Removing Pesticides from Water Using Ultraviolet Irradiation
Received: 26-Sep-2019 / Manuscript No. JABT-19-2968 / Editor assigned: 30-Sep-2019 / PreQC No. JABT-19-2968 / Reviewed: 14-Oct-2019 / QC No. JABT-19-2968 / Revised: 22-Aug-2022 / Manuscript No. JABT-19-2968 / Published Date: 19-Sep-2022 QI No. / JABT-19-2968
Abstract
Organophosphorus is the most common pesticides being used all over the world. These compounds are poisons which can easily enter the water table and other drinking sources during application in crop fields since using some drinking water accumulating an amount of toxins of elevated value than the standard level, results unwanted impacts on health of individuals. These studies aimed to search the effectiveness of removal of Malathion from water by method of Ultraviolet Irradiation (UV) medium pressure and with a Mercury lamp.
Methods: In this study, variants of primary pH and initial concentrations and exposure times were examined. Initial concentrations of Malathion were 0.5, 1 and 2 mg/l. The samples aliquots were then exposed to UV irradiation uncontinuously with the time periods of 10, 20, 30, 40, 50 and 60 minutes. The UV medium pressure (irradiation irradiation by HPLC instrument. Furthermore, the results achieved from the experiment were analyzed using SPSS software and ANOVA and t-test statistical trials.
Results: The minimum concentration dropped off occurs at 10 min (46%) and the maximum reduction in 60 min (87.25%) (P<0.05) Additionally, the efficiency of irradiation process is inversely proportional to the concentration of pesticide (P<0.001). However, the efficiency of the process increases with pH increase.
Conclusion: The result data show the most efficacies were attained at pH=9, at 60 min and 0.5 mg/l consequently the application of UV reactors could be regarded as an appropriate method
Keywords
Pesticide; Malathion; Water; Ultraviolet irradiation
Introduction
Organophosphate compounds are the leading and diverse groups of easily obtainable pesticides and about 40% of insecticide comes in this category in the world. From agricultural lands the pest drowns poisons go through the water resources by direct washing and irrigation from used places. Besides, rainwater on covered toxin areas enters into the water sources. In addition pesticides along water percolate into water table beneath the soil deposits and during water infiltration. During rain fall some toxins can go through to air and as a result enter to sources of surface water and soil. Percolation of these pollutants substances in sources of supply water for drinking purpose can have adverse results on individual health and surroundings. Additionally remains of these pesticides are lethal for human health, action of Malathion is to one clean hereditary inhibiter operator in human that results to uncover the complications and problems in nervous system and create symptoms like dizziness, nausea and attention disorder [1]. This poison has very critical impacts on paediatric nervous system especially in first years. So we must use sure and appropriate ways for avoidance of entrance these toxins to water source and air and land. Now days some investigations are done regarding being and remains of these pollutants in environment. These experiments proves that post practice of pesticides in short times, their quantity have been more than permissible limit. For example studies performed by on Malathion left behind in drinking water showed that remains of these toxins was present for some months in environment of that locality. Evaluated remains of Malathion poisons in rivers water source and concluded that quantity of this poison has been more than acceptable extent in each river [2].
Different technologies are there to eliminate organophosphate pesticides. Recently the modern methods have been applied for elimination or mitigation of pesticides toxins like irradiating of ultrasonic waves ultraviolet radiation and advanced oxidation technology. Since ultraviolet radiation is one of the serviceable and safe technologies that can be advantageous in treatment of surface and ground water sources which are polluted by pesticides with its consequential benefits. Definitely one of protection reasons and greeted this technology is requirement that has present for purpose of urge in laws observance and new rules of water treatment. The usefulness of this method as compared with other technique of water treatment is as follows [3].
• Addition of chemical materials is not needed.
• Absorbent media not required.
• The reactor gets profited by better malleability.
• The nature of manufactured sewage is limpid and odourless and perfect.
• Almost all pesticides are degraded in the reactor significantly.
