Research Article Open Access
Report on the First Stages in the Translation of Measures of Health-Related Quality of Life at the End of Life
Poirier AL1,2*, Kwiatkowski F3,4, Commer JM5, Swaine-Verdier A6, Montel S7, Charpy JP8, Maucourt F5, Le Pape E9, Baize N9, Villet S10, Gamblin V10, Favier L11, Berger V1, Mercier M4,12 and Bonnetain F2,41Unité de biométrie, Institut de Cancérologie de l’Ouest, Paul Papin, Angers, France
2Unité de méthodologie et qualité de vie en cancérologie, EA 3181. CHRU Besançon, France
3Unité de biostatistiques, Centre Jean-Perrin, Clermont Ferrand, France
4Plateforme nationale Qualité de vie et Cancer, Dijon, France
5Unité de soins palliatifs et de soins de support Centre Paul Papin Angers, France
6Traductrice indépendante, Mazerat-Aurouze, France
7EA 4360 Maladies chroniques, santé perçue et processus d’adaptation. Approches épidémiologiques et psychologiques. Equipe mesure et maladies chroniques, Universités de Lorraine, Paris Descartes, France
8EA 4182, Université de Bourgogne, Dijon, France
9Centre Hospitalier Universitaire, Angers, France
10Centre Oscar Lambret, Lille, France
11Service d’Oncologie médicale, Centre Georges François Leclerc Dijon, France
12EA 3181, Université de Franche-Comté, Besançon, France
- *Corresponding Author:
- Anne-Lise Poirier
Unité de Biométrie, Institut de cancérologie de l’Ouest
Paul Papin 2, rue Moll 49933 Angers cedex 9, France
Tel: 0241352700
E-mail: anne-lise.poirier@ico.unicancer.fr
Received date: April 22, 2014; Accepted date: June 20, 2014; Published date: June 27, 2014
Citation: Poirier AL, Kwiatkowski F, Commer JM, Swaine-Verdier A, Montel S, et al. (2014) Report on the First Stages in the Translation of Measures of Health-Related Quality of Life at the End of Life. J Palliat Care Med 4:178. doi:10.4172/2165-7386.1000178
Copyright: © 2014 Poirier AL, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Palliative Care & Medicine
Abstract
Objectives: To translate and adapt the English version of QUAL-E (Quality of Life at the End of Life Measure) and MVQOLI (Missoula Vitas Quality Of Life Index) into French, taking into account cultural differences.
Methods: The selected translation process for linguistic validation included independent translations to the target language (French) performed by translators who were either native English speakers or French native speakers. Then, the translations were confronted to obtain a reconciled version as close as possible to the original text. This involved taking into account specific intercultural validity problems. To evaluate these translations, 30 patients tested and commented on the French adaptations of the quality-of-life (QoL) questionnaires.
Results: Some modifications were made following patients’ comments. The QUAL-E and MVQOLI translations were then finalized by the translation team. The prospective study (randomized multicentre cohort study) was then initiated. The validation of psychometric properties in the end-of-life setting is planned. Almost all questions and response options were well understood and accepted, no cultural barrier to the use of the questionnaires in palliative care was detected.
Keywords
End of life; Cancer; Quality of life; translation; QUAL-E; MVQOLI
Background
In 1993, the WHO (World Health Organisation) placed health in a wider context. Health is not only related to disease or disability, but also to physical, mental and social well-being [1]. As a result, clinicians should not work on the disease alone but should also consider patient multidimensionality. Health-related quality-of-life evaluation has emerged as an endpoint and tool to assess this multidimensional definition of health. It should at least include each of the following dimensions: physical, social, psychological and symptomatic.
Currently, clinical cancer research requires ever larger sample sizes in trials because expected overall survival improvements for reference treatments are narrowing. Equivalence or non-inferiority trials to control for toxicity and to assess deescalating doses are increasingly required. This is why taking into account another patientcentered outcome rather than tumor-centered endpoints seems a good alternative [2]. Health-related quality-of-life (HRQL) has become the second endpoint in clinical research in cancer to demonstrate clinical benefits of new treatments for patients [3].
It can be also considered as a primary endpoint in cancer clinical trials. This is especially true in a palliative setting when no benefits in terms of overall survival can be expected [4]. Indeed, if symptom management is sought, HRQL, particularly in palliative care, is important for both patients and clinicians to validate the clinical benefits of therapeutic strategies. It can help to address several important issues: should chemotherapy be administered through terminal disease? Does looking for a few additional months of life justify toxicities experienced by the patient? What kind of supportive care should be proposed to improve patient QoL until death [5-7]?
Thus, during the end-of-life phase, improving or conserving wellbeing and patient QoL should remain a major concern and maybe the ultimate goal. Given the profoundly personal concerns involved, patient perceptions of the end of life, as well as their QoL, are difficult to assess. Clinicians, nurses and paramedical staff are required to provide individualized care adapted to the end of life in order to improve patient QoL and well-being [8,9]. To enable evaluation of therapeutic strategy in this setting, it is crucial to use appropriate specific instruments to evaluate patient needs and feelings in this terminal phase of life. In a research context, a specific QoL questionnaire can be used to compare results between studies or between treatments in a study. Some studies have found that additional specific items may be required to take into account the specific QoL concerns of the end of life [10-14]. Currently, few questionnaires specifically adapted to the palliative cancer population are available in French. We can cite the QLQ-C15-PAL [15] developed by the European Organization for Research and Treatment of Cancer (EORTC) and the McGill Quality Of Life by Cohen et al. [16].
