Removal of Toxic Heavy Metal Ions from the Dye Industries Effluents by Using Isolated and Dried A. Niger Biomass Material - The Spent Biomass Briquetts Used as Fuel Material for Small Furnacess - An Alternate Energy Approach: PART âI
Received: 01-Aug-2022 / Manuscript No. jbtbm-22-70874 / Editor assigned: 03-Aug-2022 / PreQC No. jbtbm-22-70874 (PQ) / Reviewed: 19-Aug-2022 / QC No. QC No. jbtbm-22-70874 / Revised: 26-Aug-2022 / Manuscript No. jbtbm-22-70874(R) / Published Date: 02-Sep-2022 DOI: 10.4172/2155-952X.1000293
Abstract
The present Investigation is focused to removal of the heavy metal ions of Cupper, cobalt, zinc, cadmium, chromium, mercury and arsenic toxin using the technique of bio sorption potential of the heavy metal resistant fungal biomass Aspergillus niger isolated from Dye manufacturing and dying industry effluents. The dried and briquetting fungus biomass has been utilised to optimize the different parameters like contact periods, the concentration of metal ion at initial, pH, the biomass loading etc. The heavy metal ions removed is estimated by using various chemical methods was studied and recorded. The maximum of 91% Cu and other metal oxides was from in 5 mg/L starting load of Cu and other heavy metal concentration by using dried Aspergillus Niger biomass absorbent materials. The total maximum % removal of Copper and other heavy metals using the well grooming Aspergillus Niger was detected out to be 91 %. Then the characterization studies were carried out by using Scanning Electron Microscopy and EDAX analysis for copper and other individual heavy metal separately as well as all for all mixed aqueous toxic solution. The Copper heavy metal was detected to be desorbed effectively by using 0.1Molar HCl and was found to be 89 % and the reuse of the Sterilized dried fungus biomass has been showed 90.72 % Cu removal and presented all estimated efficiencies of biomass. The spent Biomass briquetts can be used as fuel material for small furnaces and other heavy metal results are also indicate the same level of removal as individual and mixed aqua solution also, which will be presented as part - II of this investigation.
Keywords
Aspergillusniger; Biosorption; Heavy metals; characterization; Alternate fuel; SEM and EDAX
Introduction
Nowadays in most areas of manufacturing industries, the casual and careless disposal of manufacturing industrial effluents and other unwanted wastes may put in a poor quality of the water [1, 2]. The Industrial waste fluids of water in particular normally contain greater levels of heavy metal ions and build serious water contamination and pollution problems. Heavy metal ions are chemically nonbiodegradable and may be accumulate in the living organisms all the way through the food chains. A study on the impact of industrial effluent on water quality was carried out widely due to its most dangerous face on animals, humans and plants. Heavy metal ions are unconfined by a number of industrial manufacturing processes are the major pollutant and contaminants in oceanic, ground, industrial process of manufacturing and even treated the wastewaters [3]. Heavy metal ions are to be exceptionally toxic as they will spoilt and damage the nerves systems, liver, kidney and bones of human beings, and also block the functional groups of essential enzymes of human metabolism. The main intimidation to human healthiness from heavy metal ions are connected with coverage and exposure to copper and other metal ions like cobalt, zinc, cadmium, chromium, mercury and arsenic toxin. Heavy metal ions may have been used by living things such as humans for decades and decades of years. Exposure to heavy metal, and is even growing in some region of the globe, in particular to under developed nations, though the emissions have been decline in the well developed nations over the past hundreds of years.
The metal ions of heavy metals present in some of the process industrial effluents, such as paper and pulp processing, chemicals and petrochemicals, petroleum refineries, fertilizer manufacturing industries, steel manufacturing, dye chemical industries and automobile components industries have more toxic effects on the receiving surroundings and par on the performance of organic and inorganic handling processes [3]. Consequently, elimination and recuperation of heavy metal ions from the process industrial effluents will gain the considerable notice in the recent years. Copper, zinc, nickel, lead, mercury, chromium and cadmium, are the most frequently found heavy metals in industrial wastewaters [4].
The metal copper is a widely used industrial metal and which is used for electrical wiring, plumbing works, air conditioner tubing and roofing. The chemical properties of copper metal, which make it appropriate for these applications, comprise elevated electrical and thermal conductivity, high-quality deterioration resistance, easiness of fabrication and putting in place, good-looking appearance, all set availability, and elevated recyclability. as well copper, which is an necessary micro nutrient to humans and other existence forms of life, which is a biostatic/ biocide to certain small organisms. Moreover, the copper (II) is well-known to be as one among the heavy metals most of the toxicity to living organisms and it is one of the most common heavy metal pollutants of the surroundings and environment. The probable source of copper in process industrial waste effluents comprise of metal cleaning and plating baths, paper pulp and board mills, wood pulp manufacture, dye chemical industries and the chemical, fertilizer manufacturing industries, etc.
Copper ions is a necessary to living things at trace levels, greater concentration can resulted a numerous physiological and healthiness trouble or even life threat. Copper can effect severe problems such as intestinal suffering, kidney mal functioning and anaemia. The concentration of copper ions in these wastewaters can reach values of 1000 mg/L. Moreover, copper may be originate as a contaminant in foodstuff, particularly shellfish, liver, mushrooms, nuts, and chocolate toffee etc [5].
