Removal and Analysis of Mercury (II) From Aqueous Solution by Ionic Liquids
Received: 25-Mar-2016 / Accepted Date: 02-Apr-2016 / Published Date: 09-Apr-2016 DOI: 10.4172/2155-9872.1000317
Abstract
D2EHP-] and 1-methyl-imidazolium di(2-ethylhexyl) phosphate [MIm+][D2EHP-] were synthesized and tested as extractants in the batch removal of mercury (II) from aqueous solution. The influence of contact time, aqueous to organic phase’s volume ratio, initial concentration of Hg (II), IL concentration, pH levels, ionic strength, and temperature was evaluated. The extraction equilibrium was established in 30 min for [MIm+][D2EHP-] and in 15 min for [BIm+][D2EHP-]. The maximum mercury extraction was obtained at pH 5.81. For the extraction of mercury, [([MIm+] [D2EHP-])5 (HgCl2)]org, [([MIm+][D2EHP-])5 (HgClOH)]org, [([BIm+][D2EHP-])3/2 (HgCl2)]org and [([BIm+][D2EHP-])3/2 (HgClOH)]org species were formed. Regarding the ionic strength for [MIm+][D2EHP-], the results show a significant improvement of the mercury extraction yield (100%) upon the addition of sodium acetate to the aqueous phase in a Na+/Hg2+ mass ratio ranging from 0.1 to 2.0. The relationship between the percentages of the extracted species and the extraction yield was established by calculations using CHEAQS V. L20.1 software. The results revealed a decrease in the extraction yield of Hg (II) with decreasing proportions of HgCl2aq from 65.15 to 40.31% and of HgClOHaq from 31.31 to 0.1%, when NaCl was added. The very important optimal sorption capacities for ([BIm+][D2EHP-]) and ([MIm+][D2EHP]) were 58.39 mg/g and 93.23 mg/g respectively. With a longer alkyl chain on the imidazolinic ring, the decreasing of extraction yield was observed.
Keywords: Mercury; Ionic liquid; Solvent extraction; Speciation
304291Introduction
Removal of heavy metals and more particularly of mercury from wastewater has become a major environmental issue to be addressed [1]. Mercury (Hg) is one of the most toxic heavy metals commonly found in nature. Its toxicity is related to the bio concentration capacity of its derivatives in living organisms through food chain. Mercury and derivatives are considered as being hazardous contaminants with negative impact on human health and biodiversity [2-5]. Bioaccumulation in living organisms is accentuated by increasing emission sources, which has stimulated worldwide efforts aiming regulation and reduction of these emissions, especially in water sources.
Large-scale uses of mercury in industrial processes impose its recovery from secondary industrial resources. In this regard, several techniques for mercury (II) removal from aqueous solutions such as reduction process [6], chemical precipitation [7], membrane separation [8], ion-exchange [9], solvent extraction [10], coagulation [11], adsorption [12,13] and magnetic ferrites treatment [14] have been developed so far. Most of them turned out to be quite unsatisfactory in total elimination of heavy metals. The use of ionic liquids for mercury elimination (ILs) would be an interesting route to explore. ILs have recently received increased attention from both industrial and academic communities because of their unusual properties and great potential as ‘‘green’’ solvents for industrial processes [15]. They show unique properties such as non-volatility (negligible vapor pressure), thermal stability, nonflammable nature, lower reactivity, strong ability to dissolve a large variety of organic and inorganic compounds, along with a potential to extract rare earth (lanthanides plus Sc and Y) and heavy metals.
Ionic liquids, also called room temperature ionic liquids (RILS) and ambient temperature molten salts, which are liquids at ambient temperature [16], are totally composed of organic cations and inorganic/organic anions. ILs are environmentally harmless, nonvolatile, nonflammable, and display many properties of conventional organic solvents such as excellent solvation qualities, low viscosity, low vapor pressure, high ionic conductivity, appreciable thermal and electrochemical stability in a wide temperature range [17-20]. They are considered as alternatives to classical organic solvents and, as such, have been applied for many purposes such as organic synthesis, electrochemistry, and liquid phase extraction, catalysis for clean technology and polymerization processes [21-25]. A number of papers have been devoted to the description of synthesis and properties of various ILs, including imidazolium and phosphoniumbased ILs and their capacity in the extraction of organic and inorganic compounds [26-28]. The aim of this work is to investigate the ability of newly synthesized ionic liquids, namely 1-butyl-imidazolium di(2- ethylhexyl) phosphate [BIm+][D2EHP-] and 1-methyl-imidazolium di(2-ethylhexyl) phosphate [MIm+][D2EHP-], to extract Mercury (II) from aqueous chloride solutions. The effects of various parameters such as the initial mercury (II) concentration, IL concentration, initial pH ion, ionic strength and temperature on the mercury extraction yield were studied. The species extracted into the organic phase were also investigated using slope analysis method. Calculations using CHEAQS V. L20.1 software allowed determining the nature and the amounts of the species present in organic medium and establishing the relation between the nature of the extracted species and the extraction yield [29].