The QLQ-C15-PAL is a short questionnaire; there are 15 questions. Questions are focused on both autonomy and symptoms experienced by the patient (diarrhea, pain, trouble sleeping, depression, loss of appetite…) [15]. This QoL questionnaire is a reduction of the EORTC QLQ-C30 [17]. To adapt this questionnaire to a palliative population, the EORTC retained only the autonomy and symptoms items. This new questionnaire enables the amount of missing data to be limited because questions inappropriate for the palliative population were dropped.
Each question has response options on a five-point Likert scale. However, specific domains related to the end of life are not explored, such as Quality of care, Preparation for death, Spirituality or Transcendence [10-14,18].
Another questionnaire specifically adapted to the palliative population and adapted into French caught our attention; it was the MQOL (McGill Quality Of Life questionnaire). The principal domains of MQOL are Physical Symptoms, Psychological Symptoms, Outlook on Life, and Meaningful Existence. It is composed of 3 parts: an overall question (Part A), 16 questions that address the four domains mentioned above (Part B) (all questions comprising response options on a ten-point scale) and a last part where the patient lists or describes the things which have an impact on his quality of life (Part C) [16].
Finally, neither the QLQ-C15-PAL nor the MQOL were chosen for our study. The first does not explore specific domains related to the end of life; the second uses open questions that cannot be reduced to a score, it was not specifically adapted to the cancer population and the use of a ten-point response scale complicates administration (patient completion) and interpretation of the results [19].
In English language there are a lot of interesting questionnaires specifically adapted to our population [20-22]. After a literature review, we identified two questionnaires that take into account the specific QoL concerns of the end of life: the Missoulas-Vitas Quality Of Life Index (MVQOLI) and the QUAL-E (Quality of Life at the End of Life Measure). At this time neither of the two tools have been developed or culturally adapted in French. It therefore seemed relevant to perform a cultural adaptation of these English language QoL questionnaires to enable clinical trials in an end-of -life setting with a patient-oriented primary endpoint.
For this purpose, we launched the cohort study CEOLE to perform a cross-cultural validation of the MVQOLI and QUAL-E questionnaires for advanced cancer patients in a palliative setting. This study aims to enable us to validate these two questionnaires in two steps, culturally and psychometrically.
We report here the first step of the study: the translation of QUAL-E and MVQOLI QoL questionnaires and the assessment of their cultural relevance and acceptability.
Material and Methods
Design
The French adaptation of the two questionnaires (MVQOLI and QUAL-E) comprises two steps. The first step is the cultural adaptation, and the second is the psychometric validation. The Protocol of the study has been extensively described elsewhere [23].
This paper describes the first step: the cultural adaptation of the two questionnaires: MVQOLI and QUAL-E in a French-language version.
Population
To be eligible, patients were to present with advanced cancer (whatever the cancer location) and be treated or not with palliative intent only (chemotherapy, analgesic radiotherapy, surgery without curative intent). They were to have an ECOG (Eastern Cooperative Oncology Group) or a WHO performance status 2, a life expectancy ≥1 month and be older than 18 years.
Patients were to be informed about the palliative stage of the disease and were to have been followed for at least one month by a palliative caregiver to be included in the study.
The non-eligibility criteria were: a psychiatric disease compromising understanding of the study objectives and/or informed consent, and/or the ability of patients to meet the study requirements for psychological, social, family or geographical reasons.
Written informed consent was required before inclusion.
Ten hospitals were contacted to participate in the first step of the study. They were selected on the basis of the qualification of the medical team in palliative care. Five of these hospitals account for the totality of the sample (Integrated Center for Oncology Paul Papin - Angers, Cancer Care Center Georges François Leclerc - Dijon, Cancer Care Center Oscar Lambret - Lille, Angers University Hospital Center and Cholet Hospital Center).
Protection of Human subjects
This project was reviewed by a national committee of patients consulted in July 2009 and the project obtained the approval of the local ethics committee (CPP Ouest II Angers) in April, 2010.
In 2011, the Project was funded by a grant (PHRC: Programme Hospitalier de Recherche Clinique 2011) from the French national cancer institute (INCa :Institut National du Cancer).
Assessment tools
Missoula Vitas Quality Of Life Index (MVQOLI): The Missoula- Vitas Quality Of Life Index developed by Byock and Merriman was specifically designed for the evaluation of the end of life [12]. This QoL tool provides an exhaustive assessment of major dimensions in that setting. The longer version includes 25 items, and the shortened version includes only 15 items. Both investigate 5 dimensions: Symptoms, Function, Interpersonal Relationships, Well-being and Transcendence [12].
Every dimension is composed of three response modes: Assessment, Satisfaction and Importance. Assessment has a score from -2 to +2, Satisfaction a score from -4 to +4 and Importance from 1 to 5. A scoring algorithm is applied to obtain a QoL score for each dimension and for global quality-of-life [12,24].
The possible scoring range is -30 to 30 for each of the five subscores (Symptoms, Function, Interpersonal Relationships, Well-being and Transcendence), 0 to 30 for Total score and 1 to 5 for Global score. When the sub-score is negative, quality-of -life is reduced while positive sub-score imply an increasing quality of life.
The MVQOLI can be completed by the patient himself (selfadministered questionnaire) or with the help of a clinician, nurse or paramedical staff (interview) if required by the patient. A user guide was created to administer the questionnaire.
The shortened version was used in the study.