Most of these techniques suffer from some setbacks in process, such as greater investment and working cost or the removal of the remaining metal sludge, and are not appropriate for small-scale process industries [6]. For the above said reasons, new methodology of these heavy metal ions removals is developed as chemi-sorption processes. The biological absorption processes have been widely studied in recent years. Biosorption is proved to be an attractive method with large surface area, effective, selective adsorption of heavy metal ion, cheap and valuable, operated over broad range of environmental conditions, quick and end to end reversible chemical reaction, and eco-friendly natural and outstanding performance.
The utilization of bio sorption technology for the treatment of heavy metal contaminated wastewaters has become an alternative method to conventional treatments [7, 8]. Metal ions absoption by the microorganism involves some chemical processes together with adsorption process, ion exchange process, covalent bindings and coordinations. Fungi organisms have been known to capable of removing metal ions from industrial waste effluent liquids. Many fungus species like Aspergillusniger, Mucorsp, Phanerochaetecrysosporium, Rhizopus sp. and Saccharomyces sp., has been extensively studied as a more prospective biosorbent in heavy metal ions removal as it is cheap and abundant in nature. Fungi are also been recognized as a promising low-cost biosorbent for heavy metals biosorption from aqueous solution [9]. The more activated fluid sludge provides an outstanding prospectus for the removal of heavy metal ions by the technique biosorption because of its accessibility on the plant site and free to use it. The used sludge after biosorption can be recycled by acid or alkaline treatment of desorption and reused [10]. The spent Biomass briquetts can be used as fuel material for small furnacess.
The Biosorption technique and mechanisms can be separated into: Metabolism reliant and Non-Metabolism reliant. According to the place where the metal ions separated from solution to originate biosorption can be classified as 1.Extra cellular accumulation / precipitation technique 2.Cell surface sorption/precipitation technique and 3. Intracellular accumulation methodology. Transportation of the metal ions across the cellular yields this type of biosorption may take place only in the feasible cells. It is often connected with an active resistance system of the microorganism which reacts in the occurrence of toxic metal ions. This is based on the process physical adsorption, ion exchange process and chemical biomass, mainly comprise of polysaccharides, protein and lipids which have abundant metal binding groups like carboxyl, sulphate, phosphate and amino groups etc.. These types of biosorption, i.e., non-metabolism related and is a rapid reversible processes. Alternatively, heavy metals may be recovered from the waste sludge after biosorption by solvent extraction and the sludge may be reused. Some of those studies were done using pure cultures of bacteria, yeasts and molds [11, 12].
Materials and Methods
Collection of fungal culture
The Fungal material culture (Aspergillusniger MTCC No.478) was collected by Microbial Type fungus Culture Collection (MTTC), originated from Chandigarh. This parent culture of fungus has been used for further investigation studies as Biosorbent materials. The fungal culture was maintained in sophisticated place without any chemical and biological contaminations.
Preparation of media
Mostly the micro-organisms are grown-up or cultured in a aqueous liquid medium (broth) or on solid/semi solid medium (agar plates mostly or slants).
Composition of potato dextrose agar
Potato Dextrose Broth 2.4 g
Agar Agar 2.0 g
pH 5.6±0.2
Distilled Water 100 ml
Potato Dextrose Broth preparations
About 24.5g of potato dextrose broth was weighed and added to 1000 ml pure distilled water. The potato dextrose broth medium was taken in a conical flask and lid with cotton plug and then autoclaved at 121°C for about 15 minutes to avoid any outside contaminations. The medium was then kept ready for inoculation.
Sub-Culturing of the biosorbent
The freshly inoculated medium is then incubated at temperature 37°C appropriate for growing the organism to aging period of seven full days, it become necessary changes due to the nutrient diminution and medium aeration, to transfer the fungal culture to fresh media.
The Slants were prepared by using organic potato dextrose agar medium with the same composition as already composed in the media preparation and put it into test tubes and these medium was allowed to solidify itself. The fungal culture was then streaked on the slants under aseptic conditions to avoid contamination. The slants were replaced and then kept it for incubation at 33-37°C for three to four full days.
Mass Culturing of the biosorbent
Under sterilized state a loop full of fungal mass culture was taken and diluted in 1 ml volume of the distilled aqueous medium as water then the diluted fungal biomass culture medium was inoculated into the prepared broth and incubated at about 37°C for 4 to 5 days. Proper sterilization procedure was followed to avoid contamination. After completion of the incubation time periods the materials of fungal biomass was obtain and which is used for further investigation.
Filtration of the biosorbent
Filtration process is a physical operation through which the solid materials of Biosorbent were separated from the fluids. The fungal biomass was obtained by mass culturing in potato dextrose broth. The fungal mat was then filtered using a filter.
Drying of the biosorbent
Through the process of drying the moisture content of biomass Martials were removed. The fungus biomass materials obtained through mass culture was then placed in a petriplates and dried in an oven at about 80°C for 4-5 hours to facilitate dry it. The dried biomass was then obtained and utilized for the present investigation. The dry biomass was then scrapped and collected from the plates, weighed and stored in a air tight container. This dried biomass material was taken for further studies as a bio-sorbent material.