Experimental
Reagents
The reagents used in this study were 1-methylimidazole (98%, Aldrich), 1-butyl-imidazole (98%, Aldrich), Di(2-ethylhexyl) phosphate (97%, Fluka), Chloride of mercury(II) (99.5%, Aldrich), Chloride of sodium (99.5%, Aldrich), sodium acetate (99%, Aldrich), 4-(2-pyridylazo)-resorcinol (PAR) (97%, Aldrich), chloroform (99%, Fluka), hydrochloric acid (37%, Fluka) and buffer solution at pH=13.0 (Riedel-Dehaen AG).
Apparatus
Analytik Jena SPECORD 210 Double Beam UV-VIS was used for spectra recording and absorbance measurements. Spectra were recorded in the range from 400 to 800 nm with 0.2 nm resolution in 10 mm quartz cells. Data were processed with WinLab software.
Sodium was analyzed in aqueous solutions before and after extraction on JENWAY PFP7 Flame Photometer. pH measurements for all solutions were taken on a potentiometer Consort C831, with combined glass electrode, that was calibrated with pH 4.00, 7.00 and 10.00 buffer standards. The logarithmic n-octanol = water partition coefficients (log P) were calculated with Clog P program of the commercial available software ChemDraw® ultra (Cambridge Soft).
Ionic liquids synthesis
A mixture of D2EHPA (6.448 g, 20 mmol) and 1-methylimidazole (1.640 g, 20 mmol) or 1-butylimidazole (2.483 g, 20 mmol) to a 250 mL round-bottom flask fitted for 24 h at room temperature till the formation of a yellow and viscous liquids.
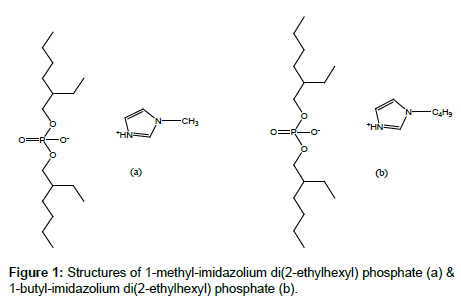
The viscous liquids were washed three times with 50 mL portion of ethyl acetate in a separation funnel. The washed ILs were dried with anhydrous magnesium sulfate and heated under vacuum at 70°C to remove the solvent. The purity of final products was characterized with 1H NMR, 13C NMR, 31P NMR and FTIR (Figure 1). The yields were about 98%.
For [MIm+][D2EHP-] the attributions were:
1H NMR: δ/TMS (ppm) = 0.96 (m, 15H, CH3), 1.33 (m, 16H, CH2), 1.56 (m, 4H, CH), 3.93 m, 4H, CH2O P), 5.0 (t, 1H, NH), 5.72 (q; 1H, CHar), 5.89 (q; 1H, CHar), 6.36 (q; 1H, CHar). 13C NMR: δ/TMS (ppm)= 11.6; 14.1; 68.8; 117.8; 119.1; 142.5, 21.7; 23.0; 23.3; 29.3; 30.4; 30.6; 32.6; 34.2; 22.4; 40.3; 108.1; 108.5; 118.0; 141.5. 31P NMR: δ/H3PO4 (ppm) = 0.02. IR: ν (cm−1) = 895 (w), 1045 (P OC, vS, L), 1240 (P=O, S, L), 1380 (w), 1470 (m), 1685 (w, L), 2855 (S), 2955 (vS).
For [BIm+][D2EHP-] the attributions were:
1H NMR: δ/TMS (ppm) = 0.95 (m, 15H, CH3), 1.33 (m, 20H, CH2), 1.55 (m, 4H, CH), 3.93 (m, 4H, CH2O P), 5.1 (t, 1H, NH), 5.72 (q; 1H, CHar), 5.89 (q; 1H, CHar), 6.36 (q; 1H, CHar). 13C NMR: δ/TMS (ppm)= 11.6; 14.1; 68.8; 117.8; 119.1; 142.5, 21.7; 23.0; 23.3; 29.3; 30.4; 30.6; 32.6; 34.2; 22.4; 40.3; 108.1; 108.5; 118.0; 141.0. 31P NMR: δ/H3PO4 (ppm) = 0.021. IR: ν (cm−1) = 895 (w), 1045 (P OC, vS, L), 1240 (P=O, S, L), 1380 (w), 1470 (m), 1685 (w, L), 2855 (S), 2955 (vS).
δ, chemical shift; ν, wave number; s, singulet; d, doublet; t, triplet; q, quadruplet; quint, quintuplet; sext, sextuplet; m, multiplet; J, coupling constante; S, strong; L, large; w, weak.
Procedure
The liquid/liquid extraction was carried out by contacting volumes of ionic liquid in chloroform and mercury solution (with known concentration and fixed pH (2.2-6.4), under stirring at constant temperature (20°C). The biphasic system was stirred for homogenization for few minutes, and then both phases were separated by centrifugation. The upper aqueous phase was analyzed through spectrophotometry UV-Visible to determine the concentration of the residual unremoved metal cation.
The mercury concentrations in the aqueous phase were determined, before and after extraction by UV-Visible with PAR as ligand for Mercury with buffer solution at pH=13.0 [28,30]. The absorbance of PAR-Hg(II) complex was measured at 580 nm.
