the ovary in the crayfish. Procambarus clarkii. Amer Zool 31: I I5A.
- Nagabhushanam R, Sarojini R, Reddy PS, Devi M, Fingerman M (1995) Opioid peptides in invertebrates: localization, distribution and possible functional roles. Curr Sci 69: 659-671.
- Chan SM, Chan KM (1999) Characterization of the shrimp eyestalk cDNA encoding a novel fushi tarazu-factor 1 (FTZ-F1). FEBS Lett 454: 109-114.
- Reddy PS (2000) Involvement of opioid peptides in the regulation of reproduction in the prawn Penaeus indicus. Naturwissenschaften87: 535-538.
- Kishori B, Reddy PS (2000) Antagonistic effects of opioid peptides in the regulation of ovarian growth of the Indian rice field crab, Oziotelphusa senex senex Fabricius. Invertebr Reprod Develop 37: 107-111.
- Kishori B, Reddy PS (2004) Influence of leucine-enkephalin on moulting and vitellogenesis in the freshwater crab, Oziotelphusa senex senex (Fabricius, 1791)(Decapoda, Brachyura). Crustaceana 76: 1281-1290.
- Fingerman M (1997) Roles of neurotransmitters in regulating reproductive hormone release and gonadal maturation in decapod crustaceans. Invert Reprod Dev 31: 47-54.
- Fingerman M, Julian WE, Spirtes MA, Kostrzewa RM (1974) The presence of 5-hydroxytryptamine in the eyestalks and brain of the fiddler crab Uca pugilaror. its quantitative modification by pharmacological agents, and possible role as a neurotransmitter in controlling the release of red pigment-dispersing hormone. Corny Gen Pharmac 5: 299-303.
- Sarojini R, Nagabhushanam R, Fingerman M (1995) Mode of action of the neurotransmitter 5-hydroxytryptamine in stimulating ovarian maturation in the red swamp crayfish, Procambarus clarkii: An in vivo and in vitro study. J Exp Zool 271: 395 - 400.
- Ragunathan MG, Arivazhagan A (1999) Influence of eyestalk ablation and 5-hydroxytryptamine on the gonadal development of a female crab, Paratelphusa hydrodromous (Herbst). Curr Sci 76: 583-587.
- Vaca AA, Alfaro J (2000) Ovarian maturation and spawning the white shrimp, Penaeus vannamei, by serotonin Injection. Aquaculture 182: 373-385.
- Kumlu M (2005) Gonadal maturation and spawning in Penaeus semisulcatus de Hann, 1844 by hormone injection. Turk J Zool 29: 193-199.
- Falck B, Hillarp NA, Thieme G, Torp A (1982) Fluorescence of catechol amines and related compounds condensed with formaldehyde. Brain Res Bull 9: xi-xv.
- Cahansky AV, Medesani DA, Chaulet A, Rodr√?¬≠guez EM (2011) In vitro effects of both dopaminergic and enkephalinergic antagonists on the ovarian growth of Cherax quadricarinatus (Decapoda, Parastacidae), at different periods of the reproductive cycle. Comp Biochem Physiol A Mol Integr Physiol 158: 126-131.
- Hardeland R (1997) New actions of melatonin and their relevanc to biometeorology. Int J Biometeorol 41: 47-57.
- Vivien-Roels B, P√?¬©vet P (1993) Melatonin: presence and formation in invertebrates. Experentia 49: 642-647.
- Balzer I, Espinola IR, Fuentes-Pard B (1997) Daily variations of immunoreactive melatonin in the visual system of crayfish. Biol Cell 89: 539-543.
- Sainath SB, Reddy PS (2010) Effect of selected biogenic amines (dopamine, serotonin and melatonin on ovarian maturation in the fresh water edible crab, Oziotelphusa senex senex. Aquaculture 313: 144-148.
- Fingerman M, Nagabhushanam R, Sarojini R, Reddy PS (1994) Biogenic amines in crustaceans: identification, localization and roles. J Crust Biol 14: 413-437.
- Sarojini R, Nagabhushanam R, Fingerman M (1997) An in vitro study of the inhibitory action of methionine enkephalin on ovarian maturation in the red swamp crayfish, Procambarus clarkii. Comp Biochem Physiol 115: 149-153.
- Kulkarni GK, Nagabhushanam R, Joshi PK (1981) Neuroendocrine regulation of reproduction in the marine female prawn, Parapenaeopsis hardwickii (Miers 1878). Ind J Mar Sci 10: 350-352.