• The fewer connection with pH variability and water temperature in compared with other chemical substances [4].
• To have protection and satisfactoriness from the perspective of refineries employees and the general public and be proposed as a green technology.
A variety of studies have been performed on elimination of pesticides by ultraviolet radiation, like on impact of medium pressure lamp on Atrazine. The results were symptomatic of ideal influence this way in Atrazine pesticide degradation. Perfectly oxidized mineral products were obtained by the photo-oxidation method using visible and ultraviolet light. Conducted research to evaluate the effects of the photo-oxidation procedure on the carbamate. This experiment was performed by average pressure mercurial lamp. The consequences were indicative of ideal influence ultraviolet irradiation in degradation of this object. Main aim of this experiment were to review the effectiveness of UV irradiation for degradation of Malathion pesticide from water and the effect of irradiation time, initial concentration and pH alters impact on power of ultraviolet radiation efficiency in degradation of Malathion in water [5].
Materials and Methods
This research is the experimental-applicable study that performed In Institute for Industrial Research and Toxicology Ghaziabad U.P India. Malathion compound was prepared in the different concentrations and estimated. In this study, Malathion toxin has prepared and analyzed [6]. The confrontation period to ultraviolet wave were selected on the ground of performed pre examinations in lab and also the considered concentrations in this research judging to done studies about the present leftover of Malathion Toxin in country upper layer of water [7].
The method of samples preparation
The extraction steps and samples preparation prepared for valuation were: First, 500 ml of selected water sample is added to separator funnel that has 1 L of capacity [8]. In some cases from the Atman filter (0.45 μm) used to pass the sample because it had cloudily suspended particles; then, 30 ml dichloromethane was treated with noted sample. For better separation two stages 15–20 g NaCl was mixed to contents of separator funnel; next, funnel substance was blended and left its valve in repeated times for lessening of the funnel inside pressure. In order to sorting out the organic phase from watery phase while passing from column that consist the sodium sulphate for removal of water molecule from organic phase. In this stage, 30 ml dichloromethane was again added to that and mixed to separate organic phase from watery phase. During evaporation of extracted fluid by the Rotary set, the evaporation of under vacuum, thus the bulk of the obtained liquid is reduced 2–3 ml. At the end of the procedures 1 ml organic solvent chloroform was applied for extracting [9].
The concentration reading
Considering to the proposed method of Standard Method Book for measuring of Malathion toxin because the considering to the conditions of HPLC set following stages was completed. The standard solution was prepared from noted poison by HPLC grade material and with high purity percent in the various concentrations and then it was injected to HPLC set. 2.1 μL was removed and injected to HPLC set.The thermal program of this set was as follows
• Initial temperature will be 110°C for 1 min.
• To reach to temperature of 188°C with addictive velocity 25° C/min for 4 min.
• To reach to temperature of 190°C with addictive velocity 20° C/min for 1 min.
• To reach to temperature of 244°C with addictive velocity 25° C/min for 6.21 min.
• Keeping noted temperature for samples concentration reading.
• Using of helium gas with>99% purity % and velocity of flowing 0.7 cm/s.
The specifications of HPLC
High Performance Liquid Chromatography device (HPLC), capable with visible-ultraviolet disclosure and auto sampler. The used column for separating of these compounds is C18 made in Water Company in USA, with 25 mm length and 4.6 mm diameter in 30°C temperatures [10]. The moving phase flowing velocity and the used moving phase were considered in order 1 mL/min and methanol, acetonitric and water with ratio (15: 45: 40). The used wave length was ascertained 140 nm for disclosure of these compounds. The used separating column of HPLC set was capillary column DB5.6.25 kind. By the performance of correct thermal program and the set preparing was observed the pertaining vertex to Malathion in 10.23 minutes. The used set exactness in research was estimated at nearly 1 μg/L.
