Research Article Open Access
Removal of Dimethyl Sulphide in a Biotrickling Filter under ThermophilicConditions
| Wei ZS*, Ye QH, Li BR, Chen ZY and Huang QR | |
| School of Environmental Science and Engineering, Sun Yat-sen University, Guangdong Provincial Key Laboratory of Environmental Pollution Control and Remediation Technology (Sun Yat-sen University), Guangzhou 510275, China | |
| Corresponding Author : | Wei ZS School of Environmental Science and Engineering Sun Yat-sen University Guangdong Provincial Key Laboratory of Environmental Pollution Control and Remediation Technology (Sun Yat-sen University) Guangzhou 510275, China Tel: +8620 84037096 Fax: +8620 39332690 E-mail: weizaishan98@163.com |
| Received: October 08, 2015; Accepted: October 31, 2015; Published: November 02, 2015 | |
| Citation: Wei ZS, Ye QH, Li BR, Chen ZY, Huang QR (2015) Removal of Dimethyl Sulphide in a Biotrickling Filter under Thermophilic Conditions. J Bioremed Biodeg 6:320. doi:10.4172/2155-6199.1000320 | |
| Copyright: © 2015 Wei ZS, et al. This is an open-a ccess article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | |
| Related article at Pubmed, Scholar Google | |
Visit for more related articles at Journal of Bioremediation & Biodegradation
Abstract
Biotrickling filter (BTF) of odorous containing dimethyl sulfide (DMS) emitted from the sewage sludge drying under thermophilic conditions was investigated. DMS removal efficiencies in the BTF achieved 50%, 65%, 75% at 40, 50, 60°C , respectively. The elimination capacities at 60°C was higher than that of biotrickling filter at 50°C or 40°C. At a bed contact time of 34.4 s, the elimination capacities at 60°C in the BTF was 14 g-DMS.m-3.h-1, which was higher than that of mesophilic biofilter. Sulfur dioxide (SO2) has inhibitory effect to the microbial activity for dimethyl sulfide degradation. Bacterial communities in the BTF, which were assessed by PCR-DGGE, play the dominant role in the biological processes of metabolism, sulfur oxidation, sulfate-reducing and carbon oxidation under thermophilic conditions. These results show that thermophilic biotrickling filter is achievable and open new possibilities for applying BTF to hot odour gas streams from sewage sludge drying.
| Keywords |
| Biotrickling filter; Dimethyl sulphide; Thermophilic bacterial; PCR-DGGE; Degradation mechanism. |
| Introduction |
| Wastewater treatment results to the production of large quantities of sewage sludge [1]. Thermal drying of sludge makes it possible to stabilize the sludge, reduce its volume and hygienize the product. However, it is inevitable to produce odor compounds, such as dimethyl sulfide (DMS), trimethylamine (TMA), NH3, SO2, H2S, volatile fatty acids (VFAs), and PCDD/Fs [2,3]. Hot odours emitting from sewage sludge drying cause an odour problem, ranging from annoyance to documented health effects [4]. The use of thermophilic microorganisms active at temperatures over 40°C would offer great savings and would greatly extend the applicability of biofilter and biotrickling filter. The high-temperature biotrickling filter exhibited a higher degree of ethanol mineralization to CO2, and hosted a process culture composed of both mesophilic and thermotolerant or thermophilic microorganisms, whereas the ambient-temperature reactor lacked microorganisms capable of growing at high temperature [5]. Hot H2S-containing gas at 60°C was successfully treated in a thermophilic biofilter inoculated with Bacillus sp. [6]. Three biotrickling filter (BTF) systems was the successful treatment of hydrogen sulphide gas at temperatures of 40, 50, 60 and 70°C using a microbial community obtained from a hot spring. Addition of glucose and monosodium glutamate enhanced thermophilic biofiltration of hydrogen sulphide gas and a removal rate of 40 g.m-3.h-1 was achieved at 70°C [7]. Methanol removal rates over 100 g.m-3.h-1 were achieved at temperatures up to 70°C . α-Pinene removal was achieved at temperatures up to 60 °C with optimal treatment occurring at 55 °C at rates up to 60 g. m-3.h-1 [8]. Thermophilic biotrickling filter was used for a-Pinene biodegradation at 45°C [9]. Long-term stable performance of the BTF inoculated with C. daeguensis TAD1 for the effective treatment of NO was accomplished in an oxygen stream of 8% under aerobic condition at 50°C [10]. A thermophilic biofilter achieved high performance to remove gaseous toluene at 55°C [11]. The