Extraction efficiencies of mercury with ILs
The biphasic system was shaken to ensure it was fully mixed and then centrifuged to separate the two phases after extraction. The upper aqueous phase was taken out and measured with spectrophotometer UV-Visible to determine the concentration of metal that was left in the aqueous phase. The extraction experiments results are discussed in term of extraction yield (Y) defined as follows:
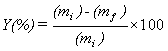 (1)
(1)
where (mi) and (mf) are the mass of Hg (II) ions in aqueous phase before and after extraction, respectively [31].
Optimization experiments
Different extraction tests were run to optimize the effect of the operating parameters for maximum extraction efficiency, namely the phase volume ratio, Hg (II) initial concentration, IL concentration, initial pH of the aqueous solution, ionic strength (salt concentration) and temperature. This allowed assessing the role of the ionic liquid as a simple medium or as an active factor in a molecular interaction. In the absence of [BIm+][D2EHP-] and [MIm+][D2EHP-], no extraction of Hg(II) was observed.
Results and Discussion
Effect of volume ratio
The effect of the aqueous organic volume ratio (Vaq/Vorg) was investigated by varying the Aq/Org ratio from 1 to 6 keeping the total volume of phase’s constant and initial pH of 5.81. The results are presented in Figure 2.
The best extraction yields was obtained to Vaq/Vorg = 1. The curves were decreasing and the extraction yields stabilized around Vaq/Vorg = 6. We have noticed that for Vaq/Vorg = 1, the difference of the extraction yields was 29% between IL [BIm+][D2EHP-] (63%) and IL [MIm+] [D2EHP-] (92%). Whereas, for the others volume ratio, the difference was only 11%. The hydrophobic character was decreased with increasing of water volume, when the both phases were in contact. In conclusion, the volume ratio Vaq/Vorg = 1 will be retained for the continuation of our study.
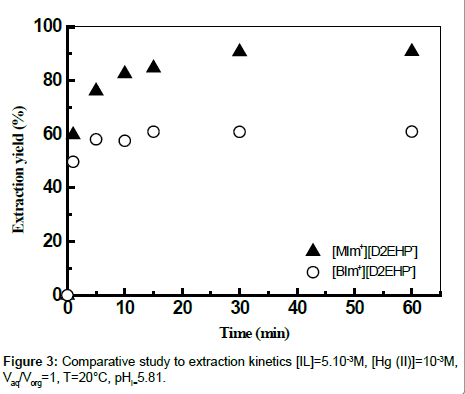
Effect of contact time
The results presented in Figure 3 showed that a minimum of 30 minutes for [MIm+][D2EHP-] and 15 minutes for [BIm+][D2EHP-] of shaking time is needed for extraction of mercury (II). The prolonged shaking had no positive effect on the extraction of Hg (II).
The hydrophobic character of IL was estimated by calculating log P, defined as the partition coefficient between two phases of a substance, generally n-octanol and water. This parameter developed by Hansch [32] is widely used in medicinal chemistry for QSAR studies, but was scarcely used in chemistry to the best of our knowledge. We recently reported the use of this parameter in the correlation between the reagent solubility and reactivity in phase transfer Heck reaction [33]. Log P values can be easily assessed using ChemDraw® ultra (Cambridge Soft) with Ghose’s method [34], are respectively Log P=0.81 to [BIm+] [D2EHP-] and Log P=-0.43 to [MIm+][D2EHP-]. [BIm+][D2EHP-] is more hydrophobic than [MIm+][D2EHP-]. With a longer alkyl chain on the imidazolinic ring, the decreasing of extraction yield was observed [35].
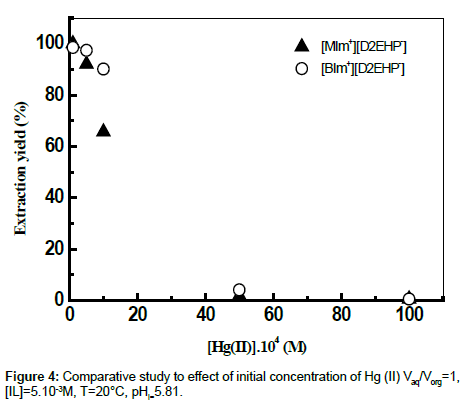
Effect of Hg (II) initial concentration
Increasing initial concentration of mercury (II) in the range 10-4M-10-2M was found to induce a decrease of the extraction yield (Figure 4). Further investigations were achieved using a medium value of 10-3M of the initial concentration of Hg (II).
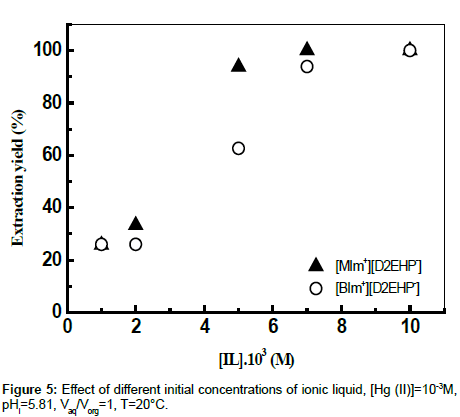
Effect of IL concentration
The role of the ionic liquid was studied by varying the IL amount in the range 10−3M-10−2M at 293 K under 900 rpm stirring. The results showed that the extraction yield of Hg (II) increases significantly with increasing IL concentration (Figure 5), thereby demonstrating the extraction of Hg (II) ions by a biphasic ILaqueous chloride system.
