Research Article Open Access
Renal Oncocytoma and Cromophobe Renal Cell Carcinoma: Main Morphological Differences and Proposal of a Simple Histochemical and Immunohistochemical Panel to separate them
Gallegos Ivan*, Carrasco Gonzalo, Fernandez Cristina, Castillo Octavio and Valdevenito RaulHospital Clinico, University Of Chile, Clinica Indisa, Chile
- *Corresponding Author:
- Ivan Gallegos MD
Hospital Clinico, University Of Chile, Clinica Indisa, Chile
Tel: 56-2-9788641
E-mail: igallegosmendez@gmail.com
Received date: August 11, 2014; Accepted date: October 08, 2014; Published date: October 12, 2014
Citation: Ivan G, Gonzalo C, Cristina F, Octavio C, Raul V (2014) Renal Oncocytoma and Cromophobe Renal Cell Carcinoma: Main Morphological Differences and Proposal of a Simple Histochemical and Immunohistochemical Panel to separate them. J Clin Exp Pathol 4:195. doi: 10.4172/2161-0681.1000195
Copyright: © 2014 Ivan G, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Clinical & Experimental Pathology
Abstract
Introduction: Renal Oncocytoma (RO) and Chromophobe Renal Cell Carcinoma (ChCCR) are within spectrum of “eosinophilic renal neoplasms” that can share morphological features. In some instances, it can be challenging differentiate both entities based only on the HE. For this reason, complementary ancillary techniques are needed.
Methodology: Sixteen RO cases and 21 ChCCR cases were evaluated for macroscopic and microscopic features, defining their architectural, nuclear and special stains criterion. Hale`s Coloidal Iron (HCI), Citokeratin 7 and CD15 were performed.
Results: Significant (p<.001) morphological differences were the pattern of grown (16/16 RO nested type vs 19/21 ChCCR diffuse type), nuclear morphology (“raisinoid” nuclei: 0/16 RO vs. 19/21 ChRCC) and presence of mitotic figure (0/16 RO vs. 16/21 ChCCR). Special stains showed that HCI was positive in 2/16 cases of RO and 20/21 of ChCCR, CK7 was positive in 1/16 cases of RO and 18/21 of ChCCR and CD15 was positive in 13/16 RO and 4/21 of ChCCR (p<.001).
Conclusion: Main differences beetwen RO and ChCCR are respectively the pattern of grown (nested/diffuse), raisinoid nucleus (-/+), and presence of mitosis (-/+). Besides ancillary techniques show HCH (-/+), CK7 (-/+) and CD15 (+/-). The ancillary panel of stains is very easy to perform and useful to achieve the correct diagnosis.
Keywords
CD15; Cromophobe carcinoma; Hale`s iron colloidal; Keratin 7; Renal oncytoma
Introduction
Renal oncocytoma (RO) was first described in 1942 by Zippel [1], but it was not considered as a distinct tumor until many years later, being classified together with malignant tumors of the kidney for more than four decades. Currently, RO is regarded as a benign kidney tumor [2,3]. RO has several features that overlap with other renal neoplasms with a preponderance of granular cytoplasm, such as Chromophobe Renal Cell Carcinoma (ChRCC). The lack of knowledge about this entire spectrum of eosinophilic renal cell neoplasms has led to several misconceptions in the literature regarding renal oncocytoma. These include the "grading of oncocytomas," "metastatic oncocytomas," “malignant oncocytoma”, and the impression that RO is usually low grade and lacks prominent nucleoli. However if hematoxylin and eosin-based morphology alone cannot render a definitive diagnosis of aforementioned tumors, pathologists resort to ancillary techniques, including histochemistry, immunohistochemistry, electron microscopy (EM), cytogenetics, as well as other molecular studies. Of them, Hale’s colloidal iron stain has long been used to differentiate renal tumors [4,5], but sometimes, this stain is technically difficult to perform and the results can be difficult to interpret. EM has been used to differentiate ChRCC and RO [6], but its use is expensive, work intensive, time-consuming, and not easily available to practicing pathologists. Several immunohistochemistry markers have been used to differentiate between both tumors, especially in cases difficult to assess only with H&E. We propose a simple panel of histochemistry (Hale’s colloidal iron) and two common immunohistochemistry markers (CD15 and Cytokeratin 7), to differentiate the majority of these tumors.