- Sarojini R, Mirajkar MS, Nagabhushanam R (1983) Bihormonal control of oogenesis in the freshwater prawn, Macrobrachium kistnensis. Acta Physiol Hung 61: 5-10.
- Takayanagi H, Yamamoto Y, Takeda N (1986) An ovary-stimulating factor in the shrimp, Paratya compressa. J Exp Zool 240: 203-209.
- Yano I, Wyban JA (1993) Induced ovarian maturation of Penaeus vannamei by injection of lobster brain extract. Bull Natl Res Inst Aquacult 21: 1-7.
- Otsu T (1960) Precocious development of the ovaries in the crab, Potamon dehaani, following implantation of the thoracic ganglion. Annot Zool Japan 33: 90-96.
- Medesani DA, L√?¬≥pez Greco LS, Rodr√?¬≠guez EM (2004) Interference of cadmium and copper with the endocrine control of ovarian growth, in the estuarine crab Chasmagnathus granulata. Aquat Toxicol 69: 165-174.
- Laufer H, Biggers WJ (2001) Unifying concepts learned from methyl farnesoate for invertebrate reproduction and post-embryonic development. Amer Zool 41: 442-457.
- Borst DW, Ogan J, Tsukimura B, Claerhout T, Holford K (2001) Regulation of the crustacean mandibular organ. Amer Zool 41: 430-441.
- Borst DW, Wainwright G, Rees HH (2002) In vivo regulation of the mandibular organ in the edible crab, Cancer pagurus. Proc Biol Sci 269: 483-490.
- Nagaraju GP, Suraj N, Reddy PS (2003) Methyl farnesoate stimulates gonad development in Macrobrachium malcolmsonii (H. Milne Edwards) (Decapoda, Palaemonidae). Crustaceana 76: 1171-1178.
- Tobe SS, Young DA, Khoo HW (1989) Production of methyl farnesoate by the mandibular organs of the mud crab, Scylla serrata: validation of a radiochemical assay. Gen Comp Endocrinol 73 : 342-353.
- Landu M, Laufer H, Homola E, (1989) Control of methyl farnesoate synthesis in the mandibular organ of the crayfish Procambarus clarkii: evidence for peptide neurohormones with dual functions. Invertebr Reprod Dev 16: 165-168.
- Sagi A, Homola E, Laufer H (1991) Methyl farnesoate in the prawn Macrobrachium rosenbergii: Synthesis by the mandibular organ in vitro, and titers in the hemolymph. Comp Biochem Physiol 99: 879-882.
- Wilder MN, Okumura T, Suzuki Y, Fusetani N, Aida K (1994) Vitellogenin production induced by eyestalk ablation in juvenile giant freshwater prawn Macrobrachium rosenbergii and trial methyl farnesoate administration. Zool Sci 11: 45-53.
- Prestwich GD, Bruce MJ, Ujv√?¬°ry I, Chang ES (1990) Binding proteins for methyl farnesoate in lobster tissues: detection by photoaffinity labeling. Gen Comp Endocrinol 80: 232-237.
- Li H, Borst DW (1991) Characterization of a methyl farnesoate binding protein in hemolymph from Libinia emarginata. Gen Comp Endocrinol 81: 335-342.
- Tamone SL, Chang ES (1993) Methyl farnesoate stimulates ecdysteroid secretion from crab Y-organs in vitro. Gen Comp Endocrinol 89: 425-432.
- Laufer H, Borst D, Baker FC, Reuter CC, Tsai LW, et al. (1987) Identification of Juvenile hormone-like compound in a crustacean. Science 235: 202-205.
- Homola E, Sagi A, Laufer H (1991) Relationship of claw form and exoskeleton condition to reproductive system size and methyl farnesoate in the male spider crab Libinia emarginata. Invert Reprod Dev 20: 2190-2225.
- Sagi A, Homola E, Laufer H (1993) Distinct reproductive types of male spider crabs, Libinia emarginata differ in circulating and synthesizing methyl farnesoate. Biol Bull 185: 168-173.
- Laufer H, Biggers WJ, Ahl JS (1998) Stimulation of ovarian maturation in the crayfish Procambarus clarkii by methyl farnesoate.Gen Comp Endocrinol 111: 113-118.
- Tsukimura B, Kamemoto FI (1991) In vitro stimulation of oocytes by presumptive mandibular organ secretions in the shrimp, Penaeus vannamei. Aquaculture 92: 59-66.