Quality of Life at the End of Life measure (QUAL-E): The Quality of Life at the End of Life measure was developed by Steinhauser and colleagues. It was developed for various advanced illness trajectories, including cancer. According to the authors, the QUAL-E can be proposed to patients who may or may not define their health status as terminal disease. The first version of QUAL-E contained 31 items and investigated 5 domains: Life completion, Relations with the health care system, Preparation for end of life, Symptom severity and Affective social support and one question on the overall quality of life [18].
Each question in answered on a five-point Likert scale. The QUAL-E is completed with a clinician’s help (interview).
The scoring algorithm is provided to obtain a score for four dimensions: Life Completion (Q23, Q24, Q25, Q26, Q27, Q28, Q14), Healthcare (Q7, Q8, Q9, Q10, Q11), Symptoms (Q1, Q2, Q3, Q4) and Preparation (Q17, Q18, Q19, Q21). The totality of the 31 items was not employed to the scoring process [25].
Ranges possible for sub-scores are 4 to 20 for Symptom Impact, 5 to 25 for Relationship with Healthcare System, 4 to 20 for Preparation, 7 to 35 for Life Completion. The Symptom impact and Preparation sub-scales are reverse-scored. The analysis of the global score with the sub-scales as a total score has not yet been developed [25].
Translation process (Phase I according EORTC)
The Food and Drug Administration (FDA) and the European Organization for Research and Treatment of Cancer (EORTC) have recommended forward-backward translation to adapt a HRQL questionnaire into another language. Forward-backward translation procedures can be divided into 4 steps: (1) a translation (Text 1) into the target language (French) is made by one translator who is a native English speaker; (2) a translation of text 1 into the source language (English) is made by a translator who is a native French speaker (Text 2); (3) confrontation of the two translations and debriefing; (4) final translation reconciliation. [26,27].
However, no study has demonstrated the superiority of this method [26]. The use of the forward-backward translation method is based on a consensus. Some studies have underlined that the backward translation method could generate a “word for word” translation instead of fluent, natural and appropriate language [28].
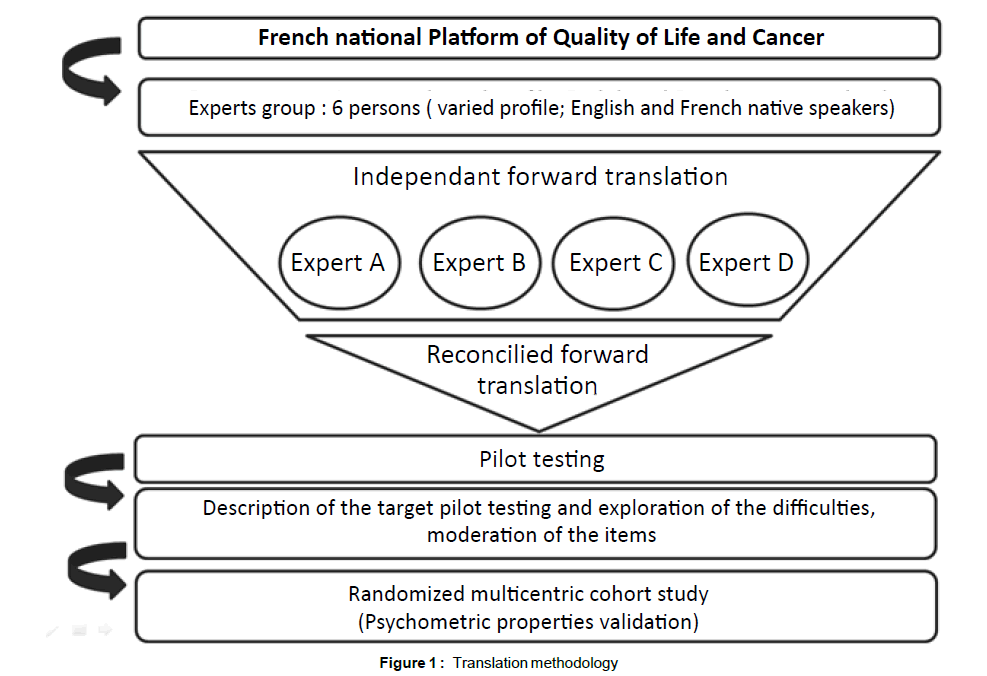
Another methodology to translate and adapt a questionnaire into another language exists. It is the « Dual Panel Approach » (DPA) [28]. This alternative method showed advantages in terms of preference by a target population, and complied with the psychometrics properties [29]. The translation of the two HRQL questionnaires (MVQOLI and QUAL-E) proposed in this trial is based on the DPA method including 3 stages: Translation, patient testing, and finalization of translation (Figure 1).
Stage 1: Translation: An expert group was created to translate the questionnaires from English into French The group included varied profession (linguists, psychologists, methodologists). Some of them were English native speakers, others French native speakers. The concept and methodological requirements were presented by the French National Platform of Quality of Life and Cancer in a workshop.
This Platform assembles experts from mathematics and statistics, public health, epidemiology and psychology. The main topic is the development of quality of life measurement and analysis methodology [29].
Each of the 6 experts (Angela Swain-Verdier, Jean-Pierre Charpy, Fabrice Kwiatkowski, Sébastien Montel, Mariette Mercier and Franck Bonnetain) worked independently on the translation before face-toface meeting and/or teleconference. In the course of a working meeting, the experts discussed the weight and meaning of the words. At the end, they agreed on a translation.
Stage 2: Patient testing: Thirty patients, representative of the target population, tested the two questionnaires. It was considered that beyond thirty patients in number, there was no new information, and empirical saturation occurred (see statistical analysis).