Materials and Methods
Tumor specimens
Renal tumors from 38 patients with the diagnosis of RO or ChRCC were pointed from the tissue registry files at the Clinical Hospital of the University of Chile and Indisa Clinic from 1991 to 2009, to form the basis of this study. All cases had histological material available to be reviewed by the urologic pathologist (IG) of which 18 cases of renal oncocytoma and 20 cases of Chromophobe Renal Cell Carcinoma were obtained. One case of ChRCC was dismissed because it was reclassified as Granular cell variant of conventional renal cell carcinoma and two cases originally classified as “atypical oncocytomas”, with large irregular nuclei and variations in cell size and configuration were reclassified as ChRCC. Two cases of RO was moved to the ChCCR group after the pathologic re-evaluation (IG) because have nuclear and architectural features of malignant lesion. Finally two groups of 16 RO and 21 ChRCC were formed. Information of macroscopic findings was obtained from surgical pathology reports, and the clinical data was retrieved from medical records. The Clinical information includes age and gender. All carcinomas were classified as ChRCC with no differentiation between classic or eosinophilic variants.
Macroscopic and histologic features
The noted macroscopic features included size, laterality and the presence or absence of a central scar. The contour of the tumor was separated into rounded or infiltrative forms. The dominant architectural pattern was defined as the most common pattern found in the tumor. Besides there are two separate groups: “Nested type” which forms acinar or tubular structures separated by many delicate vascular septum, and “Sheet type” tumor cell which forms diffuse masses, with only few delicate vascular septum. The involvement of perinephritic fat was defined as the presence of tumor cells in contact with adipose tissue, even in the cortical-peripheral zone or in the renal sinus. The vascular invasion was defined as tumor cells in the vascular lumen associated to reactive changes like cytoplasmic eosinophilia, fibrin deposits and endothelial cells over tumor cells. The presence of tumor necrosis was of the ischemic type, with karyorrectic debris. The presence of mitosis was defined as any mitotic figure within the tumor in a 50 consecutive high power fields (HPF). The nuclear features were assessed, separating them into the two types: rounded and “raisinoid” (defined as very irregular and with wrinkled nuclear contour). Although this is not applicable for oncocytomas, an equivalent Fuhrman's grade was assigned based on its nuclear characteristics.
Histochemistry
Hale’s Colloidal iron stains were performed using the modified Mowry staining method, and deep blue staining was interpreted as positive, separating cases into predominant cytoplasmic and negative or apical positivity. This stain was performed on whole tumor slides.
Tissue microarray
Regarding other ancillary studies, a manual Tissue Microarray (TMA) of viable fraction of the tumors was made, separating RO and ChRCC groups. Manual TMA was constructed as we previously reported [7].
Immunohistochemistry
Immunohistochemical stains were performed using mouse monoclonal antibody to CK-7 (Clone OVTL 12/30, DAKO Corporation, USA), and mouse monoclonal antibody to CD15 (clone BRA4F1, Biogenex, USA) according to the manufacturers’ instructions, using 4 microns sections placed on Silane coated slides. All cases were subjected to microwave antigen retrieval and incubated in Buffer PBS for 10 minutes. The slides were then loaded onto a DAKO Cytomation Autostainer (Dakocytomation Inc, Carpinteria, CA). Upon completion of the incubation with secondary antibodies, a chromogenic solution of aminoetilcarbazol was added, and the slides were counterstained with hematoxylin. Current external control was used. Qualitative method was used to evaluate immunostains. Staining for CK7 was considered to be positive if groups of several cells have cytoplasmic and membrane positivity, even if they were located in a focal area of the tumor (“diffuse stain pattern”) and it was considered to be negative if cells were negative or isolated cells were positive (“single cells positivity pattern”), as previously described [8]; the intensity of the immunostain was not relevant. Staining for CD15 was considered to be positive if only few cells had cytoplasmic or membrane positivity, regardless its intensity [9]. The statistical analysis was done in an invariable form with chi-squared (X2) test and the statistical significance was defined with a p-value<0.001.