Chemical modification of the Biosorbent
The bio-sorbent was subjected to pre-treatments by using a variety of chemicals. The pre-treated biomass can be used in wet and dry conditions. The bio-sorbent was pre-treated using 1Normal HNO3, 1N NaOH, NaOH Doping solution, HNO3 Doping and Sterilization techniques [13].
Chemical Pre-Treatment
The obtained fungal biomass material was boiled at about 80°C in 1N HNO3 and 1N NaOH for about 30 minutes. This form of pretreated biomass was then used for further investigation of Copper removal in both wet and dry conditions.
Sterilization
The obtained fungal biomass material was sterilized in an autoclave at about 121°C for 12-15 minutes. This well sterilized biomass was then utilized for further process of Copper removal in both wet and dry conditions.
Aqueous copper solution preparation
Stock solution
The stock solution of aqueous copper of concentration 1000 mg/L was prepared by dissolving 3.9 g of Copper sulfate salt of AR grade in 1000 ml distilled water. The test solution containing copper was prepared by diluting 1 ml of stock solution of metal to the desired concentrations. The Copper concentration was varied in the range of 5 to 25 mg/L.
Working solution
Copper working solution was prepared at different concentrations to carry out batch experiments.
5 mg/L: 0.5 ml of prepared stock solution was then taken and was made upto100 ml using distilled water.
10 mg/L: 1 ml of prepared stock solution was then taken and was made upto100 ml using distilled water.
15 mg/L: 1.5 ml of prepared stock solution was then taken and was made upto100 ml using distilled water.
20 mg/L: 2 ml of prepared stock solution was then taken and was made upto100 ml using distilled water.
25 mg/L: 2.5 ml of prepared stock solution was then taken and was made upto100 ml using distilled water.
Biosorption using aqueous copper Solution
Batch experiments were carried out using non-living mycelia suspensions. Batch studies were carried out in Erlenmeyer flasks by adding 1g of the bio-sorbent in 100ml of aqueous liquid copper solution at preferred strength of concentrations. The flasks were agitated on a reciprocating shaker at constant temperature and at constant pH. The samples were collected at regular intervals of 14 minutes up to the equilibrium stages was attained. Once the equilibrium state was attained, the bio-sorbent was separated and the top supernatant liquid was subjected to characterisation [14].
Effect of Contact Time
The effect of contact time was conducted in a batch reactor for different contact periods of time like (0, 15, 30, 45, 60, 120, 180, 240, 270, 300 minutes) at an initial starting concentration of 5mg/l of CuSO4 at pH of 2.0 [Ahmed, 2007: Pareshet al., 2010]. The adsorbent material dosage was 1gram in 100 ml of aqueous solution in 100 ml conical flask at room temperature (370C) [15]. The samples were then stirred continuously in a rotary shaker at around 140-150 rpm and at regular intervals the samples were collected, separated by filtration and characterized by using Atomic Absorption Spectroscopy (AAS). Each trial was repeated three times in order to get accurate and concordant results [16].
Effect of Initial Metal Ion Concentration
The outcomes of initial copper metal ion concentrations were carried out for the initial copper load concentration (5, 10, 15, 20, 25 mg/L) in a batch type reactor at pH level of 2.0 at room temperature (370C) [Ahmed et al., 2007]. 1g of biomass material was added in 100 ml aqueous solution in 100 ml flask and well agitated in a rotary type shaker at 120-150 rpm. The samples were removed at regular time intervals until the equilibrium stage was attained and characterized for the remaining copper metal concentration as indicated earlier [17].
Effect of pH
The equilibrium batch type trials were conducted out to study the effect of pH studies, 50ml of aqueous copper solution with 5 mg/L concentration was modified to various pH levels (1, 2, 3, 4, 5, 6, 7, 8 and 9) and 1g of biomass materials at room temperature was added to 100ml of aqueous metal ion solution and well agitated in a rotary type shaker at 120-150 rpm. The samples were collected at regular time intervals until the equilibrium was reached and supernatant solution was characterised for remaining copper metal ion concentration as mentioned earlier [18].
Effect of Biosorbent Dosage
The effect of biomass loading on trial studies 100 ml of 5 mg/l initial concentration of aqueous copper solution was added with different Biosorbent material dosages (0.5,1.0, 1.5, 2.0, 2.5 and 3g ) by keeping the pH range and temperature as 2.0 and 370C correspondingly [19].
Effect of Pre-Treatment
The biomass material was then subjected to modification process. The resultant effect of this pre-treated fungal biomass materials on percentage elimination in 5 mg/l of the initial concentration of copper metal ion was calculated at pH 2.0 and 37°C respectively.
Characterization of the Biosorbent- SEM Analysis
Scanning Electron Microscope (SEM) was used for the characterization studies of the Biosorbent material [20]. The samples for SEM characterisation analysis were prepared by drying and powdering the fungal materials that obtained from the technique mass culturing. 0.5 gram of the dried fungal biomass material was taken for SEM character analysis. 0.5g of fungal sample was further added to 50 ml of the aqueous copper solution for biosorption process. The bios orbed fungal sample materials was then collected, dried and taken for SEM characterization analysis. The surface of the fungal biomass material was analyzed before and after Copper biosorption process. The changes in the surface of materials morphology was studied by SEM analysis.