- Nagaraju GP, Ramamurthi R, Reddy PS (2002) Methyl farnesoate stimulates ovarian growth in Penaeus indicus. In: Harikumar VS (Ed.), Recent Trends in Biotechnology.Agrobios, India. 1: 85-89.
- Reddy PS, Ramamurthy R (1998) Methyl farnesoate stimulates ovarian maturation in the freshwater crab Oziotelphusa senex senex. Curr Sci 74: 68-70.
- Zapata V, Lopez GLS, Medasani D, Rodriguez M (2003) Ovarian growth in the crab Chasmagnathus granulata induced by hormones and neuroregulators throughout the year. In vitro and in vivo studies. Aquaculture 224: 339-352
- Rodriguez EM, Medesani DA, Greco LS, Fingerman M (2002) Effects of some steroids and other compounds on ovarian growth of the red swamp crayfish, Procambarus clarkii, during early vitellogenesis. J Exp Zool 292: 82-87.
- Mak AS, Choi CL, Tiu SH, Hui JH, He JG, et al. (2005) Vitellogenesis in the red crab Charybdis feriatus: hepatopancreas-specific expression and farnesoic acid stimulation of vitellogenin gene expression and farnesoic acid stimulation of vitellogenin gene expression. Mol Reprod Dev 70: 288-300.
- Chan SM, Mak AS, Choi CL, Ma TH, Hui JH, et al. (2005) Vitellogenesis in the red crab, Charybdis feriatus contributions from small vitellogenin transcripts (CfVg) and farnesoic acid stimulation of CfVg gene expression. Ann N Y Acad Sci 1040: 74-79.
- Adiyodi KG, Subramanian T (1983) Arthropoda Crustacea. In: Adiyodi KG, Adiyodi RG (Eds.). Reproductive biology of Invertebrates. Wiley Chichester. 1: 443-495.
- Chang ES (1997) Chemistry of crustacean hormones that regulate growth and reproduction. In: Fingerman M, Nagabhushanam R, Thompson MF (Eds.), Recent Advances in Marine Biotechnology. Oxford and IBH Publishing, Co., New Delhi. 163-178.
- Charniaux-Cotton H, Toui, A (1973) Contrale de la prgvitellogenese et de la vitellogenese chez la crevette hermaphrodite Lysmata seticaudata Risso. C R Acad Sci Ser D 276: 2717-2720.
- Subramoniam T (2000) Crustacean ecdysteroids in reproduction and embryogenesis. Comp Biochem Physiol125: 135-156.
- Meusy JJ, Blanche, MF, Junera H (1977) Molting and vitellogenesis in the Crustacean Amphopod Orchestia gammarella Pallas. II. Synthesis of vitellogenin (female protein fraction of hemolymph) after the destruction of Y organs. Gen Comp Endocrinol 33: 35-40.
- Okumura T, Han CH, Suzuki Y, Aida K, Hanyu I (1992) Changes in hemolymph vitellogenin and ecdysteroid levels during the reproductive and non-reproductive molt cycles in the freshwater prawn Macrobrachium nipponense. Zool Sci 9: 37-45.
- Demeusy N (1962) Role de la gland de mue dans l√ʬ?¬?evolution ovarianne du crabe Carcinus maenas Linn Can Biol Mar 3: 37-56.
- Steel CGH, Vafopoulou X (1998) Ecdysteroid titres in haemolymph and other tissues during moulting and reproduction in the terrestrial isopod, Oniscus asellus (L.). Invertebr Reprod Dev 34: 187-194.
- Lafont R, Dauphin-Villemant C, Warren J, Rees H (2005) Ecdysteroid chemistry and biochemistry. Compr Mol Insect Sci 3 : 125-195.
- Gunamalai V, Kirubagaran R, Subramoniam T (2006) Vertebrate steroids and the control of female reproduction in two decapod crustaceans, Emerita asiatica and Macrobrachium rosenbergii. Curr Sci 90: 119-123.
- Brown M, Sieglaff D, Rees H (2009) Gonadal ecdysteroidogenesis in Arthropoda: occurrence and regulation. Annu Rev Entomol 54: 105-125
- Burns BG, Sangalang GB, Freeman HC, McMenemy M (1984) Isolation and identification of testosterone from serum and testes of the American lobster (Homarus americanus). Gen Comp Endocrinol 54: 429-432.