To assess the face validity and content validity of the questionnaire, one-to-one interview is recommended. In the course of the interview, patients indicated when they had not understood every part of the questionnaires, identified disturbing words or response options and highlighted subjects that were not relevant to their disease.
Stage 3: Finalization of the translation: The expert group met again once all data from patient tests had been collected and analyzed. Patients’ comments were used to modify the final translation as required.
The final translation was agreed on under the supervision of the national Quality of Life and Cancer clinical research platform (France). This version will be retained for the psychometric validation.
Data collection
Questionnaires were completed only once after reading the information letter and signing the informed consent. All questionnaires were in paper version. A member of the medical team administered the two QoL questionnaires to the patient. Our methodology for QoL questionnaire administration complied with the users’ guide of the two questionnaires [2,12]. Indeed, for QUAL-E, it was necessarily a member of the medical team who interviewed and recorded the patient’s responses. Ideally, for MVQOLI it was the patient himself who completed the questionnaire, but the clinician could also administer the MVQOLI as an interview and record the patient’s responses.
Data collected included: demographic data, education level, and completion times. Patient opinions were collected via an evaluation questionnaire: this included questionnaire length, complexity of questions, disconcerting questions, relationship between topics and their health situation, personal interest, their general comments. Patients’ comments also highlighted other subjects they would like to see in future questionnaires.
The Quality of Life questionnaires were completed only once at this stage of the study.
Questionnaire completion or response times were recorded by a member of the medical team (Start time – End time). Other collected data included: clinical characteristics, demographic data and education level.
Statistical analysis
The aim was to evaluate the translations in the targeted population: the consensus recommends a sample size between 10 and 40 individuals [27,28,30]. It was estimated that with a sample size of 30 patients, empirical saturation is reached and no new information emerges. Therefore, we retained the minimum requirement of 30 patients to test the translations (reconciled versions) of the two questionnaires.
The primary aim was a descriptive approach. All data collected were described. Continuous variables were presented by means (standard deviation) or median [range]. Qualitative variables were described by frequencies and percentages.
Ranges of dimensional subscores and total scores were calculated according to manual scoring methods recommended by the Missoulas- Vitas team for MVQOLI and Steinhauser team for QUAL-E. Each score was presented and described using median, range and mean (SD) and percentage of patients with a low score (negative score).
Missing data were presented using frequencies and percentages.
Statistical analysis was conducted with the SAS v9.2 (SAS Institute Inc., Cary, NC, USA)
Results
Patient characteristics
Between December 15, 2010 and April 5, 2011 30 patients were included in five centers and completed the two questionnaires.
The analysis was conducted on data from the 30 patients. Demographic and medical history data are shown in Table 1.
| n (%) | |
|---|---|
| Investigator sites Integrated Center for Oncology Paul Papin, Angers, France Cancer Care Center Georges François Leclerc, Dijon, France Cancer Care Center Oscar Lambret, Lille, France Angers University Hospital Center, France Cholet Hospital Center, France |
10 (33.3) 3 (10.0) 5 (16.7) 10 (33.3 2 (6.7) |
| Sex Male Female |
17 (56.7) 13 (43.3) |
| Performance status 0 1 2 3 4 Not known |
0 (0) 0 (0) 15 (62.5) 2 (8.3) 3 (12.5) 4 (16.7) |
| Age in years [18-60[ [60-90] Median [Min ; Max] |
6 (20.0) 24 (80.0) 69 [53 ; 87] |
| Cancer Location Colon Rectum Lung Melanoma Breast Endometrium Ovary Prostate Kidney Brain Other |
1 (3.3) 1 (3.3) 8 (26.7) 1 (3.3) 6 (20.0) 1 (3.3) 1 (3.3) 3 (10.0) 2 (6.7) 1 (3.3) 5 (16.6) |
| Chemotherapy | 13 (43.3) |
| Therapeuticradiotherapy | 1 (1.3) |
| Palliative radiotherapy | 5 (16.7) |
| Hormone therapy | 4 (13.3) |
| Supportive care No specific treatment Psychological help Social welfare officer Management of pain Nutrition Respiratoryphysiotherapy Not known |
11 (36.7) 5 (16.7) 4 (13.3) 9 (30.0) 4 (13.3) 1 (1.3) 1 (1.3) |
Table 1: Patient characteristics (n=30)
The patients who completed the questionnaires represented a wide range of ages (53-87) median 69 years. There was a slight predominance of males (56.7%). The principal cancer locations were lung (26.7%), breast (20.0%) and prostate (10.0%). Eleven patients (36.7%) had no supportive care and 5 (16.7%) had psychological help only.
MVQOLI results
There were no missing answers to the MVQOLI questionnaire.
The median time to complete the questionnaire was estimated at 10 minutes (5,40). Only 5 patients (16.6%) found the questionnaire too long.
26.7% of patients reported needing help to complete the MVQOLI.
Six questions were indicated as complicated (Q1, Q6, Q11, Q13, Q14, Q15) and 5 as disturbing (Q7, Q10, Q11, Q12, Q15) by some patients. Two questions were reported to be both complicated and disturbing. We can note that all questions in the Well-being domain were presented as disturbing at least once. Overall perceptions by the patients are shown in Table 2.
| N (%) | ||
|---|---|---|
| MVQOLI | QUAL-E | |
| Median time [Min; Max] (minutes) | 10 [5 - 40] | 20 [10 - 40] |
| Complicated item | 3 (10.0) | 8 (26.7) |
| Disturbingitem | 2 (6.7) | 7 (23.3) |
| Questionnaire relevant | 27 (90.0) | 28 (93.3) |
| Needed help | 8 (26.7) | 13 (43.3) |
Table 2: Duration of completion and patients’ comments on questionnaires (n=30)
The range for the Symptoms domain was (2 -24) and there were 4 patients with a negative sub-score (13.3%).
