Removal of Polyacrylamide Hydrogel Injected in Different Layers for Breast Augmentation
Received: 01-Dec-2017 / Accepted Date: 21-May-2018 / Published Date: 25-May-2018 DOI: 10.4172/2476-2024.1000141
Abstract
Background: The complications of polyacrylamide hydrogel (PAAG) injected for breast augmentation have captured the attention of the physicians and patients. More and more patients are seeking for the removal of it. In this article, we analyzed the effectiveness of the removal of PAAG injected for breast augmentation by using an inferior periareolar incision under the direct visualization. We got to know the extents of the removal of PAAG injected in different layers.
Methods: Fifty patients (99 breasts) for the removal of PAAG were randomly selected. On the basis of the preoperative and postoperative MRI, the patients were divided into four groups according to whether the PAAG infiltrated to the subcutaneous tissue and muscles or not. In each group, the volumes of PAAG before and after the removal were calculated to analyze the removal amount of the PAAG injected in different layers.
Results: The mean volume of injected PAAG was 264.81 ml. The mean volume of residual PAAG was 9.18 ml. The mean percentage of the removed PAAG was 96.49%. There was no significantly difference in preoperative volume of injected PAAG among different groups p=0.992). There was significantly difference in postoperative volumes of residual PAAG after removal among different groups (p=0.000).
Conclusions: The PAAG injected for breast augmentation and degenerated tissue could be removed using the direct visualization method to obtain successful removal of the great amount of PAAG. The PAAG without infiltrated to the subcutaneous tissue and muscles was most easily removed. The infiltration of the subcutaneous tissue and muscles increased the difficulty of the removal the PAAG.
Keywords: Polyacrylamide hydrogel; Removal; Direct visualization
Introduction
Polyacrylamide hydrogel (PAAG) has been widely used for injection augmentation mammoplasty in Ukraine, Russia, China, and Iran for more than 2 decades [1]. Although numerous reports have indicated that polyacrylamide hydrogel injection for soft-tissue augmentation leads to a good result [2-4]. Many other studies have reported many complications after the use of polyacrylamide hydrogel. The reported adverse effects associated with PAAG injection for augmentation mammoplasty include indurations and lumps, hematoma, inflammation, infection, persistent mastodynia, poor cosmetic results, glandular atrophy, gel migration, loss of ability for breastfeeding and even delayed diagnosis of breast cancer [1,5-10].
The complications of the use of PAAG for breast augmentation had attracted the attention not only of the physicians but of the government. In April 2006, the China state Food and Drug Administration announced that PAAG would be prohibited from production and clinical application in plastic surgery. It was reported by a lot of media which made people pay much more attention on this matter. Many patients came to our hospital asking for removal of the polyacrylamide hydrogel in their breasts regardless of whether they had complications or not.
There were several approaches reported for the removal of PAAG. Some reports suggested that PAAG in breast could be removed by the suction method with a cannula [11,12]. However, there were some physicians who believed that blunt aspiration not only left too much gel in breast, but also gave rise to new complications such as the migration and grow of the PAAG, blunt trauma and causing several scattered spaces which enhanced the degree of muscle tissue degeneration [13,14]. To remove as much PAAG as possible, it was recommended by several physicians to remove the infiltrated fascia and capsule together with the free PAAG using the direct visualization method [13,15,16]. But there were few studies on the analysis of the removal amount of PAAG using this method. In this article, we reported our experience in the removal of PAAG injected in breast by using an inferior periareolar arc incision under the direct visualization. We calculated the volume of PAAG injected for breast augmentation before and after the removal. The purpose of the study was to get to know the effectiveness of the method for the removal of PAAG injected for breast augmentation.
Material and Methods
Between January 2005 and November 2012, there were 407 patients who admitted to our hospital for the removal of the PAAG because of the complications after PAAG injected for breast augmentation. Fifty patients (99 breasts) were randomly selected form them. All patients were scanned before the operative procedures by magnetic resonance image (MRI) (Siemens Magneton Vision 1.5T).