Desorption Studies
Desorption studies was conducted out so that the fungal biomass materials can be reused for biosorption purpose. Desorption was also performed by desorpting agents like 1Normal HCl, 1N NaOH and 0.1N EDTA solution. The desorption agents was scraped away the Copper (So4) metal bound with the surface of fungus materials and hence to make the fungal biomass materials free from metal ions which could be further used for biosorption [Naliniet al, 2007]. The fungus biomass material that has been utilised for the biosorption is collected and calculated mass of the materials before desorption process. After continuous shaking overnight the solution was centrifuged to separate the supernatant. The supernatant decanted was subjected to AAS to estimate the amount of desorbed fungal biomass obtained as semi solid pellet was estimated.
Results
Collection of fungus culture from MTCC (Chandigarh)
Mother-culture of Aspergillusniger
(Figure 1)
Sub culture of Aspergillusniger
(Figure 2)
Mass culture of Aspergillusniger
(Figure 3)
Biosorption using Aqueous Solution
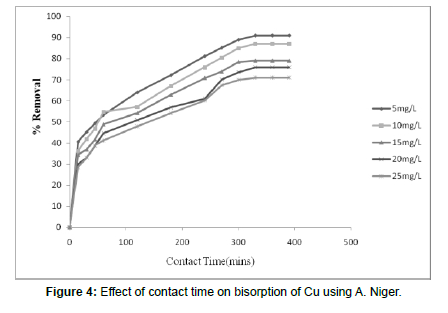
Effect of Contact Time
(Table 1), (Figure 4)
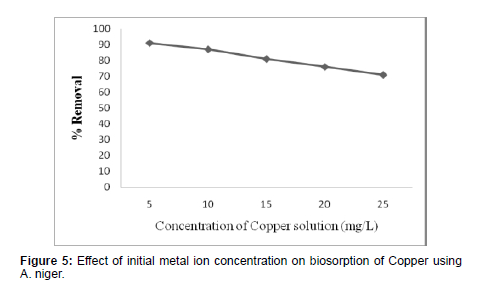
Effect of Initial Metal Ion Concentration
(Table 2)
| Time (mins) |
Initial Conc. of Cu(II) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 5 mg/L | 10 mg/L | 15mg/L | 20 mg/L | 25mg/L | ||||||
| Conc. Of Cu (mg/L) |
Copper Removal (%) |
Conc. of Cu (mg/L) |
Copper Removal (%) |
Conc. Of Cu (mg/L) | Copper Removal (%) |
Conc. of Cu (mg/L) |
Copper Removal (%) |
Conc. of Cu (mg/L) | Copper Removal (%) |
|
| 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 10 | 2.95 | 40.46 | 6.57 | 36.35 | 9.75 | 34.13 | 14.12 | 30.14 | 17.59 | 28.54 |
| 20 | 2.73 | 45.52 | 5.62 | 41.48 | 9.45 | 36.89 | 13.54 | 33.40 | 16.65 | 33.03 |
| 35 | 2.53 | 49.4 | 5.32 | 46.8 | 8.74 | 41.7 | 12.25 | 38.75 | 15.3 | 38.8 |
| 50 | 2.33 | 53.4 | 4.53 | 54.7 | 7.67 | 48.8 | 11.08 | 44.6 | 14.71 | 41.16 |
| 110 | 1.80 | 64.0 | 4.23 | 57.1 | 6.87 | 54.2 | 9.83 | 50.85 | 13.05 | 47.8 |
| 160 | 1.39 | 72.2 | 3.29 | 67.1 | 5.57 | 62.8 | 8.63 | 56.85 | 11.46 | 54.16 |
| 220 | 0.94 | 81.2 | 2.41 | 75.9 | 4.38 | 70.8 | 7.21 | 60.95 | 9.96 | 60.16 |
| 250 | 0.74 | 85.2 | 1.95 | 80.5 | 3.92 | 73.8 | 5.96 | 70.20 | 8.15 | 67.4 |
| 310 | 0.55 | 89.0 | 1.51 | 84.9 | 3.24 | 78.4 | 5.28 | 73.6 | 7.52 | 69.92 |
| 320 | 0.45 | 91.0 | 1.30 | 87.0 | 3.15 | 79.0 | 4.83 | 75.85 | 7.25 | 71.0 |
| 350 | 0.45 | 91.0 | 1.30 | 87.0 | 3.15 | 79.0 | 4.83 | 75.85 | 7.25 | 71.0 |
| 380 | 0.45 | 91.0 | 1.30 | 87.0 | 3.15 | 79.0 | 4.83 | 75.85 | 7.25 | 71.0 |
Table 1: Effect of Contact Time.
Initial Metal Ion Concentration (mg/L) |
Concentration of Copper (mg/L) | Copper Removal (%) |
|---|---|---|
| 5 | 0.45 | 91 |
| 10 | 1.3 | 87 |
| 15 | 2.85 | 81 |
| 20 | 4.8 | 76 |
| 25 | 7.25 | 71 |
[100 ml Aqueous Metal Solution of Initial Concentration = 5 mg/L, 10 mg/L, 15 mg/L,20mg/L and 25 mg/L, pH=2.0, Weight of Biosorbent Dosage = 1 g, and Temperature = 370C].
