Make the best use of Scientific Research and information from our 700+ peer reviewed, Open Access Journals that operates with the help of 50,000+ Editorial Board Members and esteemed reviewers and 1000+ Scientific associations in Medical, Clinical, Pharmaceutical, Engineering, Technology and Management Fields.
Meet Inspiring Speakers and Experts at our 3000+ Global Conferenceseries Events with over 600+ Conferences, 1200+ Symposiums and 1200+ Workshops on Medical, Pharma, Engineering, Science, Technology and Business
Research Article Open Access
Removal of Heavy Metals from Water (Cu and Pb) Using Household Waste as an Adsorbent
| Mrinalini Kanyal and Anuja Ashok Bhatt* | |
| School of Biosciences and Biotechnology, Vellore Institute of Technology, Vellore, India | |
| Corresponding Author : | Anuja Ashok Bhatt School of Biosciences and Biotechnology Vellore Institute of Technology, Vellore, India Tel: 0416 224 3091 E-mail: anuja_bhatt92@yahoo.com |
| Received August 21, 2014; Accepted December 29, 2014; Published December 31, 2014 | |
| Citation: Kanyal M, Bhatt AA (2015) Removal of Heavy Metals from Water (Cu and Pb) Using Household Waste as an Adsorbent. J Bioremed Biodeg 6:269. doi:10.4172/2155-6199.1000269 | |
| Copyright: © 2015 Kanyal M, et al. This is an open-a ccess article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | |
Related article at Pubmed Pubmed  Scholar Google Scholar Google |
|
Visit for more related articles at Journal of Bioremediation & Biodegradation
Abstract
Chicken eggshells, Banana peels and Pumpkins are used as good adsorbents for removing heavy metals from contaminated water, which have been observed and studied currently. The effects of various parameters such as pH, agitation speed and contact time was studied and good results were obtained at pH 7, 100 rpm and 90 mins of contact time. The results indicate that usage of household waste such as these can be used as a good biosorbent for removal of heavy metals on a large scale and create effective, cheap and efficient methods in treating wastewater.
| Keywords |
| Eggshell; Banana peel; Pumpkin; Biosorbent |
| Introduction |
| Now a day’s various toxins are released into water leading to a great deal of water pollution. Many heavy metals from various industries like battery plants, metal processing industries, pharmaceuticals, hospitals, mining fields etc. are being released into the water bodies leading to unsafe water for normal consumption. The most common heavy metals found are copper and lead, which when present in high concentrations may be very fatal to the health and the surrounding environment as well [1]. In order to obtain clean and safe water it is required that these toxic chemicals and metals should be removed. |