The results seemed to show that specific molecular interactions between Hg (II) ions and the ionic liquids established and the hydrophobic/hydrophilic features of the whole system affect the metal ion association and extraction processes. The observed properties may be then related, at least for a hydrophobic ion as Hg (II), to the structural characteristics of ionic liquids.
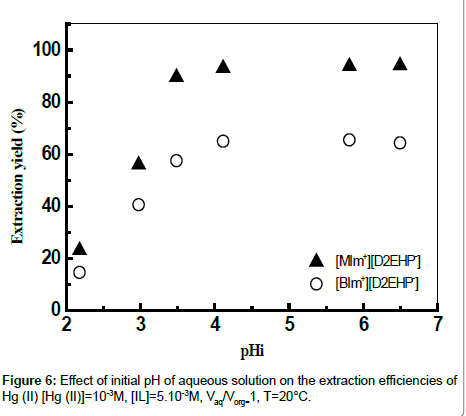
Effect of initial pH
For any extraction process, initial pH of the feed is an important parameter which governs the efficiency of the separation process. Extraction of metal from aqueous solution depends on the strength of the acid/base. Effect of pH in the aqueous phase on extraction of mercury was studied by varying initial pH in the range 2.2-6.4; pHi was adjusted by adding necessary moieties of HCl or NaOH 0.01 mol L-1 (Figure 6). Percentage extraction of Hg (II) increased with increase in the initial pH of the aqueous phase. As can be seen, extraction of mercury was optimum at pHi = 5.81, the extraction yield was (94%) and (65%) for [MIm+][D2EHP-] and [BIm+][D2EHP-] respectively. The optimal sorption capacities for ([BIm+][D2EHP-]) and ([MIm+] [D2EHP]) were 58.39 mg/g and 93.23 mg/g respectively. Other authors showed that the best extraction of Hg (II) by the tetraethyleneglycolbis( 3-methylimidazolium) diiodide Ionic liquid, was obtained at optimal initial pH between 6.0-8.0 [36].
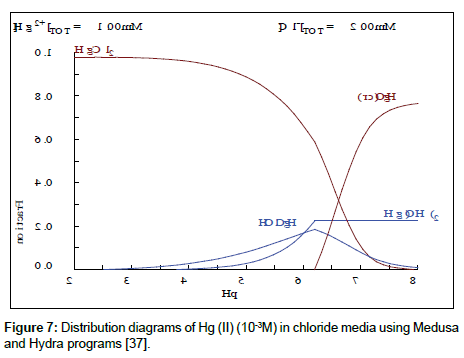
From Figure 7, it can be seen that maximum mercury extraction obtained at the pHi range 4.1-5.81 could be due to the presence of Hg (II) with a high percentage. The increase of the yield of extraction with an increase of initial pH was related with a relative increase of percentage of HgClOHaq and Hg(OH)2 species, however the HgCl2aq decreases substantially. The uptake of mercury ion beyond pH >8 is attributed to the formation of metal hydroxide species such as insoluble precipitate of Hg(OH)2.
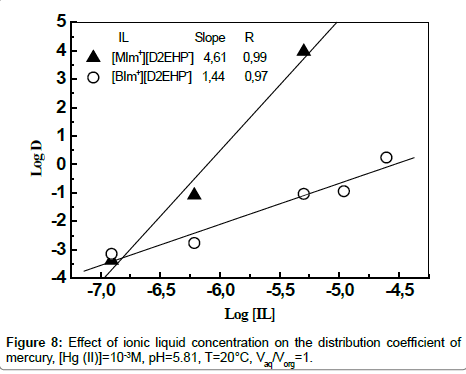
Determination of the metal-organic complex Species
The stochiometric coefficients for the extraction reaction can be determined from the intercept of the plot of log D vs. log [IL] [37,38]. Figure 8 shows a plot of log D vs. log [IL] at initial pH 5.81. The plots illustrates the slopes of nearly 5 moles of [MIm+][D2EHP-] and 1.5 moles of [BIm+][D2EHP-] for extraction of one mole of Hg(II) into the organic phase.