thermophilic biofilter could effectively degrade Methyl tert-butyl ether (MTBE) at a constant bed temperature of 52 ± 3°C [12]. Removal of isobutyraldehyde and 2-pentanone was investigated in BTF at higher temperature (52-65°C ) [13]. The thermophilic bioreactor showed a lower DMS elimination capacity. Temperature changes to 21 and 59°C decreased the removal efficiency of BTF52 by 90 and 30%, respectively. Liquid-phase sulfate concentrations exceeding 2.2 g.L−1, decreased the removal rate by 50% at 52°C [14]. The dimethyl sulfide removal is possible in a thermophilic membrane bioreactor [15]. A mechanistic model based on energy and mass balances was developed to predict the performance of a trickling biofilter as a function of temperature and the temperature variation along the height of the tricking biofilter [16]. |
| A variety of DMS degrading microorganism for dimethyl sulphide removal has been intensively studied. Most of them are mesophilic bacteria such as B. sphaericus [17] chemolithotrophic Thiobacilli [18], A. thiooxidans and T. thioparus [19], Proteobacteria, Firmicutes, and Actinobacteria [20] Actinobacteria, γ- Proteobacteria, β-Proteobacteria [21], Bacillus sphaericus, Pseudomonas putida, Thiobacillus thioparu [17,22] in the biofilter could play an important role in sulfur oxidation, sulfate reduction, carbon oxidation, and fermentation [21]. |
| The objectives of this work were to investigate the biotrickling filter of DMS emitted from the sewage sludge drying at different temperature, 40, 50 and 60°C and to examine the microbial community developed at high temperatures. The study evaluates the factors such as inlet DMS high load, empty bed residence time (EBRT) on the performance of the biotrickling filtration under thermophilic conditions, analyses microbial community by PCR-DGGE, and speculates DMS degradation mechanism by IC and GC-MS. |
| Materials and Methods |
| Experimental procedure |
| The biotrickling filter (BTF) (Figure1) was used in this study, which was operated in three phases: one at temperature of 40°C , the second at 50°C and the third at 60°C . BTF was packed with ceramsite to a height of 450 mm, which was set up to study treatment stimulated odorous containing dimethyl sulfide (DMS) emitted from the sewage sludge drying. The BTF was made of transparent rigid plexiglass with an inner diameter of 90 mm and a height of 1000 mm. It was divided into three phases with the filter medium, biodegrading bacterials adhere to the surface of ceramsite to form the biofilm. As no source of high temperature gas was available, high temperatures were achieved by heating the recycle liquid through a coiled copper tubing submerged in a thermostated water bath at 40~60°C . The DMS supplied from the gas cylinders, was first diluted with the compressed air, passed through an air mixture bottle, then flowed upwards the bottom of the biotrickling filter, at a flow rate of 100 to 600 L.h-1 (EBRT,17.2 to 103s). In the process of the biodegradation of DMS experiments, nutrient containing aqueous solutions was sprayed downward with a peristaltic pump from the top of column to maintain the moisture of the BTF and supply nutrients to the microbial population. The trickling solution was recycled to supply nutrients for microbial growth and to supply water to compensate for water evaporation. |
| Analytical methods |
| Bacterial community compositions in the thermophilic biotricking filter of DMS were assessed by polymerase chain reaction-denaturing gradient gel electrophoresis (PCR-DGGE), and identify the colonies of the predominant microorganisms by the procedures of total DNA extraction, polymerase chain reaction (PCR) amplification of 16S rDNA, and sequencing and comparing results with those in the GenBank database by using the BLAST server of nucleotide sequence similarity in the NCBI website. MiniRAE PLUS PGM-7600 Photo- Ionization Detector analysis device was used for analysis of dimethyl sulfide concentration, which was made in USA (RAE systems Company, USA). Gas flow rates were measured using Model LZB-1 flow meters with units of 1 L.h. The pH value in the recirculating liquid was determined by a Model pHS-3C pH Tester (INESA, Shanghai, China). |
| Results and Discussion |
| Performance of the thermophilic trickling biofilter |
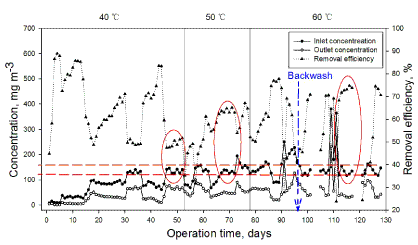