- Ollevier F, De Clerck D, Diederik H, De Loof A (1986) Identification of nonecdystroid steroids in hemolymph of both male and female Astacus leptodactylus (Crustacea) by gas chromatography- mass spectrometry. Gen Comp Endocrinol 61: 214-228.
- Fairs N, Quinlan P, Goad L (1990) Changes in ovarian unconjugated and conjugated steroid titers during vitellogenesis in Penaeus monodon. Aquaculture 89: 83-99.
- Quinitio ET, Hara A, Yamauchi K, Nakao S (1994) Changes in the steroid hormone and vitellogenin levels during the gametogenic cycle of the giant tiger shrimp, Penaeus monodon. Comp Biochem Physiol 109: 21-26.
- Yano I, Fingerman M, Nagabhushanam R (2000) Endocrine control of reproductive maturation in penaeid shrimp. In: Fingerman M, Nagabhushanam R (Eds.), Recent Advances in Marine Biotechnology: Aquaculture, Part A: Seaweeds and Invertebrates. Science Publishers, Enfield, NH.. 161-176.
- Shih J (1997) Sex steroid-like substances in the ovaries, hepatopancreases, and body fluid of female Mictyris brevidactylus. Zool Stud 36: 136-145.
- Kulkarni G, Nagabhushanam R, Joshi P (1979) Effect of progesterone on ovarian maturation in a marine penaeid prawn Parapenaeopsis hardwickii (Miers, 1878). Ind J Exp Biol 17: 986-987.
- Warrier SR, Tirumalai R, Subramoniam T (2001) Occurence of vertebrate steroids, estradiole 17√?¬≤ and progesterone in the reproducing females of the mud crab Scylla serrata. Comp Biochem Physiol A Mol Integr Physiol 130: 283-294.
- Yano I (1987) Maturation of kuruma prawns Penaeus japonicus cultured in earthen ponds. NOAA Tech Rep NMFS 47: 3-7.
- Tiu SH, Hui JH, He JG, Tobe SS, Chan SM (2006) Characterization of vitellogenin in the shrimp Metapenaeus ensis: expression studies and hormonal regulation of MeVg1 transcription in vitro. Mol Reprod Dev 73: 424-436.
- Coccia E, Lisa E, Cristo C, Cosmo A, Paolucci M (2010) Effects of estradiol and progesterone on the reproduction of the freshwater crayfish Cherax albidus. Biol Bull 218: 36-47.
- Yano I (1985) Induced ovarian maturation and spawning in greasyback shrimp Metapenaeus ensis, by progesterone. Aquaculture47: 223-229.
- Rodriguez EM, Lopez Greco LS, Medesani DA, Laufer H, Fingerman M (2002) Effect of methyl farnesoate, alone and in combination with other hormones, on ovarian growth of the red swamp crayfish, Procambarus clarkii, during vitellogenesis. Gen Comp Endocrinol 125: 34-40.
- Quackenbush L (1994) Lobster reproduction: a review. Crustaceana 67: 82-94.
- Paolucci M, Di Cristo C, Di Cosmo A (2002) Immunological evidence for progesterone and estradiol receptors in the freshwater crayfish Austropotamobius pallipes. Mol Reprod Dev 63: 55-62
- Zukowska-Arendarczyk M (1981) Effect of hypophyseal gonadotropins (FSH and LH) on the ovaries of the sand shrimp Crangon crangon (Crustacea: Decapoda). Mar Biol 63: 241-247.
- Jayaprakas V, Sambhu C (1998) Growth characteristics of white prawn Penaeus indicus (Decapoda-Crustacea) under dietary administration of protein hormones. Ind J Mar Sci 27: 389-395.
- Yano I (1993) Ultraintensive culture and maturation in captivity of penaeid shrimp. In: McVey JP (Ed.), CRC Handbook of Mariculture: Crustacean Aquaculture. CRC Press, Boca Raton. 2: 289-313.
- Laufer H, Landau M (1991) Endocrine control of reproduction in shrimp and other crustacea. In:DeLoach P, Dougherty WJ, Davidson MA (Eds.) Frontiers in shrimp research. Elsevier Science Publications, Amsterdam. 65-81.
- Souty C, Picaud JL (1984) Effect of injection of a human gonadotropin on the synthesis and liberation of vitellogenin by adipose tissue of the marine crustacean isopod Idotea balthica basteri Audouin. Gen Comp Endocrinol 54: 418-421.