The extraction mechanism of mercury in chloride medium by ionic liquid diluted in chloroform may be represented as:
(HgCl2)aq + 5([MIm+][D2EHP-])org ↔ [([MIm+][D2EHP-])5 (HgCl2)]org (2)
(HgClOH)aq + 5([MIm+][D2EHP-])org ↔ [([MIm+][D2EHP-])5 (HgClOH)]org (3)
2(HgCl2)aq + 3([BIm+][D2EHP-])org ↔ 2 [([BIm+][D2EHP-])3/2 (HgCl2)]org (4)
2(HgClOH)aq + 3([BIm+][D2EHP-])org ↔ 2 [([BIm+][D2EHP-])3/2 (HgClOH)]org (5)
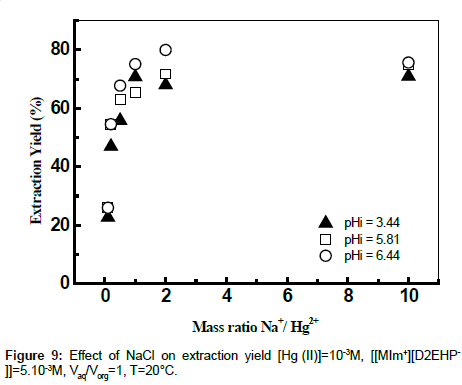
Effect of ionic strength
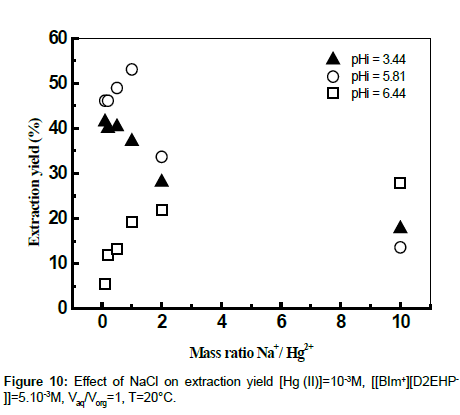
The influence of the ionic strength on the extraction yields of Hg (II) using ionic liquid was studied by adding sodium chloride and sodium acetate to the aqueous phase. The effects of some salts at various mass ratios Na+/Cd2+ on the extraction of Hg (II) were evaluated. The results showed that the addition of amount of the chloride sodium to the aqueous phase has an antagonistic effect on the yield of extraction at different values of initial pH (Figures 9 and 10). The extraction of mercury enters in competition with the sodium. The extractant goes on extracting the Na+ instead of Hg (II) [39].
The sodium complexes formed in organic phase, were ([MIm+] [D2EHP-]NaCl) and ([BIm+][D2EHP-]NaCl) respectively, and the reactions mechanism may be given by the following equations:
NaClaq + ([MIm+][D2EHP-])org ↔ ([MIm+][D2EHP-] NaCl)org (6)
NaClaq + ([BIm+][D2EHP-])org ↔ ([BIm+][D2EHP-] NaCl)org (7)
The results found by calculation program using CHEAQS V. L20.1 are summarized in Table 1. From this table it can be seen that the decrease of the extraction yield of Hg (II) is related with the decrease gradually of percentage of HgCl2aq, HgClOHaq species from 65.15 to 40.31% and from 31.31 to 0,1%, respectively with the addition of NaCl in the field of mass ratio between 0 and 10. On the other hand, the percentage of HgCl3- and HgCl4 2- species continues to increase. These observations suggest that the metal complexes of mercury (HgCl3 - and HgCl42-) formed in the aqueous phase, probably plays a role in masking the extraction of mercury (II) by the ionic liquid.
| Mass ratio Na+/Hg2+ | Extraction yield (%) pHi=5.81 |
Speciation of Hg (II) (%) | |||||
|---|---|---|---|---|---|---|---|
| [MIm+][D2EHP-] | [BIm+][D2EHP-] | HgCl2aq | HgClOHaq | Hg(OH)2aq | HgCl3- | HgCl42- | |
| 0 | 93.70 | 65.47 | 65.15 | 31.31 | 3.26 | 0.24 | 0 |
| 0.1 | 25.97 | 46.14 | 83.51 | 15.03 | 0.59 | 0.85 | 0 |
| 0.2 | 54.56 | 46.14 | 91.85 | 4.04 | 0.04 | 3.99 | 0.08 |
| 0.5 | 62.92 | 48.98 | 89.88 | 2.05 | 0.01 | 7.74 | 0.32 |
| 1 | 65.50 | 53.09 | 83.38 | 0.98 | 0 | 14.36 | 1.28 |
| 2 | 71.76 | 33.68 | 65.20 | 0.33 | 0 | 27.68 | 6.78 |
| 10 | 75.00 | 13.58 | 40.31 | 0.1 | 0 | 37.38 | 22.2 |
Table 1: Nature and percentages of extracted species at different concentration of NaCl.
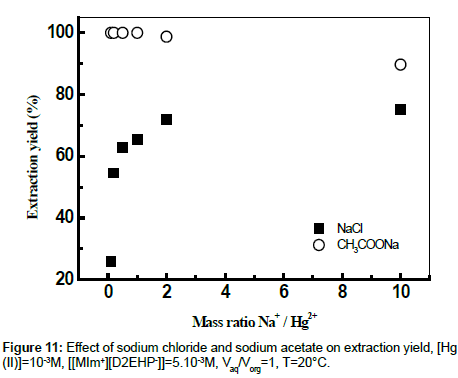
The Figure 11 shows a comparative study on the extraction behavior of mercury (II) with [MIm+][D2EHP-] from different salt medium using (sodium chloride and sodium acetate) at the same initial pH of aqueous solution. From Figure 11 it can be seen that the addition of sodium acetate in aqueous solution of Hg (II) increases the extraction yield to 100% for mNa/mHg = 0.1 to 1, Whereas the decrease from 98.73% to 89.70% is explained by the fact that the cations of salts enters in competition with Hg2+ during the extraction with [MIm+][DEHP-].