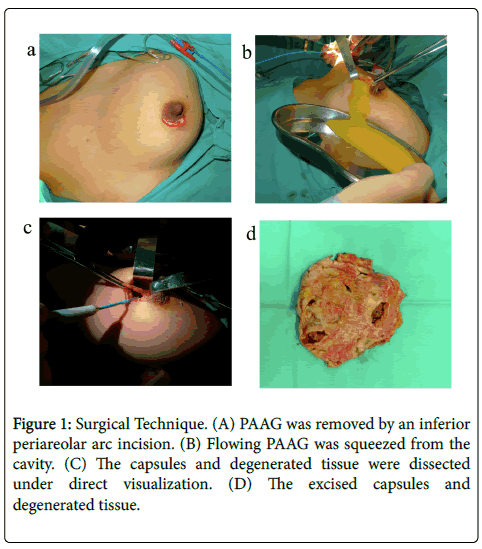
An inferior periareolar arc incision was used to remove the PAAG under direct visualization (Figure 1A). Dissection was completed along the surface of the mammary gland downward to the inferior edge of the gland. A radial incision then was made on the mammary gland. The retromammary space then was exposed and the traits of PAAG were known. The flowing PAAG can be squeezed from the cavity as much as possible (Figure 1B). After that the cavity was repeatedly irrigated with a large volume of normal saline which was sucked out by the negative pressure machine. Then the capsules were dissected away (Figures 1C and 1D). The solid PAAG and the capsules should be dissected away as intact as possible. If the PAAG had been injected into the muscles, the pectoralis major should be opened to expose the gel. Because the gel tended to be mingled with the muscle in different muscular regions and layers, the injected PAAG and degenerated tissue should be detected together. For the PAAG injected into the intercostal muscles, pneumothorax should be avoided. If the PAAG had been injected into the subcutaneous tissue, electrocautery should not be used to avoid the injury of the skin. Sporadic lumps and PAAG in independent layers should not be ignored. They also must be removed according to preoperative MRI images.
All excised infiltrated capsule tissue was sent for histopathological examination. Drainage was maintained until the total drainage was less than 20 ml. Only one seroma was found in one breast on the 6th day after the operation. The patients were advised to wear elastic garments for about 4 weeks. The patients were scanned by MRI for the postoperative check or before the repairing surgery.
On basis of the layers PAAG injected showed in the MRI, the patients were divided into four groups according to whether the PAAG infiltrated to the subcutaneous tissue and muscles or not (Table 1).
| Patient Clinical Data | Mean | SD |
|---|---|---|
| Age | 35.1 | 8.56 |
| Duration of PAHG injection (yrs) | 7.24 | 2.49 |
| Symptoms | N | |
| Nodules with pain | 11 | |
| Nodules | 8 | |
| worry | 8 | |
| Pain | 5 | |
| Hardness with nodules | 4 | |
| Displacement | 3 | |
| Hardness with pain | 3 | |
| Infection | 2 | |
| Asymmetric | 1 | |
| Upper limb discomfort with pain | 1 | |
| dissatisfaction | 1 | |
| mastoptosis | 1 | |
| Malposition with pain | 1 | |
| Residual after removal | 1 |
Table 1: Clinical characteristic of patients
Group S0G1M0: PAAG did neither infiltrate into the subcutaneous tissues nor the muscles. Group S1G1M0: PAAG infiltrated into the subcutaneous tissues but not into the muscles. Group S0G1M1: PAAG infiltrated into the muscles but not into the subcutaneous tissues. Group S0G1M1: PAAG not only infiltrated into the subcutaneous tissue but also into the muscles. In each group, the volume of PAAG injected for breast augmentation and the volume of the residual PAAG after the removal were measured using the method reported in previous article according to the MRI [17]. Preoperative volumes and postoperative volumes of the PAAG were calculated to analyze the effectiveness of the removal of the PAAG by using this method. The test was performed variance analysis and non-parametric test using a global significance level of p<0.05 using SPSS version 16 for windows (SPSS Inc, Chicago IL,USA).
