Repeated Assessment of Exercise Capacity under Goal-Oriented Sequential Combination Therapy for Pulmonary Arterial Hypertension
Received: 17-Apr-2017 / Accepted Date: 27-Apr-2017 / Published Date: 05-May-2017
Abstract
Little is known about the effects of sequential combination therapy on exercise capacity in pulmonary arterial hypertension (PAH). We monitored exercise capacity by cardiopulmonary exercise testing (CPX) and observed the benefit of using a peak oxygen uptake (VO2) cut-off of 15 mL/min/kg to guide combination therapy. The patients underwent CPX at baseline and after 3, 6, and 12 months. In patients newly diagnosed with PAH, exercise capacity progressively improved due to sequential combination therapy that was revised according to patients’ peak VO2. Therefore, repeated CPX assessment can provide useful information regarding the effectiveness of goal-oriented treatment for PAH.
Keywords: Sequential combination therapy, Cardiopulmonary exercise testing, Pulmonary arterial hypertension
74056Abbreviations
ERA: Endothelin Receptor Antagonists; PDE-5i: Phosphodiesterase-5 Inhibitors; CPX: Cardiopulmonary Exercise Testing; Cath: Cardiac Catheterization.
Introduction
Pulmonary arterial hypertension (PAH) is a life-threatening disease that is associated with poor prognosis [1]. Many potential therapeutic options are now available for patients with PAH [2], including prostanoids [3], endothelin receptor antagonists (ERA) [4], and phosphodiesterase type 5 inhibitors (PDE-5i) [5]. Current treatment algorithms [6] recommend an ERA or PDE-5i as a first-line treatment for PAH of functional class II or III. Compared with monotherapy, combination therapy improved exercise capacity and reduced the risk of clinical disease progression in patients with PAH [7]. However, the optimal strategy regarding the implementation of combination therapy has not yet been determined.
Recognition of the potential value of cardiopulmonary exercise testing (CPX) in patients with PAH is increasing [7,8]. However, little is known about how exercise capacity changes over time under “goaloriented” sequential combination therapy in patients newly diagnosed with PAH. Hoeper et al. proposed goal-oriented treatment of PAH patients according to results of CPX or 6-min walk distance [7]. In that study, the treatment goals to stratify therapeutic decisions were set according to established prognostic criteria: 6-min walk distance, 380 m; peak oxygen uptake (VO2), >10.4 mL/min/kg; and peak systolic blood pressure during exercise, >120 mm Hg. These results compared favorably with a historical control group of PAH patients treated before 2002 (i.e., before bosentan and sildenafil became available) [9,10]. However, these treatment goals might be suboptimal. For example, the most recent treatment guideline for PAH advocates that a peak VO2 cut-off value of ≥ 15 mL/min/kg is a better indicator of prognosis [6]. We therefore observed the therapeutic effect of goal-oriented therapy evaluated by using CPX. The aim of this study was to use a peak VO2 cut-off of 15 mL/min/kg to guide combination therapy and observe exercise capacity over time by monitoring the results of CPX in patients with PAH [11].
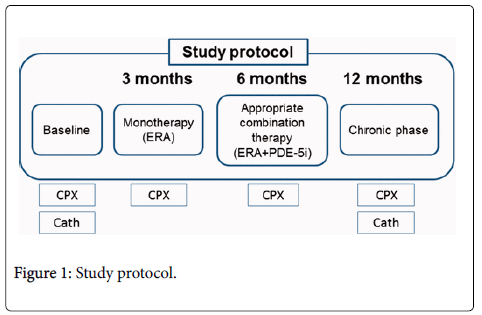
Protocol
Treatment goals were set and therapeutic decisions were made according to the established prognostic criterion of a peak VO2>15.0 mL/min/kg during CPX. Thirty patients newly diagnosed with PAH were treated with goal-oriented sequential combination therapy. ERA was the first-line treatment, with PDE-5i as the preferred combination partner. The patients underwent CPX at baseline and after 3, 6, and 12 months (Figure 1). We focused on relatively new indices, namely circulatory power (CP) and ventilator power (VP), in the CPX of PAH patients. CP was defined as the product of peak O2 uptake and peak systolic blood pressure; VP was defined as peak systolic blood pressure divided by the minute ventilation–CO2 production slope.
Discussion
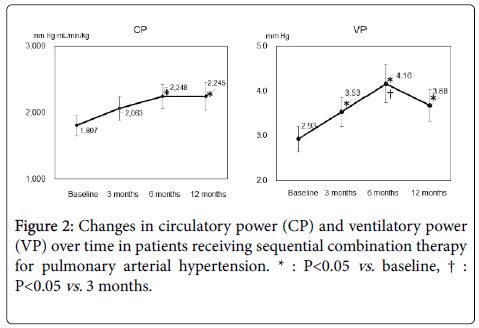
During the 12-month observation period, only one patient required intravenous epoprostenol (after 6 months). Ultimately, after 12 months, ERA had been administered to 100% of the study patients and PDE-5i to 82%. Mean CP at baseline and after 3, 6, and 12 months was 1807, 2063, 2248, and 2245 mm Hg·mL/min/kg, respectively, and mean VP was 2.93, 3.53, 4.16, and 3.68 mm Hg, respectively. CP was greater after 6 months than at baseline (P=0.047); VP was greater after 3 months than at baseline (P=0.019) and further improved at 6 months compared with 3 months (P=0.040) (Figure 2). Our CPX-guided goaloriented treatment strategy might avoid excess medication and cost but still provide appropriate intervention by enabling treatment to be tailored to the individual patient.