The experimental data of the extraction yield according to the ionic strength are presented in Table 2.
| Mass ratio Na+/Hg2+ | Extraction yield (%) pHi=5.81 |
Speciation of Hg (II) (%) | ||||
|---|---|---|---|---|---|---|
| HgCl2aq | HgClOHaq | Hg(OH)2aq | Hg(CH3COO)3- | Hg(CH3COO)42- | ||
| 0 | 93.70 | 65.15 | 31.31 | 3.26 | 0 | 0 |
| 0.1 | 100 | 64.79 | 30.77 | 3.17 | 0.27 | 0.71 |
| 0.2 | 100 | 53.57 | 13.09 | 0.69 | 2.75 | 29.46 |
| 0.5 | 100 | 26.72 | 3.72 | 0.11 | 3.03 | 66.04 |
| 1 | 100 | 2.51 | 0.26 | 0.01 | 1.80 | 95.36 |
| 2 | 98.73 | 0.04 | 0 | 0 | 0.59 | 99.37 |
| 10 | 89.70 | 0 | 0 | 0 | 0.26 | 99.74 |
Table 2: Nature and percentages of extracted species of Hg (II) with [MIm+][D2EHP-] at different concentration of CH3COONa.
It can be seen that the increase of the extraction yield is related with the decrease of percentage of HgCl2aq, HgClOHaq and Hg(OH)2aq species and the increase of Hg(CH3COO)4 2- species.
Effect of temperature
The effect of temperature on the extraction of the Hg (II) using ionic liquids was examined at 293, 303 and 318 K respectively. The effect of temperature on the extraction of Mercury (II) ions was studied under optimum conditions. Different thermodynamic parameters were computed using Van’t Hoff equation in the form [40]:
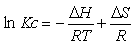 (8)
(8)
ΔG = - RT Ln Kc (9)
where ΔH, ΔS, ΔG, and T are the enthalpy, entropy, Gibbs free energy, and temperature in Kelvin, respectively.
The values of equilibrium ratio (Kc), were calculated at each temperature using the relationship.
Kc = Fe / (1 - Fe) (10)
where Fe is the fraction of Hg (II) extracted at equilibrium.
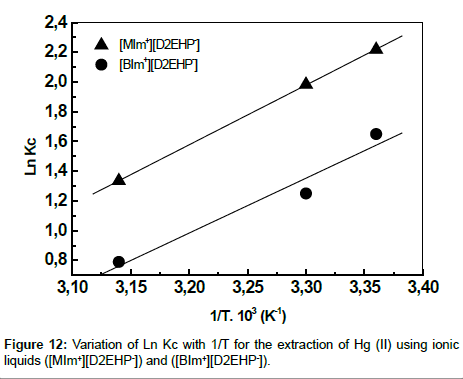
The plot of Ln Kc vs 1/T is a straight line as shown in Figure 12 with good correlation coefficient. The numerical values of ΔH, ΔS are computed from the slope. The negative value of Gibbs free energy as shown in Table 3 indicates the spontaneous nature of extraction, while negative value of ΔH reflects the exothermic extraction behavior. The negative value of ΔS indicates the complex stability.
| Thermodynamic parameters | Values ([MIm+][D2EHP-]) | Values ([BIm+][D2EHP-]) |
|---|---|---|
| ∆H (KJ/mol) | -30.6 | -33.1 |
| ∆S (J/mol K) | -89.65 | -93.5 |
| ∆G (KJ/mol) | -4.33 (T=293K) | -5.70 (T=293K) |
Table 3: Thermodynamic constants of the extraction of mercury ions.
Comparative study
In this section our results are compared with those found in the literature.
The comparison between the removal percentage in our work, which are of 94.0% for ([MIm+][D2EHP-]) and 65.0% for ([BIm+][D2EHP-]); with those found in the literature, we notice a strong increase of the quantities which we extracted (Table 4).
| Extractant | Yield (%) | [Ext] (mM) |
[Hg (II)] (mM) |
pHi | Time (min) | Volume ratio |
Reference |
|---|---|---|---|---|---|---|---|
| [BIm+][D2EHP-] | 65.0 | 5.0 | 1.0 | 5.81 | 15 | 1 | This work |
| [MIm+][D2EHP-] | 94.0 | 5.0 | 1.0 | 5.81 | 30 | 1 | This work |
| 4-methyl-N-8-quinolinyl-benzene-sulphonamide | 95.0 | 5.0 | 0.1 | 1.0 | 40 | 1 | [41] |
| LIX 34 | 75.0 | 10.0 | 0.02 | 1.1 | 40 | 1 | [41] |
| [C4MIm+][PF6-] | 70.0 | - | 5.0 | 4.68 | 240 | 1 | [35] |
| [C6MIm+][PF6-] | 62.0 | - | 5.0 | 4.68 | 240 | 1 | [35] |
| Aliquat336 | 99.9 | 7.0 | 2.0 | 2.0 | 20 | 1 | [42] |
| N-octylpyridinium tetrafluoroborate and N-octylpyridinium trifluoromethylsulfonate | 81% | 10.0 | 0.1 | 5.0 | 120 | 1 | [43] |
Table 4: Removal percentage of used extractant.