For the two domains Function and Transcendence, more than 20% of the patients had negative sub-scores and minimum scores were respectively -25 and -12. For the Interpersonal and Well-Being domains, respectively 36.7% and 33.3% of the patients reported reduced quality of life. For the Well-being domain the minimum score was reached (-30) (Table 3)
| Dimension | Median | Range | Mean | Standard deviation | Negative score n (%) |
|---|---|---|---|---|---|
| Symptoms Item : Q1,Q2,Q3 |
10 | [-20 – 24] | 9.4 | 9.6 | 4 (13.3) |
| Function Item : Q4,Q5,Q6 |
4 | [-25 – 12] | 1.0 | 9.0 | 8 (26.7) |
| Interpersonal Item : Q7,Q8,Q9 |
12 | [-25 – 30] | 7.4 | 16.1 | 11 (36.7) |
| Well-Being Item : Q10,Q11,Q12 |
6.5 | [-30 – 30] | 4.5 | 14.5 | 10 (33.3) |
| Transcendence Item : Q13,Q14,Q15 |
15.5 | [-12 – 30] | 10.5 | 14.1 | 7 (23.3) |
| Global Score | 3 | [1 – 5] | 3.4 | 0.9 | - |
| Total score Sum of Weighted Dimension Scores/10) + 15 | 19 | [6.4 – 25.5] | 18.2 | 4.2 | - |
Table 3: MVQOLI - Range of responses and means
Some patients also proposed new topics, such as causes of the disease linked to their background or lifestyle, health care relationships and death.
QUAL-E results
The median time to complete the questionnaire was estimated at 20 minutes (10-40). Eight patients (66.6%) found the questionnaire too long.
43.3% of the patients reported needing help to complete the QUAL-E.
All patients completed the questionnaires (n=30) and there were few missing items (3/21x30=0.4%).
Eight patients (26.7%) found some items complicated, and 7 (23.3%) found some items disturbing.
Nine questions were indicated as complicated (Q30, Q5, Q15, Q22, Q7, Q11, Q26, Q27, Q29). Only the items Q30, Q5, Q15 and Q22 were designated every time. Seven items were indicated as disturbing by patients (Q20, Q10, Q17, Q5, Q18, Q19 and Q21).
More than 50% of the population reported positive feelings in two domains: Relationship with Healthcare System and Life Completion.
The median sub-score for the Symptoms and Preparation domains were respectively 15 (5-20) and 20.5 (10-33). A high sub-score in the Symptoms domain demonstrates poor health status (pain, discomfort, fatigue…) and a high sub-score on the Preparation domain indicates worries (future, financial, dependence).
The median global score was 3 (2-5) (Table 4).
| Dimension | Theoretical range | Score | Median | Range | Mean | Standard deviation |
|---|---|---|---|---|---|---|
| Symptoms Item : Q1,Q2,Q3,Q4 |
[4 - 20] | Reverse | 15 | [5-20] | 13.8 | 3.8 |
| Healthcare Item : Q7,Q8,Q9,Q10,Q11 |
[5 - 25] | 21 | [11-25] | 20.1 | 3.8 | |
| Preparation Item : Q17,Q18,Q19,Q21 |
[4 - 20] | Reverse | 16 | [4-20] | 16.2 | 3.3 |
| Life completion Item : Q23,Q24,Q25,Q26,Q27,Q28,Q14 |
[7 - 35] | 20.5 | [10-33] | 20.4 | 5.7 | |
| Global score Item : Q31 |
[1 - 5] | 3 | [2-5] | 3.3 | 0.7 |
Table 4: QUAL-E - Range of responses and means
As for the MVQOLI, some patients proposed new topics such as the potential causes of the disease, health care relationships and death.
Translation results
Patients’ comments and results were used to modify the initial translation. In the course of a working meeting, the experts discussed the weight and meaning of the words.
For the MVQOLI, during the expert debriefing only 2 questions were changed: Question Q6, belonging to the Function dimension and the Importance category, and Q15 in the Transcendent dimension and the Importance category.
The question Q15 was identified as complicated and disturbing several times and Q6 seemed too negative compared to the original English version and identified as complicated. The other items were not moderated because the translations were the most adapted to the English version.
More in detail, the words chosen in the first translation of Q6 implied a notion of a constant limitation of the patient’s activity, thus a negative approach. The new version implies a dependence that is occasional and not constant.
In Q15 (“It is important to me to feel that my life has meaning”), the verb tense was switched from present subjunctive to present and the wording was simplified slightly. The present of the subjunctive underlined the hypothetical nature of the question, while the present focuses on the actual state. In the item: “It is important to me to feel that my life has meaning” the use of the present is important.
For QUAL-E, 6 items were modified: Q5, Q15, Q20, Q22, Q26 and Q30. The other items reported to be disturbing (Q7, Q11, Q26, Q27, Q28) or complicated (Q10, Q17, Q18, Q19, Q21 were not changed because only 1 patient had difficulties with each of these items, and during the review the items seemed faithful to the original English version and difficult to change in French.
More in detail, the response propositions were modified in Q5 because the responses were not adapted to the French question. In Q15, Q20, Q22, Q26 there was just one word that was changed in each item to specify or soften the meaning of them.