Results
Macroscopic and histological characteristics
Macroscopic and histological characteristics are summarized in Table 1.
| Gross and Microscopic Features | Oncocytoma (16) n (%) |
ChRCC (21) n (%) |
p |
|---|---|---|---|
| Tumoral contour | |||
| Rounded | 16 (100) | 20 (95) | ns |
| Infiltrative | 0 (0) | 1 (5) | ns |
| Central scar | 10 (62.5) | 3 (14) | <0.001 |
| Grown pattern Nested | 16 (100) | 2 (10) | <0.001 |
| Sheet | 0 (0) | 19 (90) | <0.001 |
| Vascular permeation | 3 (19) | 5 (24) | ns |
| Adipose tissue extension | 5 (31) | 1 (5) | 0.012 (ns) |
| Presence of mitosis | 0 (0) | 16 (76) | <0.001 |
| Presence of necrosis | 0 (0) | 2 (10) | ns |
| Raisinoid nuclei Fuhrman nuclear grade | 0 (0) | 19 (90) | <0.001 |
| 1-2 | 6 (37.5) | 3 (14) | ns |
| 3 | 8 (50) | 12 (57) | ns |
| 4 | 0 (0) | 6 (29) | 0.019 (ns) |
| Ancillary techniques | |||
| HCI positive | 2 (13) | 20 (95) | <0.001 |
| CD15 positive | 13 (81) | 4 (19) | <0.001 |
| CK7 positive | 1 (6) | 18 (86) | <0.001 |
| Concordance of 3 markers | 10 (63) | 13 (62) | ns |
| Concordance of 2 markers | 16 (100) | 21 (100) | <0.001 |
Table 1: Macroscopic and microscopic characteristics of 16 Renal Oncocytomas and 21 Chromophobe Renal Cell Carcinomas.
RO cases average age was 59 (30-76 yrs.), 9 females and 7 males. ChRCC cases average age was 51 (28-82 yrs.), 12 females and 9 males. Grossly, RO and 20 out of 21 ChRCC had the classic macroscopic appearance (well delimited, rounded contour, tan-brown tumor); 10 out of 16 RO and 3 out 21 ChRCC had the characteristic fibrous scar (p<0.0001). RO and ChRCC’s average sizes were 4, 5 cm (range 1.6-9 cm) and 5 cm (range 1.6-16 cm) (ns); RO and ChRCC’s laterality was 5 left and 11 rights v/s 10 left and 10 rights (ns).
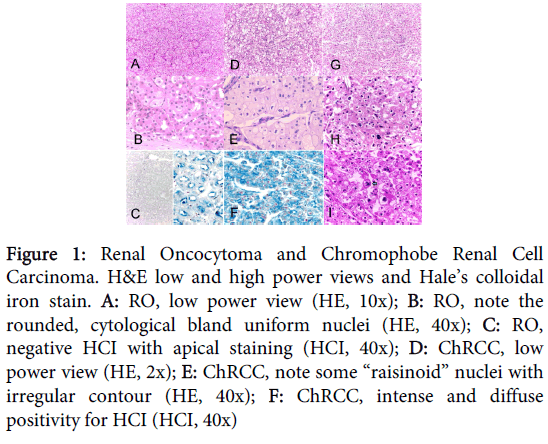
Histological characteristics were divided in architectural and cytological features, and are represented in figure 1.
Figure 1: Renal Oncocytoma and Chromophobe Renal Cell Carcinoma. H&E low and high power views and Hale’s colloidal iron stain. A: RO, low power view (HE, 10x); B: RO, note the rounded, cytological bland uniform nuclei (HE, 40x); C: RO, negative HCI with apical staining (HCI, 40x); D: ChRCC, low power view (HE, 2x); E: ChRCC, note some “raisinoid” nuclei with irregular contour (HE, 40x); F: ChRCC, intense and diffuse positivity for HCI (HCI, 40x)
Architectural features
The growth pattern was exclusively “nested” in all RO cases v/s only 2 out of 21 (9.6%) ChRCC cases (p<0.0001). The rest of the ChRCC cases (19 out of 21, 90.4%) had “sheet” growth pattern. ChRCC’s histological type was the “classic” and “eosinophilic” variant in 10 (47.6%) and 11 (53.4%) cases. Spread to perirenal fat was present in 6 out of 16 (37.5%) RO cases vs. 1 out of 21 (4.8%) ChRCC cases (ns). Necrosis and renal vein and pilocaliciary system compromise by tumor was present only in 2 and 1 ChRCC cases respectively; none of the RO had them (ns). Vascular permeation was present in 3 (19%) and 5 (24) RO and ChRCC cases respectively (ns).