Effect of Initial Metal Ion Concentration
(Figure 5)
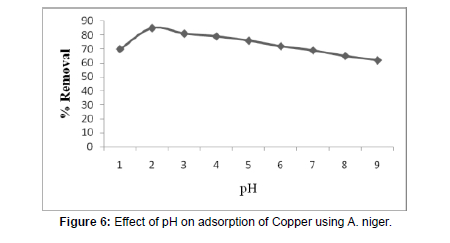
Effect of pH
(Table 3), (Figure 6)
| pH | Concentration of Copper (mg/L) | Copper Removal (%) |
|---|---|---|
| 1 | 1.5 | 70 |
| 2 | 0.75 | 85 |
| 3 | 0.95 | 81 |
| 4 | 1.05 | 79 |
| 5 | 1.2 | 76 |
| 6 | 1.4 | 72 |
| 7 | 1.55 | 69 |
| 8 | 1.75 | 65 |
| 9 | 1.9 | 62 |
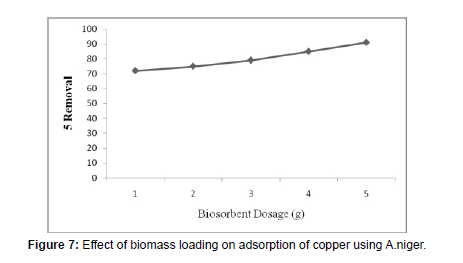
Effect of Biosorbent Dosage
(Table 4), (Figure 7)
Biosorbent Dosage (g) |
Concentration of Copper (mg/L) | Copper Removal (%) |
|---|---|---|
| 1 | 1.4 | 72 |
| 2 | 1.25 | 75 |
| 3 | 1.05 | 79 |
| 4 | 0.75 | 85 |
| 5 | 0.45 | 91 |
[Weight of Biosorbent = 1.0, 2.0, 3.0, 4.0, and 5.0 g, Volume of Solution = 100 ml, Initial Copper Concentration = 5 mg/L, Weight of Biosorbent Dosage = 1 g, pH = 2.0,Temperature =370C].
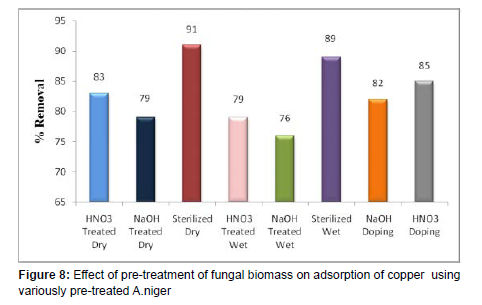
Effect of Pre-Treatment
(Table 5), (Figure 8-12)
| Pre-Treatment | Concentration of Copper (mg/L) | Copper Removal (%) |
|---|---|---|
| HNO3 Treated Dry | 0.85 | 83 |
| NaOH Treated Dry | 1.05 | 79 |
| Sterilized Dry | 0.45 | 91 |
| HNO3Treated Wet | 1.05 | 79 |
| NaOH Treated Wet | 1.2 | 76 |
| Sterilized Wet | 0.55 | 89 |
| NAOH Doping | 0.9 | 82 |
| HNO3 Doping | 0.75 | 85 |
[Volume of Copper solution = 100 ml, Initial Copper Concentrations = 5 mg/L, pH = 2.0, Temperature = 370C].
Desorption Studies
(Table 6, 7)
Reagents |
Weight of Biosorbent (g) | Concentration of Cu (mg/L) | Desorbed Copper (%) | |
|---|---|---|---|---|
| Before Desorption | After Desorption | |||
| 1 N HCl | 1.25 | 1.09 | 0.6 | 88 |
| 1 N NaOH | 1.274 | 1.11 | 0.75 | 85 |
| 0.1 N EDTA | 1.29 | 1.13 | 0.65 | 87 |
[Initial Cu Concentration = 5 mg/L, pH 2.0, Volume of solution = 100 ml, Weight of Biosorbent Dosage =1 g]
Reagents |
Concentration of Cu (mg/L) | Copper Removal (%) |
|---|---|---|
| 1 N HCl | 0.55 | 89 |
| 1 N NaOH | 0.60 | 88 |
| 0.1 N EDTA | 0.65 | 87 |
[Initial Cu Concentration = 5 mg/L, pH 2.0, Volume of solution= 100 ml, Weight of Biosorbent Dosage = 1 g]
5.3 Biosorption of Dye industry effluent
Batch Experiment
(Table 8)
| Copper Solution | Biosorbent Used | Copper Removal (%) |
|---|---|---|
| Using Aqueous Solution | Dry Fungal biomass | 92.48 |
| Sterilized Dry Fungal Biomass | 96.99 | |
| Using Dye industry effluent | Dry Fungal biomass | 91.6 |
| Sterilized Dry Fungal Biomass | 94.2 | |
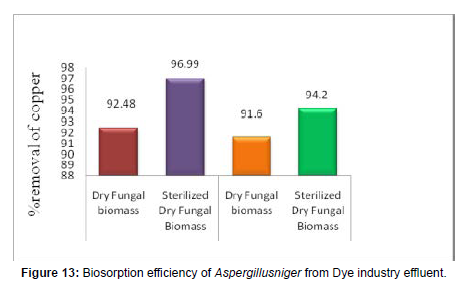
Table 8: Percentage Removal of Copper from using dye industry effluent Aspergillusniger.
[100 ml aqueous metal solution, pH=2.0, Initial metal ion concentration (C0) = 5 mg/L, Weight of biosorbent dosage = 1 g, and Temperature = 370C.].