Conclusion
The extraction equilibrium was reached after 30 min. This novel extraction system can be employed for separation and preconcentration of metal ions. This work showed that ionic liquid [BIm+][D2EHP-] has not a stronger extracting power for Hg(II) than [MIm+][D2EHP-]. This fact is related to the more hydrophobic character due at longer alkyl chain on the imidazolinic ring. It is noticeable that the ionic liquid is better when the alkyl chain is a butyl or even longer to reinforce the hydrophobicity.
The extraction yield increased with ionic liquid concentration. The stoichiometry between the metal and the extractant is 5/1 and 3/2 for ([MIm+][D2EHP-]) and ([BIm+][D2EHP-]) respectively. The extraction percentage increases with an increase of initial pH of the aqueous phase and it is better to extract Hg(II) in the pH range of 4.1 to 5.81, or HgCl2aq, HgClOHaq and Hg(OH)2aq species exist with larger percentages.
Total Hg(II) removal (100%) was achieved by adding sodium acetate in Na+/Hg2+ masse ratio ranging from 0.1 to 2. However, the addition of NaCl induced an antagonistic effect on the yield of extraction at different initial pH. The extraction process turned out to be exothermic, and the negative value of Gibbs free energy (ΔG) indicates the spontaneous nature of the Hg extraction. Among the two ionic liquids studied in the present work, [MIm+][D2EHP-] seems to be the most interesting candidate for the extraction of Hg(II) from aqueous media.
References
- OJEC (2008) Directive 2008/105/EC of the European Parliament and of the Council of the European Union. 348: 84-97.
- Clifton JC 2nd (2007) Mercury exposure and public health. Pediatr Clin North Am 54: 237-269.
- Clarkson TW, Magos L (2006) The toxicology of mercury and its chemical compounds. Crit Rev Toxicol 36: 609-662.
- Hylander LD, Goodsite ME (2006) Environmental costs of mercury pollution. Sci Total Environ 368: 352-370.
- Oehmen A, Fradinho J, Serra S, Carvalho G, Capelo JL, et al. (2009) The effect of carbon source on the biological reduction of ionic mercury. J Hazard Mater 165: 1040-1048.
- Matlock MM, Howerton BS, Atwood DA (2001) Irreversible precipitation of mercury and lead. J Hazard Mater 84: 73-82.
- Barron-Zambrano J, Laborie S, Viers P, Rakib M, Durand G (2004) Mercury removal and recovery from aqueous solutions by coupled complexation-ultrafiltration and electrolysis. J Membr Sci 229: 179-186.
- Anirudhan TS, Divya L, Ramachandran M (2008) Mercury(II) removal from aqueous solutions and wastewaters using a novel cation exchanger derived from coconut coir pith and its recovery. J Hazard Mater 157: 620-627.
- Li Z, Wei Q, Yuan R, Zhou X, Liu H, et al. (2007) A new room temperature ionic liquid 1-butyl-3-trimethylsilylimidazolium hexafluorophosphate as a solvent for extraction and preconcentration of mercury with determination by cold vapor atomic absorption spectrometry. Talanta 71: 68-72.
- Terashima Y, Ozaki H, Sekine M (1986) Removal of dissolved heavy metals by chemical coagulation, magnetic seeding and high gradient magnetic filtration. Water Res 20: 537-545.
- Basha S, Murthy ZVP, Jha B (2009) Sorption of Hg(II) onto Carica papaya: experimental studies and design of batch sorber. Chem Eng J 147: 226-234.
- Rae IB, Gibb SW, Lu S (2009) Biosorption of Hg from aqueous solutions by crab carapace. J Hazard Mater 164: 1601-1604.
- Prashant DZ, Dattatray MD (2007) Retrieval of Mercury from Wastewater as Stable Mercury Ferrite. Water Qual Res J Canada 42: 311-318.
- Rogers RD, Seddon KS (2002) Ionic Liquids Industrial Applications for Green Chemistry. ACS Symposium series, ACS 818.
- Earle MJ, Seddon KR (2000) Ionic Liquids, Green Solvents for the Future. Pure Appl Chem 72: 1391-1398.
- Noda A, Hayamizu K, Watanabe M (2001) Pulsed-Gradient Spin-Echo 1H and 19F NMR ionic diffusion coefficient, viscosity, and ionic conductivity of non-chloroaluminate room-temperature ionic liquids. J Phys Chem B 105: 4603-4610.
- Tokuda H, Hayamizu K, Ishii K, Susan MA, Watanabe M (2005) Physicochemical properties and structures of room temperature ionic liquids. 2. Variation of alkyl chain length in imidazolium cation. J Phys Chem B 109: 6103-6110.
- Anderson JL, Ding J, Welton T, Armstrong DW (2002) Characterizing ionic liquids on the basis of multiple solvation interactions. J Am Chem Soc 124: 14247-14254.
- Duchet L, Legeay JC, Carrié D, Paquin L, Vanden Eynde JJ, et al. (2010) Synthesis of 3,5-disubstituted ,2,4-oxadiazoles using ionic liquid-phase organic synthesis (IoLiPOS) methodology. Tetrahedron 66: 986-994.
- Zanoni MV, Rogers EI, Hardacre C, Compton RG (2010) The electrochemical reduction of the purines guanine and adenine at platinum electrodes in several room temperature ionic liquids. Anal Chim Acta 659: 115-121.