The final translation was agreed on by the QoL and Cancer Platform.
Discussion
In France, research on palliative care has been slow to develop [31]. An indication of this is that the number of health-related quality-of-life questionnaires available for French palliative practice is small [32]. For this reason, it is important to develop adapted tools.
From the outset, it seemed better to adapt an existing tool created by an expert team to the French environment, because developing a totally new instrument would require much more work and time. Moreover it would allow comparison of study results between countries. Therefore, it was not thought desirable to create a new instrument, since tools adapted to the palliative population existed. Besides, in a clinical research context it is important to compare treatments and populations using standardized tools.
We therefore decided to translate and evaluate existing foreign end-of-life HRQL questionnaires. It will later be possible, if required, to create a new questionnaire.
In palliative care, patients are often tired and in pain, with reduced mobility; frequently, they are in the lying position, where it is not easy to write; some patients can have visual disturbances. This is why, in this context, administering the measure face to face with an interviewer is often considered the best option [33]. But it is important to know who the interviewer is and his role with the patient, to assess any possible bias.
These questionnaires were not developed specifically for cancer patients, but cancer patients participated in their validation: 68% of the validation population were cancer patients for the MVQOLI and 64% for the QUAL-E [12,21]. Thus as more than 2/3 of the two validation populations were cancer patients, we hope that the two QoL questionnaires are adapted to our specific population of cancer patients.
Moreover, in the two questionnaires, the item response options were all on a five-point Likert scale. This simplifies administration (patient completion) and interpretation of the results [19].
We did not want to select only one of these tools because the structure of the two questionnaires is different and we did not know which was better suited to the French population. The results of the cohort study will enable us to determine which the more adapted tool is for patients with cancer at an advanced palliative stage.
In terms of content, the two questionnaires are quite similar. Indeed, the two domains in the QUAL-E: Completion and Preparation for end of life are equivalent to three domains in the MVQOLI: Interpersonal, Well-being and Transcendence. However there are differences in the amount of information attributed to the different domains (Symptoms is more developed in QUAL-E) whereas two other domains are covered by only one questionnaire (Healthcare in QUAL-E, Function in MVQOLI).
The format of QUAL-E and MVQOLI are different. After perusal of the two questionnaires, the feeling is substantially divergent. The MVQOLI has a scientific style. All domains have the same structure (3 questions for each dimension), questions are both short and direct. Sometimes the negative form is employed. The QUAL-E is more focused on the human aspect. Syntactically, sentences are longer; perhaps more “positive” or at least the negative form is less frequently used. We have the feeling that the QUAL-E team was at first primarily interested in creating a questionnaire that could improve the patient‘s actual well-being, while the Missoula-Vitas Team was mainly focused on the production of a standardized psychometric tool.
Which is more important: the humanist aspect of a questionnaire or its scientific usefulness?
In terms of methodology, translating a health-related quality-oflife questionnaire with appropriate attention to the cultural adaptation is hard work. It is necessary to be sure of the weight and meaning of the words, and to take into account the cultural specificity of the target population and medical team, especially concerning the subject of Death. A dual panel (DP) approach was chosen to translate MVQOLI and QUAL-E into French. Even if the forward-backward method is the “gold standard” [26,27]. The DP approach respects the major focus that was proposed by Acquadro et al. [34] i.e. a multistep approach. It is important too, that all independent translators could discuss and were agreed on one cultural adaptation before the pilot testing. DP enabled the translator team to exchange views more easily and intensely on both weight and meaning of the words.
The interviews with each of the patients who participated in the pilot testing were conducted very carefully. Realized on the target population, this step was very important to identify disturbing, complicated questions and if the questionnaires were well accepted. It was also necessary to take care to avoid disturbing medical teams with particular words. The subject of death is still taboo in France [30]. The quality of the future adaptation depends on that.
Indeed, if there is a disturbing word for the patient, the patient can not completed (the question or the whole questionnaire if the disturbing question is at the beginning), and if there is a disturbing word for the medical team, the questionnaire cannot be offered to the patient.
During the study, workers in the palliative unit accepted the use of questionnaires. In fact, the questionnaires enabled them to establish a more open dialogue with patients. They hoped that the use of these questionnaires would enable them to improve patients‘quality of life, and particularly the management of supportive care. Generally speaking, clinicians need standardized tools to evaluate their practice. Score and sub-score analysis confirmed this need.
The two questionnaires, QUAL-E and MVQOLI are not yet validated in French (step 2 of the study), but we used their scoring guidelines. For MVQOLI, the Symptoms dimension yielded good results (only 13.3% of the patients had a negative score). For the Function and Transcendence dimensions more than 20% of patients had a negative sub-score (poor quality of life for these dimensions). For the Interpersonal and Well-Being domains respectively 36.7% and 33.3% of patients reported reduced quality of life.
For QUAL-E, more than 50% of the population reported good feelings in the two dimensions: Relationship with Healthcare System and Life completion. A high sub-score on the Symptoms domain reflects poor health status (pain, discomfort, fatigue…) and a high subscore in the Preparation domain demonstrates certain worries (future, financial, dependence). The median sub-score for the Symptoms and Preparation domains were respectively 15 (5-20) and 20.5 (10-33).
Thus, according to these results, some patients needed help on several dimensions. The use of the two questionnaires enabled caregivers to identify patient difficulties and to modify health care (psychological and social support, pain…).