Cytological features
Nuclear contour was mainly rounded, circular in shape with smooth borders in all RO cases. Isolated atypical cells were found forming little groups in some tumors, but all showed the characteristic degenerative “blurry” chromatin. Nuclear indentation was a striking feature in ChRCC, with some folding of the nuclear membrane giving them an irregular contour, called “raisinoid” nuclei; this was observed in 19 cases (90.5%). No mitotic figures were identified in RO cases and at least one mitotic figure was observed in 16 (76.2%) ChRCC cases.
Fuhrman nuclear grading system Grade 1 to 2 was present in 6 out of 16 (37.5%) RO cases and 3 out of 21(14%) ChRCC cases (ns); grade 3 was present in 8 (50%) RO and 12 (57%) ChRCC (ns) and grade 4 was present in none of the RO cases and in only 6 (29%) of the ChRCC cases (ns). Occasional atypical degenerative cells were not considered.
Ancillary techniques:
The histochemical technique of Hale’s Colloidal Iron (HCI) was positive in the cytoplasm of just one (6.3%) RO case and 20 (95.2%) ChRCC cases (p<0.001) (Figure 1). CK7 was positive in groups of contiguous cells in 1 (6.3%) RO case and 18 (85.7%) ChRCC cases (p<0.001). CD-15 was positive in 13 (81.3%) RO cases and 4 (19%) ChRCC cases (p<0.001).
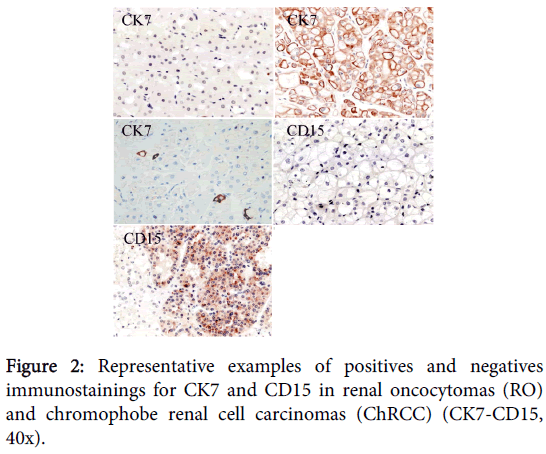
Complete results can be found on Table 1. Picture representatives of immunostains performed are available in Figure 2.
Discussion
The differential diagnosis between ChCCR and RO is a recurrent topic in surgical uropathology performance. The majority of renal tumors can be diagnosed with certain easiness; however those oncocytic tumors can be frequently a challenge to diagnose. A lot has been published in relation to the diagnostic differential between the ChRCC and the RO, with multiple works that demonstrate differences between expression markers in both tumors, however most of them correspond to markers with little diffusion or infrequent use in the anatomic-pathological routine. Among them are S100-A1, Claudins 7 and 8, Endogenous Abidin binding, PAX2, RON, LMP2, Cytochrome C Oxidase and others [10-15]. However, data suggests that a set of markers is much better that a single marker, besides a rigorous revision and meticulous analysis of the morphology even architectural and cytological parameters [16,17]. It’s worth saying that the principal critics used in this work synthesized the most outstanding elements publicly diagnosed. The diffuse pattern of growth is in our opinion the outstanding architectural factor for differentiate between RO an ChRCC, since most of our group of ChRCC possess it and was not found in any of the RO, agrees with published series [2,3]. Others topics don’t possess the same diagnostic value like the presence of tumoral focuses on fatty perirrenal tissue or the vascular permeations, that reach around 10% and 5 % respectively in diverse series [2,3,18] and ranges highest in our series, don’t reach statistical value in the differential diagnosis. Of the cytological characteristics, some well-known elements are confirmed to differentiate both entities, which are the presence of typical or atypical mitosis and necrotic focuses [2,3], however their frequency is low, like you see in this series and in others, hence its usefulness is limited. Others parameters have the problem of being subjective and depend on the personal interpretation such as the presence of perinuclear halos [2,3] or have been described by few and have not been replicated by others like the presence and thickness of fibrous capsule [19].