Results
Between 2005 and November 2012, the removal of PAAG by using the direct visualization method was performed in 407 patients. Their ages ranged from 24 to 69 years (mean age, 35.1 years). The period from injection to removal of the PAAG ranged from 2 years to 12 years (mean 7.24 years). Complications included nodules, pain, displacement, infection, asymmetry, dissatisfaction, mammary ptosis and psychological problems. The characteristic data was presented in Table 2.
| Groups | Subcutaneous(S) | Gland(G) | Muscle(M) |
|---|---|---|---|
| S0G1M0 | - | + | + |
| S1G1M0 | + | + | - |
| S0G1M1 | - | + | + |
| S1G1M1 | + | + | + |
| +:infiltrated,-:not infiltrated. | |||
Table 2: The breasts were divided into four groups according to the layers PAAG infiltrated.
The mean volume of injected PAAG was 264.81 ml. The mean volume of residual PAAG was 9.18 ml. The mean percentage of the removed PAAG was 96.49%. There were separately 15 breasts, 17 breasts, 34 breasts and 33 breasts in each group. The volumes of injected PAAG were 259.70 ml, 254.81 ml, 264.72 ml and 263.37 ml separately. The volumes of residual PAAG were 1.15 ml, 4.24 ml, 9.68 ml and 14.85 ml. The percentage of removed PAAG was 99.56%, 98.34%, 96.34% and 94.36%.
The results were showed in Table 3. There was no significantly difference in preoperative injected PAAG volumes among different groups (p=0.992). There was significantly difference in postoperative residual PAAG volume after removal among different groups (p=0.000). There was significantly difference between group S0G1M0 and other groups (pS0G1M0, S1G1M0=0.008, pS0G1M0, S0G1M1=0.000, pS0G1M0, S1G1M1=0.000). There was significantly difference between group S1G1M0 and group S1G1M0 (p=0.007). There was no significantly difference between group S1G1M0 and group S0G1M1 (p=0.272). There was no significantly difference between group S0G1M1 and group S1G1M1 (p=0.071).
| Groups | Preoperative volume of PAAG (ml) | Postoperative volume of PAAG (ml) | Percentage of removed PAAG | ||
|---|---|---|---|---|---|
| Average | SD | Average | SD | Average | |
| S0G1M0 | 259.7 | 96.52 | 1.14 | 2.59 | 99.56% |
| S1G1M0 | 254.81 | 97.23 | 4.24 | 3.56 | 98.34% |
| S0G1M1 | 264.72 | 111.19 | 9.68 | 13.61 | 96.34% |
| S1G1M1 | 263.37 | 133.59 | 14.85 | 21.3 | 94.36% |
| Average | 264.81 | 4.43 | 9.18 | 6.05 | 96.49% |
Table 3: Preoperative and postoperative volume of PAAG and the Percentage of PAAG removed.
Discussion
PAAG consists of 2.5% polyacrylamide, which is a non-degradable synthetic polymer, suspended in 97.5% water [18]. It is believed to be a nontoxic, non-allergenic, non-teratogenic, nonembryotoxic, nonmutagenic, and non-biodegradable watery gel [3,19-21]. However, it has been shown to alter the physician parameters of human fibroblasts and increase the expression of c-myc RNA [21]. Although there was no cause-effect relationship between breast cancer and PAAG mammoplasty, it was clear that the diagnosis of breast cancer can be delayed and the prognosis can be affected with PAAG: the hyper dense gel and lumps may interfere with identification of the malignant lesions [22]. On the other hand, acrylamide monomer can’t be totally avoided during the manufacture of the PAAG, although the monomer was typically present at a low level [9]. Acrylamide has inherent toxic properties such as neurotoxicity, genotoxicity and carcinogenicity, and is classified as a group 2A substance (probably carcinogenic to humans) by the International Agency for Research on Cancer and a category 2 mutagen by the European Union [23]. There are concerns about possible degradation of polyacrylamide in the human body into monoarylamide, which is a tumor initiator that increases the incidence of mammary gland tumors in female rats [19,24]. There are many complications reported after the use of polyacrylamide hydrogel. Treatment of the complications needs to remove as much PAAG as possible. The direct visualization method was suggested by several surgeons [15,24-26].