The two questionnaires were well accepted by the patients as well. Indeed, few items were frequently classified as complicated and/or disturbing. In general, patients were open to communicating with the medical team about their QoL. Some of them even proposed new topics such as elements in their background or personal history that could have caused the disease, health care relationships and death. We believe that via these new topics the patients were trying to obtain answers. For factors contributing to causing the disease, the question for patients was whether the environment, food, household products and other products, or cosmetics could have caused their cancer. For Death, they probably they needed as much information as possible in order not to be afraid of the moment (feelings, pain, mental state…).
For the topic of health care relationships, addressed by the QUAL-E questionnaire, we believe that patients wanted to talk about their caregivers in more detail (nurses…).
This first step of the study highlighted the importance for patients of being helped with reading or writing to complete the questionnaires.
These aspects confirmed that in palliative setting, face-to faceinterview seem the best option for the patient. Moreover these QoL questionnaires could be used as a communication tool to facilitate discussion about difficult subjects that the patient and/or the clinician would not have discussed spontaneously [35].
From these results, a randomized multicentre cohort study is underway for the psychometric validation of the French versions of QUAL-E and MVQOLI. The main criterion to assess the reliability of the questionnaires is their reproducibility (test-retest method) using intraclass correlation coefficients. Under the assumptions considered and considering the number of dimensions for each questionnaire for which intraclass correlation coefficients will be calculated, it is necessary to include 372 patients. To conduct a complete psychometric validation, other specific objectives such as sensitivity to change, discriminant abilities and convergent validity are being investigated.
The cohort study has been underway since November, 2011
Conclusion
We found that the MVQOLI and QUAL-E questionnaires are both valid and usable for assessment of palliative advanced cancer patient QoL.
The two questionnaires in their French version were well accepted by both patients and healthcare professionals, making it reasonable to conclude that the questionnaires are culturally adapted for the French environment. A cohort study will enable us to validate the psychometric properties of these two questionnaires. This cohort study is underway.
References
- (1993) Study protocol for the World Health Organization project to develop a Quality of Life assessment instrument (WHOQOL). Qual Life Res 2: 153-159.
- Fiteni F, Westeel V, Pivot X, Borg C, Vernerey D, et al. (2014) Endpoints in cancer clinical trials. J ViscSurg 151: 17-22.
- Beitz J, Gnecco C, Justice R (1996) Quality-of-life end points in cancer clinical trials: the U.S. Food and Drug Administration perspective. J Natl Cancer InstMonogr 20:7-9.
- Bonnetain F (2010) [Health related quality of life and endpoints in oncology]. Cancer Radiother 14: 515-518.
- Kao S, Shafiq J, Vardy J, Adams D (2009) Use of chemotherapy at end of life in oncology patients. Ann Oncol 20: 1555-1559.
- Kadakia KC, Moynihan TJ, Smith TJ, Loprinzi CL (2012) Palliative communications: addressing chemotherapy in patients with advanced cancer. Ann Oncol 23 Suppl 3: 29-32.
- Lee HR, Yi SY, Kim do Y (2013) Evaluation of Prescribing Medications for Terminal Cancer Patients near Death: Essential or Futile. Cancer Res Treat 45: 220-225.
- Wright AA, Zhang B, Ray A, Mack JW, Trice E, et al. (2008) Associations between End-of-Life discussions, patient mental health, medical care near death, and caregivers bereavement adjustment. Journal of the American Medical Association 300: 1665-1673.
- Patrick DL, Curtis JR, Engelberg RA, Nielsen E, McCown E (2003) Measuring and improving the quality of dying and death. Ann Intern Med 139: 410-415.
- Aspinal F, Hughes R, Dunckley M, Addington-Hall J (2006) What is important to measure in the last months and weeks of life?: A modified nominal group study. Int J Nurs Stud 43: 393-403.
- Steinhauser KE1, Christakis NA, Clipp EC, McNeilly M, McIntyre L, et al. (284) Factors considered important at the end of life by patients, family, physicians, and other care providers. JAMA 284: 2476-2482.
- Byock IR, Merriman MP (1998) Measuring quality of life for patients with terminal illness: the Missoula-VITAS quality of life index. Palliat Med 12: 231-244.
- Teno JM, Byock I, Field MJ (1999) Research agenda for developing measures to examine quality of care and quality of life of patients diagnosed with life-limiting illness. J Pain Symptom Manage 17: 75-82.
- Stewart AL, Teno J, Patrick DL, Lynn J (1999) The concept of quality of life of dying persons in the context of health care. J Pain Symptom Manage 17: 93-108.
- Groenvold M, Petersen MA, Aaronson NK, Arraras JI, Blazeby JM, et al. (2006) The development of the EORTC QLQ-C15-PAL: a shortened questionnaire for cancer patients in palliative care. Eur J Cancer 42: 55-64.
- Robin Cohen S, Balfour M Mount, Michael G Strobel, France Bui (1995) The McGill Quality of Life Questionnaire: a measure of quality of life appropriate for people with advanced disease. A preliminary study of validity and acceptability. Palliat Med 9: 207-219.
- Osoba D, Aaronson N, Zee B, Sprangers M, te Velde A (1997) Modification of the EORTC QLQ-C30 (version 2.0) based on content validity and reliability testing in large samples of patients with cancer. The Study Group on Quality of Life of the EORTC and the Symptom Control and Quality of Life Committees of the NCI of Canada Clinical Trials Group. Qual Life Res 6:103-108.
- Steinhauser KE, Bosworth HB, Clipp EC, McNeilly M, Christakis NA, et al. (2002) Initial assessment of a new instrument to measure quality of life at the end of life. J Palliat Med 5: 829-841.