| The biotrickling filter was operated for 128 days in three phases at different temperature, 40, 50 and 60°C , respectively. Phase I at 40°C , lasted from days 1 to 52; in phase II at 50°C , from days 53 to 77; the third phase at 60°C , from days 78 to 128. In each phase, effect of different temperature on total performance of the biotrickling filter was investigated. The performance of the biotrickling filter was evaluated in terms of the removal efficiency (RE, %) and the elimination capacity of the filter bed (EC, g.m-3 h-1) as defined elsewhere [23]. Figure 2 shows the performance of the biotrickling filtration for DMS removal during the 128-d continuous running test. Three red ovals and parallel dotted lines are shown in Figure 2, RE of DMS are about 50%, 65% and 75% at different temperature, 40, 50 and 60°C , separately, when inlet concentration in range from 120~150 mg.m-3. |
| After one day of operation in phase I at 40°C , The BTF was initially acclimated to DMS under the conditions with flowing low concentrations of 8~15 mg.m-3 and the reactor temperature at 40°C , DMS removal efficiency achieved 85% during the initial 3 days. After acclimation, the effect of DMS inlet concentration was investigated that correspond to increasing inlet concentration of 30, 60, 90, and 180 mg.m-3. At the beginning of increasing inlet concentration to 30 mg m-3 at 6th d, DMS removal efficiency fell down to 70 % and recovered fast within 1 week. DMS removal efficiency decreases, while the EC increases with increasing inlet concentration, the magnitude of the drop increases by about 30% and the time of acclimation were more than 2 weeks. The inlet concentration, RE and EC of Day 26 were 93.0 mg m-3, 52.1 %, 5.1 g m-3 h-1, respectively. RE and EC of Day 29 reached 89.9%, 5.9 g m-3 h-1, respectively while the inlet concentration and EC was 63.1 mg m-3. However, RE decreased with increasing of inlet concentration, the RE kept about 50% when inlet DMS concentration of 150~180 mg m-3. EC increased with increasing of inlet concentration, the maximum EC was 12.2 g m-3 h-1 at 40°C under inlet concentration of 180.2 mg m-3. |
| On day 53, the start of phase II, temperature was increased from 40 to 50°C . Due to sudden temperature change in the biotrickling filter, DMS removal efficiency declined to 40% at 50°C . Microbe in BTF need to a short time to adapt it due to rising temperature. RE increases from 50% at 55th d to 82.7% at 67th d, when maintained inlet concentration at a range from 80 to 150 mg.m-3 at 50°C in the BTF system. The EC was 11.1 g-DMS.m-3.h-1 at inlet concentration of 127.9 mg.m-3. With inlet concentration increased to 200 mg.m-3, RE decreased to 50%. |
| During the 50-day phase III, temperature was further increased from 50 to 60°C. Same response, similar to phase II, was observed once again. RE attained 52.1% at 78th d, when maintained inlet concentration of 125 mg.m-3 at 60°C in the BTF system. RE increased steadily, achieved 65% at 81th d with inlet concentration of 200 mg.m-3 DMS. At a bed contact time of 34.4 s, the elimination capacities at 60°C in the BTF was 14.2 g-DMS.m-3.h-1, where the RE was 66.4%, which was higher than that of biotrickling filter at 50°C or 40°C. Higher temperature condition has a better capacity to degrade hydrophobic DMS. |
| The influence of DMS inlet load and EBRT |
| The effect of DMS inlet load on DMS removal efficiency and elimination capacity is presented in Fig. 3, under the conditions of pH of 6.9, EBRT of 34.4 s and sprinkling density of 2.7 m3·m-2·h-1 at 40, 50, 60°C in the BTF system. DMS removal efficiency decrease, while the EC increases with increasing inlet load of DMS. When the inlet load was less than 10 g·m-3·h-1, DMS removal efficiency achieved 80% at 40°C , RE fell sharply to 48.9% If inlet load increased to 15.5 g.m-3.h-1, the EC was 7.6 g-DMS.m-3.h-1. RE of DMS achieved 75% with less than 15 g.m- 3.h-1 inlet load at 50°C . When inlet load increases to 19.7 g.m-3.h-1, RE fell sharply to 55.2%, the EC was 10.6 g-DMS.m-3.h-1. When inlet load is lower than 21.5 g m-3 h-1 at 60°C , RE of DMS fell down slowly with increasing of DMS inlet load, and RE is keeping above 65%, the EC was 14 g-DMS.m-3.h-1. Thus, the elimination capacities at 60°C was higher than that of biotrickling filter at 50°C or 40°C . At a bed contact time of 34.4 s, the elimination capacities at 60°C in the BTF was 14 g-DMS.m- 3.h-1, which was higher than that of mesophilic biofilter. |