Most of the PAAG injected for breast augmentation can be removed by using the direct visualization method through periareolar approach. In our cases, the mean percentage of the removal PAAG was 96.49%. It demonstrates that this method is quite effective in the removal of injected PAAG. The periareolar approach is beneficial for the removal of PAAG [16]. In the first, the PAAG can be squeezed for the flowing out freely through the periareolar approach. Secondly, the periareolar approach is near to the border of the injected PAAG. The infiltrated capsule and fascial can be dissected easily. Thirdly, the dissection is much easier through the periareolar approach and can go directly from layer to layer to remove widespread hydrogel. Some reports suggested that PAAG in breasts could be removed by suction [11,12]. But blunt aspiration left too much gel in breasts; reoperation may be needed in most of the patients. The capsule and degenerated tissue only can be removed using the direct visualization method [13].
Before the surgical techniques, MRI scanning was meaningful for us to ascertain the distribution of the PAAG. The risk and difficulty of the surgery were also reckoned. At the same time, we can predict the postoperative breast shape on the basis of preoperative MRI images. But there were few articles about the relationship between the layers of PAAG injected and the difficulty of the removal. In this article, we analysis the difficulty of the removal of PAAG infiltrated in the breast. In accordance with our clinical experience, it can be most easily removed for the PAAG injected into the anatomical space between the gland and the pectoralis major. The infiltration of the subcutaneous tissue or the muscle increased the difficulty of the surgical technique. According to the results of the study, we can know the residual volumes of PAAG. Before the surgery, the patients must be informed that the PAAG could not be completely removed.
It is worth noting that different extents of breast deformities can be caused after the removal of PAAG injected in different layers. These deformities would make adverse effects on breast shapes; therefore it is meaningful to improve breast shape and life quality to correct the secondary deformities after the removal of the PAAG injected. There are different reconstruction methods in correspondence with different extents of breast deformities and it will be reported in another article.
Conclusion
The PAAG injected for breast augmentation and degenerated tissue could be removed using the direct visualization method. Successful removal of the great amount of PAAG can be obtained by this method. It is necessary to have a MRI scanning to know the distribution of PAAG before the removal of it. By MRI, we also can estimate the difficulty of the surgery. The PAAG without infiltrated to the subcutaneous tissue and muscles was most easily removed. The infiltration of the subcutaneous tissue and muscles increased the difficulty of the removal the PAAG.
References
- Zhen-Xiang W, Dong-Lin L (2012) Polyacrylamide Hydrogel Injection for Augmentation Mammaplasty: Loss of Ability for Breastfeeding. Aesthet Plast Surg 69: 123-128.
- Christensen LH, Breiting BV (2003) Long-term effects of polyacrylamide hydrogel on human breast tissue. Plast Reconstr Surg 111: 1883-1890.
- Galatenko NA (1992) Toxicological Estimate of Hydrogel PAAG (for endoprosthesis) Kiev, Ukraine: Institute of Chemistry of High-Molecular Compounds, National Academy of Sciences of Ukraine.
- Kovanskaya VA (2000) Statistic analysis of the results of soft tissue contour plastics with the use of hydrogel PAAG. Presented at the Scientific-Practical Conference on ‘‘10 Years Experience in the Wide Clinical Use of Hydrophile Polyacrylamide Gel in Plastic, Aesthetic, Reconstructive Surgery,’’ Kiev, Ukraine.
- Acarturk S, Gencel E, Tuncer I (2005) An uncommon complication of secondary augmentation mammaplasty: bilaterally massive engorgement of breasts after pregnancy attributable to postinfection and blockage of mammary ducts. Aesthet Plast Surg 29: 274-279.
- Cheng NX, Wang YL, Wang JH (2002) Complications of breast augmentation with injected hydrophilic polyacrylamide gel. Aesthet Plast Surg 26: 375-382.
- Cheng NX, Liu LG, Hui L (2009) Breast cancer following augmentation mammoplasty with polyacrylamide hydrogel (PAAG) injection. Aesthet Plast Surg 33: 563-569.
- Khan UD (2009) Breast autoinflation with sterile pus as a marker of implant rupture: single-stage treatment and outcome of five consecutive cases. Aesthet Plast Surg 33: 58-65.