- Toullec JY, Van Wormhoudt A (1987) Variations quantitatives de peptides apparent√?¬©s √?¬† l'hormone de croissance humaine chez Palaemon serratus. CR Acad Sci Paris 353: 265-269.
- Bomirski A, Klek-Kawi√?¬?ska E (1976) Stimulation of oogenesis in the sand shrimp, Crangon crangon, by a human gonadotrophin. Gen Comp Endocrinol 30: 239-242.
- Charmantier-Daures M, Charmantier G, Janssen KP, Aiken DE, vanHerp F (1994) Involvement of eyestalk factors in the neuroendocrine control of osmoregulation in adult American lobster Homarus americanus. Gen Comp Endocrinol 94: 281-293.
- Spanings-Pierrot C, Soyez D, Van Herp F, Gompel M, Skaret G, et al. (2000) Involvement of crustacean hyperglycemic hormone in the control of gill ion transport in the crab Pachygrapsus marmoratus. Gen Comp Endocrinol 119: 340-350.
- Lovett D, Tanner C, Glomski K, Ricart T, Borst DW (2006) The effect of seawater composition and osmolality on hemolymph levels of methyl farnesoate in the green crab Carcinus maenas. Comp Biochem Physiol A Mol Integr Physiol 143: 67-77.
- Nagaraju GP, Borst DW (2008) Methyl farnesoate couples environmental changes to testicular development in the crab Carcinus maenas. J Exp Biol 211: 2773-2778.
- Henry R, Borst DW (2005) Effects of eyestalk ablation on carbonic anhydrase activity in the euryhaline blue crab Callinectes sapidus: neuroendocrine control of enzyme expression. J Exp Zool A Comp Exp Biol 305: 23-31.
- Olmstead A, LeBlanc G (2007) The environmental-endocrine basis of gynandromorphism (intersex) in a crustacean. Int J Biol Sci 3: 77-84.
- Laufer H, Homola E, Landau M (1987) Control of methyl farnesoate synthesis in crustacean mandibular organs. Amer Zool 69: 27.
- Sarojini R, Nagabhushanam R, Fingerman M (1995) In vivo effects of dopamine and dopaminergic antagonists on testicular maturation in the red swamp crayfish, Procambarus clarkii. Biol Bull 189: 340-346.
- Reddy PR, Nagaraju GPC, Reddy PS (2004) Involvement of methyl farnesoate in the regulation of molting and reproduction in the freshwater crab Oziotelphusa senex senex. J Crust Biol 24: 511-515.
- Gunamalai V, Kirubagaran R, Subramoniam T (2004) Hormonal coordination of molting and female reproduction by ecdysteroids in the mole crab Emerita asiatica (Milne Edwards). Gen Comp Endocrinol 138: 128-138.
- Martin-Creuzburg D, Sperfeld E, Wacker A (2009) Colimitation of a freshwater herbivore by sterols and polyunsaturated fatty acids. Proc Biol Sci 276: 1805-1814.
- Heckmann LH, Sibly RM, Connon R, Hooper HL, Hutchinson TH, et al. (2008) Systems biology meets stress ecology: linking molecular and organismal stress responses in Daphnia magna. Genome Biol 9: 40.
- Medeiros MN, Mendonca LH, Hunter AL, Paiva-Silva GO, Mello FG, et al. (2004) The role of lipoxygenase products on the endocytosis of yolk proteins in insects: Participation of cAMP. Arch Insect Biochem Physiol 55: 178-187.
- Meusy JJ, Martin G, Soyez D, van Deijnen JE, Gallo JM, (1987) Immunochemical and immunocytochemical studies of the crustacean vitellogenesis-inhibiting hormone (VIH). Gen Comp Endocrinol 67: 333-341.
Relevant Topics
- Algal Blooms
- Blue Carbon Sequestration
- Brackish Water
- Catfish
- Coral Bleaching
- Coral Reefs
- Deep Sea Fish
- Deep Sea Mining
- Ichthyoplankton
- Mangrove Ecosystem
- Marine Engineering
- Marine Fisheries
- Marine Mammal Research
- Marine Microbiome Analysis
- Marine Pollution
- Marine Reptiles
- Marine Science
- Ocean Currents
- Photoendosymbiosis
- Reef Biology
- Sea Food
- Sea Grass
- Sea Transportation
- Seaweed
Recommended Journals
Article Tools
Article Usage
- Total views: 22729
- [From(publication date):
June-2013 - Mar 31, 2025] - Breakdown by view type
- HTML page views : 17370
- PDF downloads : 5359