The most outstanding cytological aspect according to our results is the morphology of the nucleus, those which are described in the ChRCC as partly rounded with nucleoli, partly ovoid, with straight contours or indentations of the nuclear membrane [1-3] that gives them the “raisinoid” aspect similar to the raisins of grapes. In our series, this point clearly differentiates the two groups, not finding any cases of RO with these characteristics. However, this type of nuclei has been reported in RO by others [20]. It is important to point out that in certain cases, they are not the most predominant in the tumor, but they are frequently dispersed among others of more rounded contour in most of the fields. Besides they possess vital aspects, with chromatin that maintains their details and not with the degenerative aspect of the atypical cells of the oncocytomas, that show vacuolated cytoplasm is and the chromatin has blurred aspect or simply "blurry" [3]. The histochemical technique of HCI has been used for many years to identify the ChRCC and to differentiate it from the RO. However the exclusive use of this method has been questioned because there is enough variability in its results according to the ph to the one that is carried out, and to the positivity that can be given in other types of renal carcinomas as that of clear cells or even some cases of RO [4]. For that reason apart from the positivity or negativity of the marker, is important to the staining pattern, where the positivity in the ChRCC is characteristically at cytoplasmic level and comprising the majority of the neoplastic cell [4]. On the other hand in the RO, the staining is variable, being totally negative in a variable percentage among 16% [4] and 25% in our own series. In addition, call our attention find a high number of RO with positive stain for the HCI in a denominated "luminal pattern” [4], located in the cellular membrane that couches to give in the lumen of the tubules or centre of the acinar structures that conform the tumor. These results also replicated in our series, reaching 75% of cases of RO.
The CK7 is an intermediate filament classically described in the ChRCC, with a diffuse expression in cytoplasm and membrane [4,8,11,16,17] with expression levels that fluctuate among the 63% and 100% of the cases. This pattern is found in almost all cases of our series, but sometimes is observed only in a part of the neoplasm, being negative in other areas. In RO, a pattern of negative stain is classically described; however the stain is not completely negative, but rather a special pattern can be observed with "single positive cells", described by Skinnider et al. [8].
In relation to the marker CD15, also known as Lewis X or Leu M1, there are not many published data of their use to differentiate ChRCC and RO; however its main reference is the recommendation of being part of the diagnostic panel in the last edition of Urologic Surgical Pathology from Bostwick [9]. In general, CD15 stains epithelial lesions, especially some carcinomas like those from colon or bile ducts. In our series, CD15 expression statistical differs between RO and ChRCC (80% v/s 20%, respectively), however the stain is sometimes difficult to interpret, since its positivity is usually in focuses and rarely reaches a high intensity.
The panel of auxiliary techniques presents a high diagnostic agreement. In both groups the agreement of the three markers reached 62% and the agreement of at least two was 100%. This is a very significant fact because in cases with not straightforward morphologic features, we can support our diagnosis in the result of at least 2 of this easily access ancillary techniques.
Conclusion
The differential diagnosis between RO and ChRCC is a frequent situation sometimes not simple to elucidate. In our series the most categorical histological elements and ancillary techniques to achieve a correct diagnosis of ChRCC are diffuse pattern of growth, the presence of “raisinoid” nuclei, cytoplasmic positivity for HCH and positivity in grouped cells for CK7 and negativity of CD15. For RO are nested pattern of growth, absence of raisinoid nuclei, cytoplasmic negativity of HCH, negativity or positivity in isolated cells for CK7 and positivity for CD15. At least two of the three auxiliary techniques must be concordant. The suggested panel can be easily performed due to its reasonable cost and to be composed of antibodies commonly used in the routine pathology practice. We think this should be tested and proved by others to evaluate the reproducibility of our results.
References
- Zippel J (1942) Zurkenntnis der onkocyten. Virchows Arch Path Anat 308: 360-382.
- Gudbjartsson T, Hardarson S, Petursdottir V, Thoroddsen A, Magnusson J, et al. (2005) Renal oncocytoma: a clinicopathological analysis of 45 consecutive cases. BJU Int 96: 1275-1279.
- Trpkov K, Yilmaz A, Uzer D, Dishongh KM, Quick CM, et al. (2010) Renal oncocytoma revisited: a clinicopathological study of 109 cases with emphasis on problematic diagnostic features. Histopathology 57: 893-906.
- Tickoo SK, Amin MB, Zarbo RJ (1998) Colloidal iron staining in renal epithelial neoplasms, including chromophobe renal cell carcinoma: emphasis on technique and patterns of staining. Am J SurgPathol 22: 419-424.