- Mu DL, Luan J, Mu LH (2009) Reoperation for the removal of polyacrylamide hydrogel in the breast: use of periareolar approach under direct visualization. Aesthet Plast Surg 33: 473-474.
- Zhao Y, Yuan NA, Li K, Geng YI, Zhou H, et al. (2015) Bilateral breast cancer following augmentation mammaplasty with polyacrylamide hydrogel injection: A case report. [J]. Oncology letters 9: 2687-2693.
- Yang Y, Chui ZW, Li HY (2010) Removal of polyacrylamide hydrogel after injecting augmentation mammoplasty with negative liposuction technique. Chin J Aesthet Med 19: 1441-1442.
- Ying GQ, Cheng SH, Liu QF (2007) Dislodging polyacrylamide hydrogel after injecting augmentation mammaplasty by microincision vacuum absorption. J Minim Invasive Med 2: 23–24.
- Leung KM, Yeoh GP, Chan KW (2007) Breast pathology in complications associated with polyacrylamide hydrogel (PAAG) mammaplasty. Hong Kong Med J 13: 137-140.
- Zhang YL, Luo Q (2000) The complication of polyacrylamide hydrogel injection for augmentation mammoplasty. J Pract Aesthet Plast Surg 11: 173-175.
- Wei W (2016) Treatment of complications from polyacrylamide hydrogel breast augmentation. Exp Ther Med 12: 173-176.
- Yu L, Wang J (2012) Treatment of breast injection with polyacrylamide hydrogel with infiltrated fascia capsule removal: A report on 104 cases. Aesthet Plast Surg 36: 1120-1127.
- Hu J, Liu C, Chen L, Xing W, Luan J (2014) Volumetric measurement of polyacrylamide hydrogel injected for breast augmentation using magnetic resonance imaging. Experimental and therapeutic medicine 7: 681-684.
- Shimpei O, Rei O (2010) Complications after Polyacrylamide Hydrogel Injection for Soft-Tissue Augmentation. Plast Reconstr Surg 126: 1349-1357.
- (2005) Amended final report on the safety assessment of polyacrylamide and acrylamide residues in cosmetics. Int J Toxicol 24: 21-50.
- King DJ, Noss RR (1989) Toxicity of polyacrylamide and acrylamide monomer. Rev Environ Health 8: 3-16.
- Smith EA, Oehme FW (1991) Acrylamide and polyacrylamide: A review of production, use, environmental fate and neurotoxicity. Rev Environ Health 9: 215-228.
- Khedher NB, David J, Trop I, Drouin S, Peloquin L, et al. (2011) Imaging findings of breast augmentation with injected hydrophilic polyacrylamide gel: patient reports and literature review. Eur J Radiol 78: 104-111.
- Dybing E, Farmer PB, Andersen M (2005) Human exposure and internal dose assessments of acrylamide in food. Food Chem Toxicol 43: 365-410.
- Sun JM, Zhang Y, Wang JZ (2009) Postoperative management of breast augmentation by polyacrylamide hydrogel injection: a report of 157 cases. Chin J Med Aesthet Cosmet 15: 88-91.
- Qiao Q, Wang X, Sun J, Zhao R, Liu Z, et al. (2005) Management for postoperative complications of breast augmentation by injected polyacrylamide hydrogel. Aesthet plast surg 29: 156-161.
- Luo SK, Chen GP, Sun ZS, Cheng NX (2011) Our strategy in complication management of augmentation mammaplasty with polyacrylamide hydrogel injection in 235 patients. J Plast Reconstr Aesthet Surg 64: 731-737.
Citation: Removal of Polyacrylamide Hydrogel Injected in Different Layers for Breast Augmentation Using an Inferior Periareolar Incision Under Direct Visualization. Diagn Pathol Open 3: 141. DOI: 10.4172/2476-2024.1000141
Copyright: © 2018 Hu J, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Open Access Journals
Article Tools
Article Usage
- Total views: 6708
- [From(publication date): 0-2018 - Apr 03, 2025]
- Breakdown by view type
- HTML page views: 5842
- PDF downloads: 866