| Many methods have been undertaken in the process to remove these unwanted contaminants such as physio-chemical methods, various biological methods and to large extent nano-based techniques [2]. The methods that we have employed are purely based on the aim to achieve environmental sustainability by using house hold waste such as eggshells, banana peels and pumpkin which are cheap, easily available and a very effective adsorbent. Eggshells are a very reliable adsorbent due to its calcium carbonate content [2,3]. Moreover,there is no scope of any organic compounds dissolving in the solution like pumpkin powder , banana peel powder ,pomegranate powder leaving the solution colourless [4,5]. Banana peels have good adsorbent properties and may be a successful method in purification of water due to the compounds in the banana peels that contain atoms of nitrogen, sulfur and organic compounds such as carboxylic acids. These acids are charged such that their negatively charged electron pairs are exposed, meaning they can bind with metals in the water that usually have a positive charge [5,6]. Pumpkins also have good adsorbent properties due to the lignocellulosic compounds in the organic matter that contain functional groups like carboxyl, hydroxyl, ester, etc. A large number of lignocellulosic biosorbents are utilized for metal cation sorption. |
| The main objective of our project is see how efficient eggshell powder is in adsorbing heavy metals such as copper and lead at a concentration of 5 ppm at various different parameters such as pH, agitation (rpm) and contact time [2,3]. We have devised our own protocol and decided various parameters which we thought suited the best. |
| Materials and Methods |
| Materials |
| We tested our protocol using the three adsorbents on two heavy metals namely Copper and Lead. Copper (ii) Sulphate and Lead Nitrate (both S.R.L) was used to prepare a stock solution of 1000 ppm in deionised water respectively. A .1 M of HCl and NaOH solutions were used to adjust the pH. All the chemicals used were of laboratory grade used in the laboratories of our college. We measured the absorbance of heavy metals by Atomic Absorption Spectrometer (AAS) [1]. |
| Preparation of solutions |
| The solutions were self-contaminated. To prepare a 1000 ppm of stock solution of Copper sulphate we added 3.93 g of copper(II) sulphate in 1 L of deionised water. Likewise, we added 1.598 g of Lead nitrate in 1 L of deionised water to prepare the 1000 ppm stock solution. From the stock solution we prepare a 5 ppm concentration of heavy metal contaminated water. For preparation of 5 ppm solution we took 20 ml from 1000 ppm( both for Cu and Pb respectively) solution in a beaker and add deionised water upto 250 ml (till required). |
| Preparation of adsorbent |
| Around 20 eggshells were collected from daily kitchen waster and washed with normal tap water followed by distilled water (using latex gloves to avoid contamination). The eggshells were left to dry on blotting paper to absorb excess water and were then subjected to the hot air oven at 50°C for 2 days (RDB27 HIS TC-102). Once completely dried we pulverised and shred eggshells to fine particles using mortar and pestle followed by a mixer, later we sieved the pulverised adsorbent to obtained a homogenous size. Likewise 20 small banana peels and 2 small size pumpkins were taken washed thourougly, air dried then subjected to the hot air oven at 50°C for 2 days then they were sieved to obtain uniform particl size. |
| Method used |