| The influence of EBRT on DMS removal are presented in Figure 3, under the conditions of pH of 6.9, inlet concentration between 70 and 90 mg.m-3 DMS and spray rate at 3.3, 2.7 and 2.0 m3.m-2.h-1 at the different operation temperatures of 40°C , 50°C and 60°C , respectively. DMS removal efficiency increases with EBRT increasing (Figure 4). The increasing process of removal efficiency is from sharp rise to slow growth process. From the EBRT - RE curve of 60°C , EBRT 17.2 s corresponding removal efficiency is 27%, when EBRT increases to 34.4 s, RE rises to 78%. These results demonstrate that decreasing EBRT from 34.4 to 17.2 s caused a decline in the removal efficiency by around 188%. This decrease in removal efficiency was primarily due to the reduction in the contaminant retention time in the bed which did not provide sufficient time for the transfer of DMS from the gas phase to the biofilm. This consequently resulted in an increased exit DMS concentration and a consequent reduction of DMS removal efficiency. Increasing EBRT appropriately should enhance DMS mass transfer from gas phase to the biofilm. The EBRT was 34.4 s which was shorter than other thermophilic studies, significantly. |
| The influence of SO2 |
| In the thermal drying of sludge by waste gas of cement kiln, sulfur containing organic compounds in sludge are easy to be decomposed at high temperature, producing sulfur dioxide (SO2); while SO2 substance is present in the waste gas of cement kiln, SO2 is also emitted from cement production. SO2 will affect biological purifying of DMS. The effect of inlet SO2 on DMS removal is presented in Figure 5, under the conditions of EBRT of 34.4 s, inlet load between 12.1 and 14.9 g.m-3.h-1 DMS and sprinkling density of 2.0 m3·m-2·h-1 at 60 °C in the BTF system. DMS removal efficiency decreases with increasing inlet concentration of SO2. When the inlet concentration of SO2 was 22 ppm, DMS removal efficiency was 61.7%. Increase of the SO2 inlet concentration from 0 to 22 ppm caused a fall in DMS removal efficiency by 10%. When inlet concentration of SO2 is further increases to 55 ppm, DMS removal efficiency is only 49.8%. Increase of the SO2 inlet concentration from 55 to 100 ppm resulted in reduced DMS removal efficiencies of about 49.8% and 25%, respectively. This decrease in DMS removal efficiency was primarily because pH fell in recycling liquid affected by SO2, BTF is suspected that a neutral pH resulted in maximal enzyme activities for dimethyl sulfide degradation, SO2 has inhibitory effect to the microbial activity [24]. The degradation of DMS depends on sulfur oxidation, sulfate-reducing and carbon oxidation bacteria, these bacteria grow better in neutral. Thus, a bench scale system integrated with a desulfurization and a thermophilic biotrickling filter unit should eliminate SO2 influence and run stability. |
| Bacterial community composition |
| Microbial community structure in the biotrickling filter is investigated by polymerase chain reaction-denaturing gradient gel electrophoresis (PCR-DGGE), when system runs stably at 115th d. Results show that there are nine bands in PCR-DGGE profile which represents nine species microorganism (Table 1). Nine bands (A, B, C, D, E, F, G, H and I ) bacterial group that includes Hydrotalea sp., Acidobacteria bacterium, Filamentous cyanobacterium, Thermomonas sp., Arthrobacter sp., Thermoactinomycetaceae bacterium, Arthrobacter sp., Hydrogenothermophilus hirschii, Thermodesulfovibrio sp. Thermodesulfovibrio sp. is an anaerobic, thermophilic (45-70°C ), sulfate-reducing bacteria (SRB), enhancing the metabolism of DMS in the biotrickling filter. Growth occurred with sulfate as well as thiosulfate as electron acceptors [25,26]. Thermodesulfovibrio sp. takes part in the anaerobic degradation of DMS and the reduction of sulfate, the dominant bactrias in the BTF. Thermoactinomycetaceae bacterium is an aerobic thermophilic bacteria that can be isolated form compost at 65°C , which contributed to the aerobic degradation of DMS [27]. Arthrobacter sp. is a thermophilic bacteria [28], which has been reported to degrade kinds of persistent organic pollutants and atrazine [29,30]. Hydrogenothermophilus hirschii is a rod-shaped thermophilic hydrogen-oxidizing β-proteobacterium, average size about 1.0~1.5 × 0.8 μm, growth occurred on complex organic substrates such as yeast extract and peptone and on organic acids. The optimum condition of Hydrogenothermophilus hirschii