Among the parameters available through CPX testing, we particularly focused on changes in CP and VP in the present study. CP is a powerful prognostic marker in chronic heart failure [12]. In addition, VP was independently predictive of cardiac events compared with the prognostic value of standard CPX indices [13]. Whereas CP is an index that combines central and peripheral components of cardiac stroke work, VP provides a measure that combines systemic hemodynamics with CO2 production efficiency. Both of them provide important information regarding disease severity and prognosis [14]. To our knowledge, no other data are available regarding changes in CP and VP over time in PAH patients receiving sequential combination therapy. In our study, both CP and VP—like other prognostic CPX parameters—gradually improved due to sequential combination therapy for at least 6 months. Future work is needed to determine the prognostic utility of them in patients with PAH.
One important finding of this study was that, to achieve the predefined treatment goals, combination treatment eventually became necessary in more than 80% of the patients, indicating that monotherapy lacks sufficient effectiveness in many patients with PAH. The strategy we followed was based on many factors, including the interval at which to monitor potential side effects of each medication, physician experience, practicability, and economic considerations. However, further studies are needed to define the variables most useful for clinical decision-making and the treatment principles that provide the best long-term results.
Conclusion
In patients with newly diagnosed PAH, sequential combination therapy significantly improved exercise capacity, particularly CP and VP. Sequential combination therapy may be a useful treatment option in patients with PAH. Therefore, repeated CPX assessment, including measurement of CP and VP, can provide useful information regarding the effectiveness of goal-oriented treatment in patients with PAH.
References
- Thenappan T, Shah SJ, Rich S, Tian L, Archer SL, et al. (2010) Survival in pulmonary arterial hypertension: a reappraisal of the NIH risk stratification equation. Eur Respir J 35: 1079-1087
- Fukumoto Y, Shimokawa H (2011) Recent progress in the management of pulmonary hypertension. Circ J 75: 1801-1810.
- Okano Y, Yoshioka T, Shimouchi A, Satoh T, Kunieda T (1997) Orally active prostacyclin analogue in primary pulmonary hypertension. Lancet 349: 1365.
- Rubin LJ, Badesch DB, Barst RJ, Galie N, Black CM, et al. (2002) Bosentan therapy for pulmonary arterial hypertension. N Engl J Med 346: 896-903.
- Humbert M, Sitbon O, Simonneau G (2004) Treatment of pulmonary arterial hypertension. N Engl J Med 351: 1425-1436.
- Galie N, Hoeper MM, Humbert M, Torbicki A, Vachiery JL, et al. (2009) Guidelines for the diagnosis and treatment of pulmonary hypertension: The Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS), endorsed by the International Society of Heart and Lung Transplantation (ISHLT). Eur Heart J 30: 2493-2537.
- Hoeper MM, Markevych I, Spiekerkoetter E, Welte T, Niedermeyer J (2005) Goal-oriented treatment and combination therapy for pulmonary arterial hypertension. Eur Respir J 26: 858-863
- Arena R, Lavie CJ, Milani RV, Myers J, Guazzi M (2010) Cardiopulmonary exercise testing in patients with pulmonary arterial hypertension: an evidence-based review. J Heart Lung Transplant 29: 159-173.
- D'Alonzo GE, Barst RJ, Ayres SM, Bergofsky EH, Brundage BH, et al. (1991) Survival in patients with primary pulmonary hypertension. Results from a national prospective registry. Ann Intern Med 115: 343-349.
- Sandoval J, Bauerle O, Palomar A, Gomez A, Martinez-Guerra ML, et al. (1994) Survival in primary pulmonary hypertension. Validation of a prognostic equation. Circulation 89: 1733-1744.
- Hirashiki A, Adachi S, Nakano Y, Kamimura Y, Shimokata S, et al. (2017) Circulatory power and ventilatory power over time under goal-oriented sequential combination therapy for pulmonary arterial hypertension. Pulm Circ in press
- Cohen-Solal A, Tabet JY, Logeart D, Bourgoin P, Tokmakova M, et al. (2002) A non-invasively determined surrogate of cardiac power ('circulatory power') at peak exercise is a powerful prognostic factor in chronic heart failure. Eur Heart J 23: 806-814.
- Borghi-Silva A, Labate V, Arena R, Bandera F, Generati G, et al. (2014) Exercise ventilatory power in heart failure patients: functional phenotypes definition by combining cardiopulmonary exercise testing with stress echocardiography. Int J Cardiol 176: 1348-1349.
- Forman DE, Guazzi M, Myers J, Chase P, Bensimhon D, et al. (2012) Ventilatory power: a novel index that enhances prognostic assessment of patients with heart failure. Circ Heart Fail 5: 621-626.
Citation: Hirashiki A, Shimizu A, Toba K, Murohara T, Kondo T (2017) Repeated Assessment of Exercise Capacity under Goal-Oriented Sequential Combination Therapy for Pulmonary Arterial Hypertension. J Card Pulm Rehabil 1: 103.
Copyright: © 2017 Hirashiki A, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Open Access Journals
Article Usage
- Total views: 3536
- [From(publication date): 0-2017 - Apr 04, 2025]
- Breakdown by view type
- HTML page views: 2664
- PDF downloads: 872