- Fontanals N, Ronka S, Borrull F, Trochimczuk AW, Marcé RM (2009) Supported imidazolium ionic liquid phases: a new material for solid-phase extraction. Talanta 80: 250-256.
- Seddon KR (1997) Ionic liquids for clean technology. J Chem Technol Biotechnol 68: 351-356.
- Kubisa P (2009) Ionic liquids as solvents for polymerization processes-Progress and challenges. Prog Polym Sci 34: 1333-1347.
- Regel-Rosocka M (2009) Extractive removal of zinc(II) from chloride liquors with phosphonium ionic liquids/toluene mixtures as novel extractants. Sep Purif Technol 66: 19-24.
- RIVM (2004) Program for calculating chemical equilibria in aquatic systems.
- Rout A, Kotlarska J, Dehaen W, Binnemans K (2013) Liquid-liquid extraction of neodymium(III) by dialkylphosphate ionic liquids from acidic medium: the importance of the ionic liquid cation. Phys Chem Chem Phys 15: 16533-16541.
- Ann EV, Swatloski RP, Reichert WM, Sheff MRS, Wierzbicki A, et al. (2001) Task-specific ionic liquids for the extraction of metal ions from aqueous solutions. Chem Comm 135-136.
- Miralles N, Sastre AM, Aguilar M, Cox M (1992) Solvent extraction of zinc (II) by organophosphorus acids compounds from perchlorate solutions. Solvent Extr Ion Exc 10: 51-68.
- Hashem EY (2002) Spectrophotometric studies on the simultaneous determination of cadmium and mercury with 4-(2-pyridylazo)-resorcinol. Spectrochim Acta A Mol Biomol Spectrosc 58: 1401-1410.
- Didi MA, Elias A, Villemin D (2002) Effect of chain length of alkane-1-hydroxy-,1’-methyldiphosphonics acids on the Iron(III) liquid-liquid extraction. Solvent Extr Ion Exc 30: 407-415.
- Leo A, Hansch C, Elkins D (1971) Partition Coefficients and Their Uses. Chem Rev 7: 525-616.
- Nechab B, Villemin D (2000) Use of a New Hydrophilic Phosphine: DPPPA. Rapid and Efficient Heck Reaction in Aqueous Medium Under Microwave Irradiation. J Chem Res 3: 429-431.
- Viswanadhan VN, Ghose AK, Revankar GN, Robins RK (1989) Atomic Physicochemical Parameters for the Three Dimensional Structure Directed Quantitative Structure Relationships 4. Additional Parameters for Hydrophobic and Dispersive Interactions. J Chem Inf Comput Sc 29: 163-172.
- Germani R, Mancini MV, Savelli G, Spreti N (2007) Mercury extraction by ionic liquids: temperature and alkyl chain length effect. Tetrahedron Letters 48: 1767-1769.
- Bozkurt SS, Ocakoglu K, Merdivan M (2012) Separation and preconcentration of mercury in water samples by ionic liquid supported cloud point extraction and fluorimetric determination. Microchim Acta 177: 47-52.
- Puigdomenech I HYDRA (Hydrochemical Equilibrium- Constant Database) and MEDUSA (Make Equilibrium Diagrams Using Sophisticated Algorithms) Programs. Royal Institute of Technology, Sweden.
- Visser AE, Swatloski RP, Reichert WM, Mayton R, Sheff S, et al. (2002) Task-Specific Ionic Liquids Incorporating Novel Cations for the Coordination and Extraction of Hg2+ and Cd2+: Synthesis, Characterization, and Extraction Studies. Environ Sci Technol 36: 2523- 2529.
- Belkhouche N, Didi MA, Villemin D (2005) Separation of nickel and copper by solvent extraction using Di-2 ethylhexylphosphoric acid-based synergistic mixture. Solvent Extr Ion Exc 23: 677-693.
- Kadous A, Didi MA, Villemin D (2010) A new sorbent for uranium extraction: ethylenediamino tris(methylenephosphonic) acid grafted on polystyrene resin. J Radioanal Nucl Chem 284: 431-438.
- Huebra M, Elizalde MP, Almela A (2003) Hg (II) extraction by LIX 34. Mercury removal from sludge. Hydrometallurgy 68: 33-42.
- de Mendonça FF, Borges MM (2007) Liquid-liquid extraction of mercury (II) from hydrochloric acid solutions by Aliquat 336. Hydrometallurgy 87: 83-90.
- Li Z, Xia S, Wang J, Bian C, Tong J (2016) Determination of trace mercury in water based on N-octylpyridinium ionic liquids preconcentration and stripping voltammetry. J Hazard Mater 301: 206-213.
Citation: Guezzen B, Didi MA (2016) Removal and Analysis of Mercury (II) From Aqueous Solution by Ionic Liquids. J Anal Bioanal Tech 7:317. DOI: 10.4172/2155-9872.1000317
Copyright: © 2016 Guezzen B, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Open Access Journals
Article Tools
Article Usage
- Total views: 14434
- [From(publication date): 6-2016 - Jul 05, 2025]
- Breakdown by view type
- HTML page views: 13326
- PDF downloads: 1108