Biosorption of Copper from using dye industry effluent using Aspergillusniger
(Figure 13)
Desorption Studies
Discussions
Mass culturing the organism
Culturing of aspergillusniger
The Aspergillusniger fungus material which was cultured and obtained, shown in the figure 1. The fungus was cultured and grown the in Potato Dextrose Agar materials. The obtained original mother culture was again sub cultured using Potato Dextrose Broth as shown in the figure.
Mass culturing of aspergillusniger
The fungus biomass materials used for the investigation have been cultivated in greater amount in potato dextrose broth. Mass Culturing of the biomass was carried out and was obtained as mat as shown in figure 3 which was further used as wet or dried to be used as dry biomass.
Biosorption using aqueous solution
Effect of contact time
The effect of contact time and periods on Copper biosorption process using dried Aspergillusniger was characterized, studied and the results are depicted in Figure 4 and the obtained values are tabulated in Table 1. It was estimated that the adsorption effectiveness of Copper ion increased as the contact time periods was increased and equilibrium stages were reached at 330 mins with a removal of about 91%. So that for this process 330 minutes was fixed as the equilibrium standard time. At starting, the initial removal rate of the absorbate was rapid as seen, but it has steadily reached equilibrium and remains constant as time period was increased over the equilibrium time. The rate percentage of metal ion removal was greater in the opening due to the higher surface area of the adsorbent is being available for the adsorption process of the metals [20]. The adsorption phenomenon of the Copper metal was higher at earlier stages due to the availability of higher number of the active sorption sites. As over the time lapsed the metal uptake by the biosorbent surface will slow down as the contest for the lesser availability of active sites which are intensified by the metal ions remaining in the working solution [21].
Effect of initial metal ion concentration
The mass and recovery percentage of copper shows decreases with increase in initial copper concentration. The decrease in the extent of removal percentage of copper with increase in the initial metal ion concentration may be due to the reduction in immediate adsorption due to the lack of available active sites [22]. For higher concentration of copper the percentage removal decreases mainly due to the saturation of binding sites.
Effect of pH
The effect of pH on the percentage removal of copper at 5mg/L copper concentration was studied as a function of pH at constant fungal doses and the results are shown in Figure 6and the values are tabulated in Table 3. It is clear from the figure that the percentage removal of copper is 85 % at pH 2.0. The percentage removal increases with increase in pH from 1.0 to 2.0 and thereafter decreases with further increase in pH. This behavior can be explained by considering the nature of the biosorbent at different pH in copperbiosorption. The cell wall of Aspergillus species contains a large number of surface functional groups. The pH dependent to that of copper biosorption process can largely be associated to the type and ionic state of these active functional groups and also on the metal chemical in solution. The Maximum bio-absorption of copper metal occurs at the pH level of 2.0 which suggests that the negatively charged copper ion species are to bind through the electrostatic attraction to positively charged active functional groups on the surface of fungus material cell wall because at this range of pH more functional groups carrying positive charges will be recorded. The same time at pH above 3.0, it has been observed that the fungal cell wall comprises of more functional groups those carrying a net total negative charge which tend to repulse the anions in nature.
Effect of biosorbent dosage
The observed effect of biomass materials loading on percentage elimination of copper metal ions was characterised and is showed in Figure 7 and the obtained values are tabulated in Table number 4. Metal absorption with deviation in Biosorbent dosages has been observed and reported. It shows that the increase in the adsorbent biomass load from 1g to 5g increment at the rate of sorption from 72% to 91% in total. The increase in Copper metal ion adsorption may probably be due to the improved number of binding sites for copper metal ions at elevated cell loading [22].
Effect of Pre-treatment
The effect of various pre-treated biomass on percentage removal was studied at 5 mg/L of the initial copper metal ion concentration. The consequent values of percentage elimination are presented in Figure 8 and the obtained values are tabulated in the Table 5. A maximum of Copper ion elimination of 91% was obtained using Sterilized dried and chemically modified fungal biomass materials followed by about 89% elimination using the Sterilized wet and chemically modified fungal biomass material and followed by 85% elimination using HNO3 doped and chemically altered fungal biomass materials at around 37ºC, pH 2.0 and 150 rpm. These indicate that when the cell wall of fungal biomass undergo biological modifications and gets changed due to a variety of pre-treatments and hence the extent of its cu heavy metal ions adsorption may differs [23].
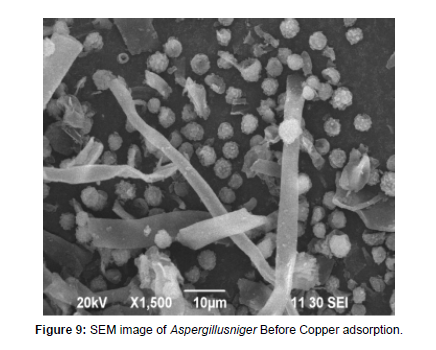
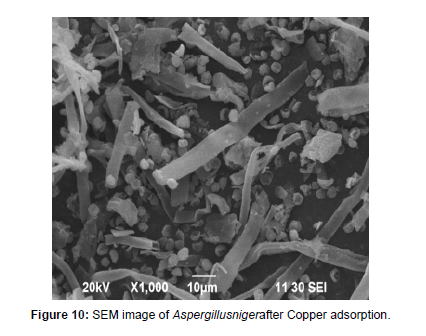
Characterization of biosorbent Using SEM Analysis
Scanning electron microscopy analysis has been used for the characterization of the adsorbent [24]. The bio adsorbent of fungal Aspergillusniger biomass was analyzed before and after it was subjected to trial of Cu metal ion adsorption process is shown in Figure 9 and figure 10 correspondingly. The Scanning electron microscopy scans the surface of the adsorbent and the change in morphology of the adsorbent surface was studied. The surface of the fungal biomass showed that the biomass appeared scattered before adsorption to Copper and the biomass appeared complexes with Copper after adsorption.