The specifications of ultraviolet reactor
The applied reactor in this experiment contains one closed bottom cylinder with 3 litres size and kind of the rust proof steel. In order to complete mixing of the sample for radiation in the reactor in this cylinder one magnetic mixer has been used. In this pilot, on account of excessive heating made the result of lamp radiation was used the chiller for decrease the temperature. For this purpose, one bocal of 4 L was used that cylinder fits its inside. The empty space of cylinder and bocal was filled by ice for temperature reducing. All the analyses were performed according to the procedures outlined in standard methods [11].
Calculation of Degradation Percentage
The definition of Malathion Degradation Percentage (DP) was as follows:
DP=(C1−C2)/C1 × 100
Where DP (%) is the degradation percentage of the ultraviolet reactor,
C1 is the initial concentration of pesticide (mg/l).
C2 is the concentration of pesticide (mg/l) after reaction for (t) time.
Statistical method
The results were analyzed by SPSS software, 11.5 version and 2003 excel. The data were analyzed using ANOVA and t-test. The variables were irradiation time, initial concentration and pH degradation was variable depended [12]. The Malathion poison was affected by ultraviolet mercurial lamp with medium pressure in 0.5, 1, 2 mg/L and 10, 20, 30, 40, 50, 60 min and pH=6, 7, 9.
Results and Discussion
Effect of pH value
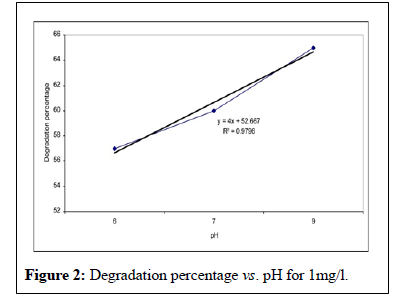
The investigation results of pH changes on the utility of Malathion concentration shows that the utility of concentration increases by pH augmentation, so that Malathion in 0.5, 1, 2 concentrations and pH=6 have the concentration utility 62%, 57%, 54.5 % but the measure of utility reaches to 70%, 65%, 63.5 % in pH=9 (Figures 1 to 6). The analysis test of anova showed in this case is the meaningful statistical difference too.
Conclusion
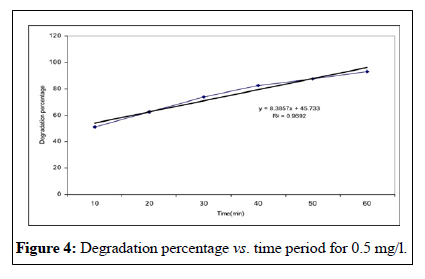
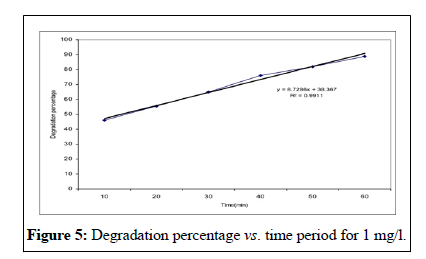
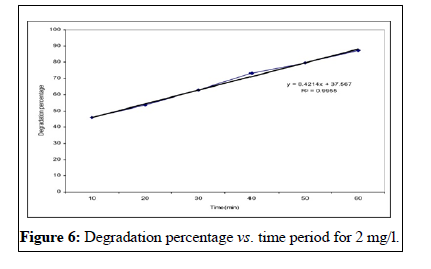
This study showed that the effectiveness of Malathion degradation from water by ultraviolet irradiation depends on three parameters; the initial concentration, the irradiation time and the pH. This study showed that initial concentration decreases with irradiation time [13]. These researches results conform to too. In other words, when the irradiation time increases, lots of free radicals has formed in the liquid and this event cause to the much decomposition of Malathion poison. Investigated pH changes in 6, 7, 9 limits on the concentration in time period of 1 hour and founded that the concentration increased by pH augmentation. The founded results of Walid’s studies confirm to the results of this research [14,15]. Besides the studies of showed that the velocity of poison decomposition increased by pH augmentation. His research results conform to this study. According to these researches, the principal reason of this event is the augmentation of OH ̄ ions producing and the hydroxyl free radicals in the alkali environment.