between 50-68°C [31]. Acidobacteria is a thermophilum sulfur oxidation bacterium (SOB), which also occurs in a large variety of other habitat types such as hot springs [32]. Filamentous cyanobacterium are regarded as one of the most successful groups of prokaryotic organisms based on a fossil record that is among the oldest for any group of organisms [33]. Hydrotalea sp. and Thermomonas sp., are thermophilic [34]. Since DMS can be metabolized to dimethyl sulfoxide, methyl mercaptan, hydrogen sulfide, and sulfide (SO42-) [35], this predominant bacterias may be attributable to the potential for sulfur oxidation, sulfate-reducing and carbon oxidation processes to occur simultaneously in the biotrickling filter system under thermophilic conditions. |
| Mechanism of DMS degradation in BTF |
| The intermediate products were identified by analysing them with a GC–MS. The gas-phase intermediate organic products were detected in the sample after the bioreaction. While DMS (CH3SCH3, m/z=91) was identified in the sample collected before biodegradation reaction, the gas-phase intermediate organic products such as carbonyl sulfide (O=C=S, m/z=60) from DMS biodegradation were identified by GCMS. Sulphuric acid (SO42-) in the DMS biodegradation was identified by ion chromatographic (IC). |
| Based on the above results, we propose the following reaction mechanism for DMS in BTF under thermophilic conditions (Figure 6). Under aerobic conditions, DMS was oxidized into OCS after demethylation, OCS was further oxidized into SO2 and H2SO4 by sulfur-oxidative bacteriums (SOB) such as Acidobacteria bacterium, Thermoactinomycetaceae bacterium. While DMS translate to S0 after demethylation, which may further reduced to S2- by sulfate-reducing bacteria (SRB) such as Thermomonas sp., Thermodesulfovibrio sp using methanol and formic acid as the electron donors under anaerobic conditions. Intermediate product methyl combine with water to generate methanol and formic acid, which finally converted to CO2 [36,37]. |
| Conclusions |
| The paper revealed that the thermophilic biotrickling filter can be used for removal of dimethyl sulphide emitted from the sewage sludge drying. The DMS removal efficiency of 60°C over 75% at an inlet load range of 120~150 gm-3, while removal efficiency of 40°C and 50°C achieve at 50% and 65% under the same inlet load. The elimination capacities at 60°C was higher than that of biotrickling filter at 50°C or 40°C . At a bed contact time of 34.4 s, the elimination capacities at 60°C in the BTF was 14 g-DMS.m-3.h-1, which was higher than that of mesophilic biofilter. SO2 has inhibitory effect to the microbial activity for dimethyl sulfide degradation. PCR-DGGE results show that thermophilic bacterial group includes Hydrotalea sp., Acidobacteria bacterium, Filamentous cyanobacterium, Thermomonas sp., Arthrobacter sp., Thermoactinomycetaceae bacterium, Arthrobacter sp., Hydrogenothermophilus hirschii, Thermodesulfovibrio sp. The predominant thermophilic bacterias play the dominant role in the thermophilic biological processes of metabolism, sulfur oxidation, sulfate-reducing and carbon oxidation under thermophilic conditions. These results show that thermophilic biological degradation of technology is achievable and open new possibilities for applying biotrickling filtration to hot odour gas streams from sewage sludge drying. |
| Acknowledgements |
| The authors gratefully acknowledge the financial support from the Project Foundation of Guangdong Province Science & Technology (2011A030700010). |
References
|
Tables and Figures at a glance
| Table 1 |
Figures at a glance
 |
 |
 |
| Figure 1 | Figure 2 | Figure 3 |
 |
 |
 |
| Figure 4 | Figure 5 | Figure 6 |
Relevant Topics
- Anaerobic Biodegradation
- Biodegradable Balloons
- Biodegradable Confetti
- Biodegradable Diapers
- Biodegradable Plastics
- Biodegradable Sunscreen
- Biodegradation
- Bioremediation Bacteria
- Bioremediation Oil Spills
- Bioremediation Plants
- Bioremediation Products
- Ex Situ Bioremediation
- Heavy Metal Bioremediation
- In Situ Bioremediation
- Mycoremediation
- Non Biodegradable
- Phytoremediation
- Sewage Water Treatment
- Soil Bioremediation
- Types of Upwelling
- Waste Degredation
- Xenobiotics
Recommended Journals
Article Tools
Article Usage
- Total views: 10336
- [From(publication date):
November-2015 - Jul 03, 2025] - Breakdown by view type
- HTML page views : 9385
- PDF downloads : 951