| After preparing 5 ppm solution (Cu and Pb) we added 100 ml of solution in 16 (250 ml each) conical flasks for Cu and Pb respectively, where each flask has 100 ml of 5 ppm solution we then adjusted the pH using 0.1 M HCl and NaOH such that flasks had a pH of 4-7 respectively in order. We labelled the flasks with respect to their pH, agitation (rpm) of 100 rpm and 165 rpm then added 0.1 g of the adsorbent (different set of trials for different adsorbents) and subjected them to a shaker with a contact time of 60 minutes and 90 minutes at room temperature. Afterwards we filtered the solutions using Whatmann filter paper 1 and observed the absorbance of metal using the AAS. The experiment was taken out in two batches on the first day the batch for Cu an Pb at 5 ppm were tested at a pH of 4 and 6 (flasks 1-4 were at pH 4 and 5-8 were at pH 6) Likewise the next day they were tested at Ph5 and ph 7 (flasks 1-4 were at pH 5and 5-8 were at pH 7). There were samples of each flask 1-pH 4 ,rpm 100,contact time-60 mins, 2-pH 4 ,rpm 100,contact time-90 mins, 3-Ph 4 ,rpm 165 ,contact time-60 mins , 4-pH 4 ,rpm 165,contact time-90 mins, 5-pH 6 ,rpm 100,contact time-60 mins, 6-pH 6 ,rpm 100,contact time-90 mins, 7-pH 4 ,rpm 165,contact time- 60 mins, 8-pH 6 ,rpm 165,contact time-90 mins). The same process is employed for pH 5 and pH 7. |
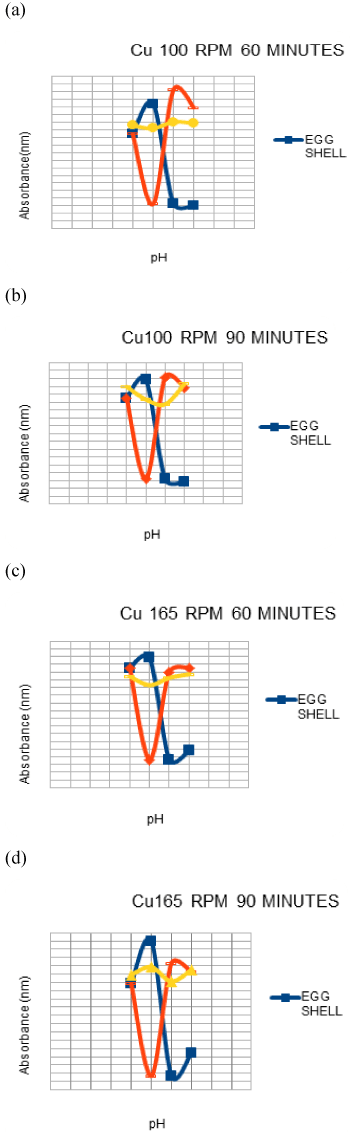
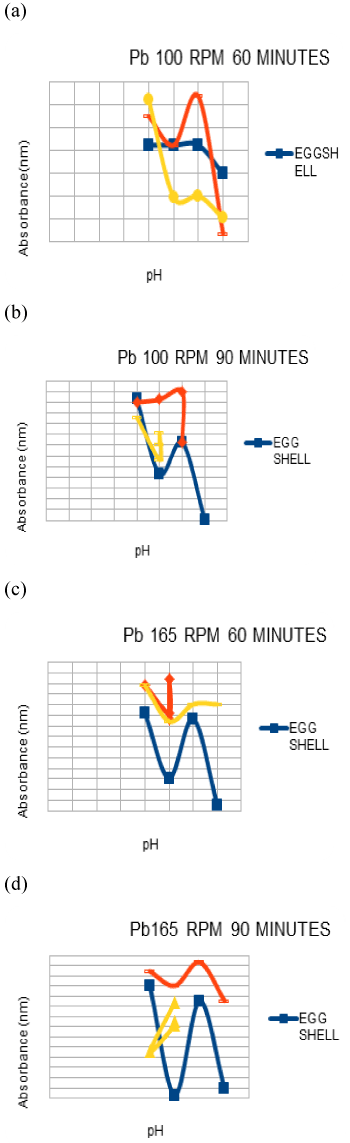
| Effect of ph on Cu and Pb adsorption |
| The range of pH used was 4-7. The 100 ml solutions which are at a concentration of 5 ppm in the conical flasks were subjected to .1 g of adsorbent the adjustments were done using a .1 M solution of HCl and NaOH [1] later the flask were subjected agitation of 100 rpm and 165 rpm(marked accordingly) with a contact time of 60 mins and 90 mins. Once the contents were taken from the mixture they were filtered using Whatmann fileter paper no. 1 and given to the AAS to check the adsorption of heavy metals by the adsorbent. |
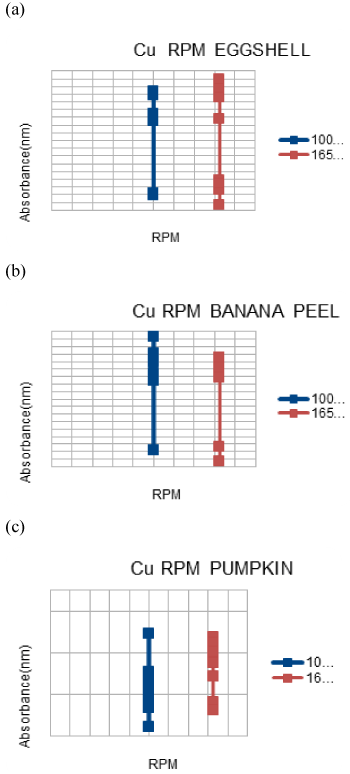
| Effect of agitation on Cu and Pb adsorption |
| The agitation on the shaker was decided to be 100 rpm and 165 rpm to make the process more efficient for a contact time of 60 mins and 90 mins after the pH adjustment and addition of .1 g of adsorbent was done. Afterwards filtration using Whatmann filter paper number 1 was done followed by giving the samples to AAS to check the adsorption. |
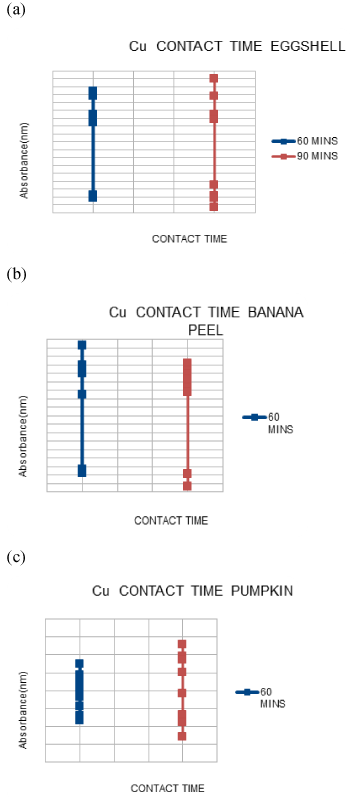
| Effect of contact time on Cu and Pb adsorption |
| The contact time used was 60 mins and 90 mins during the agitation period after which again filtration occurred and adsoption was checked by subjecting the samples to the AAS (Tables 1, 2 and Figures 1-6). |
| Results and Discussion |
| Effect of pH ON Cu and Pb adsorption |
| It was seen that at the lower pH values the absorbance was less as compared to that at the higher pH values, it could be because at the lower values the adsorption could have been compromised due to competitive interactions between the heavy metal ions and the hydrogen ions, which restricts the adsorption whereas at higher pH values like 7 the carbonate groups present in the eggshells may have resulted in attracting the heavy metals due to an increase in the negative charge on adsorbent surface area [1] In banana peel and pumpkin also the results were good at a higher pH , which may be due to the various compounds and acids present.The overall best results were obtained at pH 7 . |