- Reuter VE, Argani P, Zhou M, Delahunt B; Members of the ISUP Immunohistochemistry in Diagnostic Urologic Pathology Group (2014) Best practices recommendations in the application of immunohistochemistry in the kidney tumors: report from the International Society of Urologic Pathology consensus conference. Am J SurgPathol 38: e35-49.
- Bonsib S (1996) Renal chromophobe cell carcinoma: The relationship between cytoplasmic vesicles and colloidal iron stain. J UrolPathol 4: 9-14.
- Gallegos I, Valdevenito JP, Miranda R, Fernandez C (2011) Immunohistochemistry expression of P53, Ki67, CD30, and CD117 and presence of clinical metastasis at diagnosis of testicular seminoma. ApplImmunohistochemMolMorphol 19: 147-152.
- Skinnider BF, Folpe AL, Hennigar RA, Lim SD, Cohen C, et al. (2005) Distribution of cytokeratins and vimentin in adult renal neoplasms and normal renal tissue: potential utility of a cytokeratin antibody panel in the differential diagnosis of renal tumors. Am J SurgPathol 29: 747-754.
- Bostwick D, Cheng L (2008). Urologic Surgical Pathology (2nd Edn). Mosby Elsevier, USA: 97.
- Kim SS, Choi YD, Jin XM, Cho YM, Jang JJ, et al. (2009) Immunohistochemical stain for cytokeratin 7, S100A1 and claudin 8 is valuable in differential diagnosis of chromophobe renal cell carcinoma from renal oncocytoma. Histopathology 54: 633-635.
- Li G, Barthelemy A, Feng G, Gentil-Perret A, Peoc'h M, et al. (2007) S100A1: a powerful marker to differentiate chromophobe renal cell carcinoma from renal oncocytoma. Histopathology 50: 642-647.
- Osunkoya AO, Cohen C, Lawson D, Picken MM, Amin MB, et al. (2009) Claudin-7 and claudin-8: immunohistochemical markers for the differential diagnosis of chromophobe renal cell carcinoma and renal oncocytoma. Hum Pathol 40: 206-210.
- Kanehira K, Hu J, Pier T, Sebree L, Huang W (2008) High endogenous avidin binding activity: an inexpensive and readily available marker for the differential diagnosis of kidney neoplasms. Int J ClinExpPathol 1: 435-439.
- Zheng G, Chaux A, Sharma R, Netto G, Caturegli P (2013) LMP2, a novel immunohistochemical marker to distinguish renal oncocytoma from the eosinophilic variant of chromophobe renal cell carcinoma. ExpMolPathol 94: 29-32.
- Adam AC, Scriba A, Ortmann M, Huss S, Kahl P, et al. (2014). Immunohistochemical Analysis of Cytochrome c Oxidase Facilitates Differentiation Between Oncocytoma and Chromophobe Renal Cell Carcinoma. ApplImmunohistochemMolMorphol.
- Carvalho JC, Wasco MJ, Kunju LP, Thomas DG, Shah RB (2011) Cluster analysis of immunohistochemical profiles delineates CK7, vimentin, S100A1 and C-kit (CD117) as an optimal panel in the differential diagnosis of renal oncocytoma from its mimics. Histopathology 58: 169-179.
- Fernández-Aceñero MJ, Cazorla A, Manzarbeitia F (2011) Immunohistochemistry for the differential diagnosis of renal tumors with oncocytic features. UrolOncol 29: 545-549.
- Hes O, Michal M, Sima R, Vanecek T, Brunelli M, et al. (2008) Renal oncocytoma with and without intravascular extension into the branches of renal vein have the same morphological, immunohistochemical, and genetic features. Virchows Arch 452 :193-200.
- Demirovic A, Cesarec S, Spajic B, Tomas D, Bulimbasic S, et al. (2010) Can renal oncocytoma be distinguished from chromophobe renal cell carcinoma by the presence of fibrous capsule? Virchows Arch 456: 85-89.
- Kim SS, Choi YD, Shim MK, Kim J, Cho YM, et al. (2012) Microscopic and nuclear morphometric findings of chromophobe renal cell carcinoma, renal oncocytoma, and tumor with overlapping histology. Ann DiagnPathol 16: 429-435.
Relevant Topics
Recommended Journals
Article Tools
Article Usage
- Total views: 20877
- [From(publication date):
November-2014 - Mar 31, 2025] - Breakdown by view type
- HTML page views : 16160
- PDF downloads : 4717