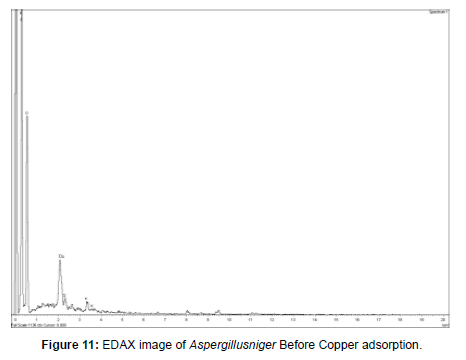
EDAX analysis
The EDAX (Energy Dispersive X-ray Spectroscopy) is an analytical and characterization technique which utilizes x-rays that are emits from the specimen when small bombard by the electron beam to spot the elemental composition of the sample. A resultant electron vacancy is filled by an electron from a privileged shell, and an x-ray is emitted to balance the energy dissimilarity between the two electrons. The EDAX detector technique, the number of emitted x-rays versus their energy band levels. The energy level of the x-ray is attributing of the elemental aspect from which the x-ray was emitted. Currently available SEM/EDS machines are to be operated by using very sophisticated installed software. These software programs allow unattended feature examination and “mapping” of the composition of the elements on the surface of the samples [25].
Desorption Studies
The adsorbed biomass material was found to be cluster with Cu metal ions. These heavy metal ions impudent the further physical adsorption of Copper metals ions to A.niger. Inorder to recycling biomass materials the metal ions adsorbed to A. nigerwas were subjected to desorption process studies which was tabulated in Table 7. The Copper metal was found to desorb effectively using 0.1 N HCl and was found to be 88 %. The recovered and Desorbed A. nigerwas was again used to trial of Copper metal ion adsorption and the mass was found out to be 88% which is higher than first cycle. The biomass material was then also weighed after adsorption of Cu heavy metal and after the process of Desorption. The mass of biomass showing a considerable difference. The mass of fungal biomass material was increased from 1g to 1.25g after the adsorption and the mass of fungal biomass diminished to 1.09 g after desorption in 1N HCl acidic medium which indicating that the desorption was efficient. Desorption technique is essential in order to reprocess and re use of the fungal biomass. The bio-sorption process using desorbed fungus material was also found to be capable agent and the obtained values are tabulated in Table 6.
Biosorption using Dye industry Effluent
Batch experiment: The characterization studies of Atomic Adsorption Spectroscopy results were established that the electroplating waste water effluents are having 0.60 mg/L strength of Cu (So4). So the dye industry effluent was made to 5 mg/L of Cu (So4) and the pH was adjusted to 2.0 and 37°C. The obtained OD values after 330 minutes are showing that the maximum removal of 91.6 % was resulted using 1g of the dried fungal biomass material. The removal performed using Sterilized dry fungal biomass also showed a maximum Cu(So4) removal of 94.2%. The percentage adsorption using the dry and pretreated fungal biomass is shown in Figure 13 and the values are shown in Table 8.
Desorption studies: In this present, the bio absorbed fungi materials mass was exactly estimated and which was established the higher and increase of mass from 1gram to 1.4 gram. Then after the desorption process by using 1Normal HCl acid the mass of fungal biomass material was decreased to 1.13 g which indicating the effectiveness of desorption process. The concentration value of the desorbed from supernatant showing 90 % elimination of Cu (So4) from the Biosorbent material. The value of desorption based on the OD and mass and also the recycling potentials of the biosorbent materials are presented in Tables.
Conclusion
The present work has been successfully carried out. The results shows that the Copper can be effectively removed upto 91% from the aqueous solution using 1g dry fungus from 5 mg/L concentration copper solution at pH 2.0, 37°C. The results also showed that when the biosorbent dosage was increased the removal was also found to increase linearly. More over when the fungus Aspergillusniger was treated previously by using a variety of chemicals and the effectiveness of their copper metal removal will be quite increased. Maximum Copper removal of about 91% was obtained when the biosorbent sterilized dry was used followed by alkali treated dry biosorbent and biosorbent sterilized wet yield 89 % and 85 % removal efficiency respectively at 37ºC, pH 2.0 and 150 rpm. The total percent elimination of Copper using the growing fungus was estimated out to be 91%. The desorption studies showed that 89 % of the Copper was effectively desorbed from the biosorbent using 1 N HCl and the reuse of the biosorbent showed a maximum of 90.72 % Copper removal. The Copper ions concentration 5 mg/L, pH value 2.0, 1g biomass material was utilised for biosorption process in dye processing chemical industrial effluent. A max ceiling removal of around 91% was estimated using dry Aspergillusniger. Further pre-treated biomass was used for removal from Dye industry effluent. Maximum Copper removal of about 94.2 % was obtained when the biosorbent doped with nitric acid was used. The desorption trials showed that 88 % of Cu was desorbed from the fungal biomass using 1 Normal HCl acid and the reuse of the Biosorbent materials shows a maximum of 91% Copper heavy metal removal and the same trend on other heavy toxic metal oxides also shown during analysis, which results and comparative discussion are all will be presented as PART - II of this present investigation as continuing research.