- Jaeschke R, Singer J, Guyatt GH (1990) A comparison of seven-point and visual analogue scales. Data from a randomized trial. Control Clin Trials 11: 43-51.
- Emery MP, Perrier LL, Acquadro C (2005) Patient-reported outcome and quality of life instruments database (PROQOLID): frequently asked questions. Health Qual Life Outcomes 3: 12.
- Kirkova J, Davis MP, Walsh D, Tiernan E, O'Leary N, et al. (2006) Cancer symptom assessment instruments: a systematic review. J ClinOncol 24: 1459-1473.
- Jordhoy MS, IngerRingdal G, Helbostad JL, Oldervoll L, Loge JH, et al. (2007) Assessing physical functioning: a systematic review of quality of life measures developed for use in palliative care. Palliat Med 21: 673-682.
- Poirier AL, Kwiatkowski F, Commer JM, D'Aillières B, Berger V, et al. (2012) Health-related quality of life in cancer patients at the end of life, translation, validation, and longitudinal analysis of specific tools: study protocol for a randomized controlled trial. Trials 13:39
- Schwartz CE, Merriman MP, Reed G, Byock I (2005) Evaluation of the Missoula-VITAS Quality of Life Index--revised: research tool or clinical tool? J Palliat Med 8: 121-135.
- Steinhauser KE, Clipp EC, Bosworth HB, McNeilly M, Christakis NA, et al. (2004) Measuring quality of life at the end of life: validation of the QUAL-E. Palliat Support Care 2: 3-14.
- U.S. Department of Health and Human Services FDA Center for Drug Evaluation and Research, U.S. Department of Health and Human Services FDA Center for Biologics Evaluation and Research, U.S. Department of Health and Human Services FDA Center for Devices and Radiological Health (2006) Guidance for industry: patient-reported outcome measures: use in medical product development to support labeling claims: draft guidance. Health and Quality of Life Outcomes 4:79
- L. Dewolf, M. Koller, G. Velikova, C. Johnson, N. Scott, et al. (2009) on behalf of the EORTC Quality of Life Group: Eortc Quality Of Life Group: Translation Procedure. EORTC Third Edition.
- Swaine-Verdier A, Doward LC, Hagell P, Thorsen H, McKenna SP (2004) Adapting quality of life instruments. Value Health 7 Suppl 1: S27-30.
- Boyer L, Baumstarck K, Michel P, Boucekine M, Anota A, et al. (2014) Statistical challenges of quality of life and cancer: new avenues for future research. Expert Rev Pharmacoecon Outcomes Res 14: 19-22.
- Hagell P, Hedin PJ, Meads DM, Nyberg L, McKenna SP (2010) Effects of method of translation of patient-reported health outcome questionnaires: a randomized study of the translation of the Rheumatoid Arthritis Quality of Life (RAQoL) Instrument for Sweden. Value Health 13:424-430.
- Rapport Onfv (2005)Devalois, Bernard. – Continuer à développer les soinspalliatifs: le coeuret la raison. La Lettre de la SFAP
- Jordhoy MS, IngerRingdal G, Helbostad JL, Oldervoll L, Loge JH, et al. (2007) Assessing physical functioning: a systematic review of quality of life measures developed for use in palliative care. Palliat Med 21: 673-682.
- Davidson SN, Murtagh FE, Higginson IJ (2008) Methodological considerations for end-of-life research in patients with chronic kidney disease. J Nephrol 21:268-282.
- Acquadro C, Conway K, Hareendran A, Aaronson N (2008) European Regulatory Issues and Quality of Life Assessment (ERIQA) Group: Literature review of methods to translate health-related quality of life questionnaires for use in multinational clinical trials. Value Health 11:509-521.
- Bredart A, Dolbeault S (2005) Evaluation de la qualité de vie en oncologie: I--définitionsetobjectifs. Revue Francophone de Psycho-Oncologie4:7-12.
Relevant Topics
- Caregiver Support Programs
- End of Life Care
- End-of-Life Communication
- Ethics in Palliative
- Euthanasia
- Family Caregiver
- Geriatric Care
- Holistic Care
- Home Care
- Hospice Care
- Hospice Palliative Care
- Old Age Care
- Palliative Care
- Palliative Care and Euthanasia
- Palliative Care Drugs
- Palliative Care in Oncology
- Palliative Care Medications
- Palliative Care Nursing
- Palliative Medicare
- Palliative Neurology
- Palliative Oncology
- Palliative Psychology
- Palliative Sedation
- Palliative Surgery
- Palliative Treatment
- Pediatric Palliative Care
- Volunteer Palliative Care
Recommended Journals
- Journal of Cardiac and Pulmonary Rehabilitation
- Journal of Community & Public Health Nursing
- Journal of Community & Public Health Nursing
- Journal of Health Care and Prevention
- Journal of Health Care and Prevention
- Journal of Paediatric Medicine & Surgery
- Journal of Paediatric Medicine & Surgery
- Journal of Pain & Relief
- Palliative Care & Medicine
- Journal of Pain & Relief
- Journal of Pediatric Neurological Disorders
- Neonatal and Pediatric Medicine
- Neonatal and Pediatric Medicine
- Neuroscience and Psychiatry: Open Access
- OMICS Journal of Radiology
- The Psychiatrist: Clinical and Therapeutic Journal
Article Tools
Article Usage
- Total views: 13711
- [From(publication date):
June-2014 - Jul 03, 2025] - Breakdown by view type
- HTML page views : 9173
- PDF downloads : 4538