| Effect of agitation on Cu and Pb adsorption |
| Agitation is very important to increase the efficiency of adsorbance and it helps speeding the process. It was observed that the best adsorbance was found at 100 rpm, although contradictory to the fact that at low rpm the matter might accumulate at the bottom of the flask, it did not hinder the adsorbance process and we got good results. |
| Effect of contact time on Cu and Pb adsorption |
| The more the surface area of adsorbent the more adsorbance occurs and if the contact time is more hence evens more adsorbance. Good and efficient results were obtained when samples were exposed for a contact time of 90 mins. |
| Conclusion |
| The removal of the heavy metals Cu and Pb using chicken eggshell powder ,banana peel powder and pumpkin powder as adsorbents was carried out using various parameters such as different contact time, agitation speed and pH values. The overall best conditions concluded from the experiment was at a pH 7, rpm 100 and contact time of 90 minutes. The size of the adsorbent was at a very small scale hence which helped in the efficiency of adsorption; also these household wastes are economical, easy to find and inexpensive making this process very sustainable. Moreover they will not harm the environment and is very safe to use. When added to the samples the eggshell left a colourless solution which made the filtration process easier. |
| By this experiment primary level of filtration was achieved and it was found that eggshells were the best adsorbents of heavy metals from water. This experiment can be used in the future probably by modifying the particle size to the nano scale to achieve better efficiency. Also chemical modifications may be done on the the domestic waste to increase the adsorbance capacity. |
| Acknowledgements |
| We would like to thank our guide Dr. C. Ramalingam Dean (Biotechnology), School of Biosciences and Technology, VIT University Vellore for his full support and guidance and Dr. Jayanthi Mahalingam, our co-guide for her efforts and support. Also we are greatful to the University for providing us with the funds and opportunity to pursue our research work. |
References
|
Tables and Figures at a glance
| Table 1 | Table 2 |
Figures at a glance
 |
 |
 |
| Figure 1 | Figure 2 | Figure 3 |
 |
 |
 |
| Figure 4 | Figure 5 | Figure 6 |
Post your comment
Relevant Topics
- Anaerobic Biodegradation
- Biodegradable Balloons
- Biodegradable Confetti
- Biodegradable Diapers
- Biodegradable Plastics
- Biodegradable Sunscreen
- Biodegradation
- Bioremediation Bacteria
- Bioremediation Oil Spills
- Bioremediation Plants
- Bioremediation Products
- Ex Situ Bioremediation
- Heavy Metal Bioremediation
- In Situ Bioremediation
- Mycoremediation
- Non Biodegradable
- Phytoremediation
- Sewage Water Treatment
- Soil Bioremediation
- Types of Upwelling
- Waste Degredation
- Xenobiotics
Recommended Journals
Article Tools
Article Usage
- Total views: 29147
- [From(publication date):
January-2015 - Apr 03, 2025] - Breakdown by view type
- HTML page views : 22824
- PDF downloads : 6323