Finally, using chemical oxidation ways are not too much safe because they can impose too much energy and costs in the process of water treatment. Because of this, is given preference that will use the integrative ways of water refining for reducing of costs and using energy in order to will founded the ideal reduction efficiency in the short time and by using low energy.
Acknowledgments
This research has been supported by Institute for Industrial Research and Toxicology Ghaziabad U.P 201015 India. The author declares that there is no conflict of interests.
References
- Alavanja MC, Hoppin JA, Kamel F (2004) Health effects of chronic pesticide exposure: Cancer and nevrotoxicity. Annu Rev Public Heal 25: 155-197.
- Walter, William G (1961) "Standard methods for the examination of water and wastewater." 940-940.
- Badawy MI, Montaser Y, Ghaly MY (2006) Advanced oxidation processes for the removal of organophosphorus pesticides from wastewater. J Desalination 194: 166-175.
- BavconKralj M, Franko M, Trebse P(2007) Photo degradation of organ phosphorus insecticides: Investigations of products and their toxicity using gas chromatography-mass spectrometry and AChE- thermal lens spectrometric bioassay. J Chemosphere 67: 99-107.
- Freed VH, Chiou CT (1979) Degradation of selected organophosphate pesticides in water and soil. J Agric Food Chem 27: 706-708.
- Hequet V, Gonzalez C, Le-Cloirec P (2001) photochemical processes for atrazine degradation: Methodological approach. J Water Res 35: 4253-4260.
- Majdzadeh R, Sadighi J, Nejat S, Mahani AS, Gholami J, et al. (2008) Knowledge translation for research utilization: Design of a knowledge translation model at Tehran University of Medical Sciences. J Con Edu Health Prof 28: 270-277.
- Huston PL, Pignatello JJ (1999) Degradation of selected pesticide active ingredients and commercial formulations in water by the photo-assisted Fenton reaction. J Water Research 33: 1238-1246.
- Maldonado MI, Malato S, Pereze- Estrada LA, Gernjak W, Oller I, et al. (2006) Partial degradation of five pesticides and an industrial pollutant by ozonation in a pilot-plant scale reactor. J Hazardous Materials 138: 363-369.
- Rauh A, Garfinkel R, Perera P (2006) Impact of prenatal chlopyrifos exposure on Neurodevelopment in the First 3 years of life Among Inner- city children. J Pediatrics 118: 1845-1859.
- Shayeghi M, Shahtaheri J, Selseleh M (2001) Organophosphorus insecticides residue in Mazandaran river and waters (Iran). Iranian J Public Health 30: 115-119.
- Shayeghi M, Darabi H (2004) Survey of Diazinon and Malathion in water of Shahpour, Mand, Dalaki, Boushehr Riverse. J South Medicine 10: 54-60.
- Safi JM (2002) Association between chronic exposure to pesticides and recorded cases of human malignancy in Gza Goveronates. J Sci Total Environ 284: 75-84.
- Videria RA, Antunes-Madiera MC, Lopes VI, Madeira Vítor MC (2001) Change induced by malathion, methylparation and parathion on membrane lipid physiochemical properties correlate with their toxicity. J Biochem Biophys Acta BBA Biomem 1511: 360-368.
- Walid K, Lafi Z, Al-Qodah (2006) Combined advanced oxidation and biological treatment processes for the removal of pesticides from aqueous solutions. J Hazardous Mat 137: 489-497.
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Citation: Ahmed S (2022) Removing Pesticides from Water Using Ultraviolet Irradiation. J Anal Bioanal Tech 13: 472.
Copyright: © 2022 Ahmed S. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Open Access Journals
Article Usage
- Total views: 1470
- [From(publication date): 0-2022 - Apr 07, 2025]
- Breakdown by view type
- HTML page views: 1150
- PDF downloads: 320