References
- Ahmed El Nemr (2007) Pomegranate husk as an adsorbent in the removal of toxic chromium from waste water. Chem Ecol23: 409-425.
- Akar T, Tunali S, Kiran T (2005) Botrytis cinereous a new fungal biosorbent for removal of Pb (II) from aqueous solutions. Biochem Eng J 25: 235-243.
- Aksu Z (2005) Application of biosorption for the removal of organic pollutants: a review. Process Biochem 40: 997-1026.
- Amit A, Pradhan Audrey D, Levine (2008) Microbial biosorption of copper and lead from aqueous Systems. Sci Total Environ170: 209-220.
- Antonio Sanchez, Antonio Ballester, Maria Luisa Blazquez (1999) Biosorption of copper and zinc by Cymodoceanodosa. FEMS Microbio Rev 23: 527-536.
- Ayhan Sengil I, MahmutOzacar (2008) Biosorption of Cu (II) from aqueous solutions by mimosa tannin gel. J Hazard Mater 157: 277-285.
- BalaKiran K, Thanasekaran (2011) Copper biosorption on Lyngbyaputealis: Application of response surface methodology (RSM). Int Biodeterior Biodegradation1-6.
- Corneliu Cojocarua, Mariana Diaconu (2009) Biosorption of copper (II) ions from aqua solutions using dried yeast biomass, Colloids and Surfaces. Physico Chem Engg Aspects 335: 181-188.
- Dean JG, Bosqui FL, Lannouette KL (1977) Removing heavy metals from wastewater. Environ Sci Technol 6: 518-524.
- El-Naas MH, Ashour I (2006) Biosorption of copper on Chlorella vulgaris from single, binary and ternary metal aqueous solutions. Process Biochem41: 457-464.
- Fergusson JE (1990) the heavy metal elements: chemistry, environmental impact and health effects. Oxford: Pergamon Press.
- Freundlich HMF (1906) over the adsorption in solution. J Phys Chem(57) A: 385-470.
- Flavia Pinto Padilha (2005) Francisca Pessoa de Franca, The use of waste biomass of Sargassum sp. for the biosorption of copper from simulated semiconductor effluents. Bioresour Technol96: 1511-1517.
- Gadd GM, Tobin JM, White C (1994) Metal accumulation by fungi: applications in environmental biotechnology. Indian J Microbiol13: 126-130.
- García-Leon AM, Soto-Regalado E (2011) Experimental design for the optimization of copper biosorption from aqueous solution by Aspergillusterreus. J Environ Manage1-6.
- Gupta VK, Arshi Rastogi, Saini VK (2006) Biosorption of copper (II) from aqueous solutions by Spirogyra species. J Colloid Interface Sci296: 59-63.
- Jia-ChuanZhenga, Hui-Min F eng (2009) Removal of Cu (II) in aqueous media by biosorption using water hyacinth roots as a biosorbent material. J Hazard Mater171: 780-785.
- Jianlong Wang Can Chen (2009) Biosorbents for heavy metals removal and their future. Biotechnol Adv 27: 195-226.
- Jyh-ping chen, Wen-renchen (1996) Biosorption of Copper from Aqueous Solutions by Plant Root Tissues. J Ferment Bioeng81: 458-463.
- Kandah M, Abu Al-Rub FA, Al-Dabaybeh N (2002) Competitive adsorption of copper-nickel and copper-cadmium binaries on SMW. Engg Life Scienc8: 237-243.
- Khormaei M, Nasernejad B, Edrisi B (2007) Copper biosorption from aqueous solutions by sour orange residue. J Hazard Mater149: 269-274.
- Kratochvil D, Volesky B (1998) Advances in the biosorption of heavy metals Tıbtech 16: 291-299.
- Langmuir I (1916) the constitution and fundamental properties of solids and liquids, Part I solids. J Am Chem Soc 38: 2221-2295.
- Mata YN, Blazquez ML, Ballester Gonzalez F (2009) Optimization of the continuous biosorption of copper with sugar-beet pectin gels. J Environ Manage90: 1737-1743.
- Mausumi Mukhopadhyay SB, Noronha (2007) Kinetic modeling for the biosorption of copper by pretreated Aspergillusnigerbiomass. Bioresour Technol98: 1781-1787.
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Citation: Bose MSC (2022) Removal of Toxic Heavy Metal Ions from the Dye Industries Effluents by Using Isolated and Dried A.Niger Biomass Material - The Spent Biomass Briquetts Used as Fuel Material for Small Furnacess - An Alternate Energy Approach: PART –I. J Biotechnol Biomater, 12: 292. DOI: 10.4172/2155-952X.1000293
Copyright: © 2022 Bose MSC. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 1645
- [From(publication date): 0-2022 - Mar 31, 2025]
- Breakdown by view type
- HTML page views: 1307
- PDF downloads: 338