Remediation of Cadmium Toxicity on Alfalfa (Medicago sativa L.) Using Biochar as a Bioadsorbent, Rhizobium meliloti and Arbuscular Mycorrhizal Fungi as Biofertilizers
Received: 24-Jan-2018 / Accepted Date: 29-Jan-2018 / Published Date: 30-Jan-2018 DOI: 10.4172/2155-6199.1000429
Abstract
Contamination of agricultural soil is becoming an increasingly serious problem because of long-term exposure to pollutants in irrigation water, sludge, fertilizers, and pesticides. Cadmium (Cd) is one of the most dangerous and potentially toxic metals. Pollution by Cd is especially significant as contaminated soils are used for agriculture; this metal is easily absorbed from soils into crops, inhibiting plant growth and nutrient uptake. So, the present study aimed to elucidate the effect of accumulation Cd over the permissible limits on growth of higher plants, e.g., alfalfa and soil microorganisms, e.g., rhizobium bacteria and arbuscular mycorrhiza fungi. In this study we also attempted to find an optimum solution for the problem of soil pollution with Cd through the application of biochar (BC) as bioadsorbent, rhizobia, (Rh) and arbuscular mycorrhizal fungi (AMF) as biofertilizers. CdCl2 was applied in a pot experiment in presence and absence of BC, AMF and Rh. Cd negatively affected alfalfa growth and yield, content of essential minerals such as N, P, K, Ca and Mg in leaves and roots, activity of nodules nitrogenase and rhizosphere dehydrogenase. AMF colonization % and the total microbial count decreased under Cd stress. Application of BC, AMF and Rh individually or in combination improved alfalfa growth, leaf and root content of essential minerals, fungal population and the microbial count. On the other side, these treatments reduced Cd uptake and translocation from roots to seeds. Solo application of BC contributed in Cd immobilization through the metal adsorption and increasing soil alkalinity. Application of BC together with AMF and Rh significantly promoted alfalfa growth and yield, the leaf and root content of mineral ions, the fungal populations and the microbial count compared to control and Cd stressed plants.
Keywords: Mycorrhizal colonization (%); Nitrogenase; Nutrient elements; Rhizosphere dehydrogenase; Total microbial count
Introduction
Environmental pollution by toxic heavy metals (HMs) has become a common concern in both developing and developed countries [1]. Cadmium (Cd) is an extremely toxic HM to entire living system. It enters into the environment, primarily by incinerators, power stations, metal working industries and automobile exhausts [2]. Also, it is among the most common contaminants in farmland soils as a result of industrial activity, over-fertilization, wastewater irrigation, and improper waste disposal [3]. It readily accumulates in soil because of unlike organic contaminants, it cannot be biodegraded. It is taken up by plants growing in contaminated soil and enters into human and animals via the food chain [4]. Its accumulation in plants inhibits photosynthesis, respiration; reduce water, nutrient uptake and the activities of key metabolic enzymes thereby inhibiting the plant growth and development [5]. It is an urgent requirement to either clean Cd from contaminated soil or control its uptake by crops, as these can be consumed by animals and humans. Various methods have been used to treat HMs pollution, which is chemically treatment such as precipitation, adsorption, nanofiltration, and reverse osmosis, but these methods are expensive, require high energy, and not environmental friendly because it cannot remove all the HMs from the environment [6]. Therefore, it should be considered a method that is eco-friendly, inexpensive, and efficient to resolve the problem of HMs pollution. A number of technological developments aimed at achieving this, including use of rhizosphere microbes such as arbuscular mycorrhizal fungi (AMF) and Rhizobium meliloti (Rh), also application of biochar to the soil. Biochar (BC) is a carbon-rich byproduct of slow pyrolysis of waste organic materials such as manures and agricultural residues [7]. Amending soil with BC is receiving increased attention because of this strategy's ability to improve soil quality and crop productivity [8]. In HMs contaminated soils, BC can adsorb and immobilize metal ions due to its porous structure, large surface area, high surface charge density and pH values [9]. The role and effectiveness of BC in soil remediation may depend on plant type, soil texture, BC production temperature, and application rate [10]. Numerous studies have reported that BC might be effective in carbon sequestration in the soil [11]. Tahir et al. [12] reported that BC application in the soil produced plants with lower Cd contents in plant compared with the plants grown in soil without BC application, also increased the growth, yield, and photosynthesis of wheat (Triticum aestivum L.) compared to the control.
Among soil microorganisms, AMF that provides plant roots with more intimate access of nutrients such as nitrogen (N), potassium (K), magnesium (Mg), cupper (Cu) and zink (Zn) [13], water, and are well known to be able to improve plant growth, reduce HMs toxicity, and decrease plant uptake and transport of HMs [14]. AMF plays an important role in metals phytostabilization by precipitating polyphosphate complexes, retaining HMs in roots and fungal structures such as extra radical mycelium, and improving plant adaptation to environmental stresses in general [15]. AMF may also help to drive the specialization of HMs and reduce metal phytoavailability by changing the microbial community structure, physical, and chemical properties of rhizosphere soil [16]. The extent of these organisms’ effects on remediation can depend on AMF species, plant tolerance to contaminants, HM concentrations [17]. Rhizobacteria is another important helpful microbial community for plants to remediate HMs in contaminated soils [18]. Rhizobacteria live in close association with plant roots and some of them, such as Rhizobium sp. are able to establish a well-known endosymbiotic relationship with roots of leguminous plants. The outcome of this symbiosis is the conversion of diatomic N into N forms that can be used by plants, since N is frequently a limiting factor of plant growth [19]. The association between nitrogen fixing bacteria (NFB) and AMF could be very effective for enhancing N-fixation under stress conditions [20]. The effect of dual inoculation of AMF and bacteria remarkably improves the HMs tolerance of plant, as AMF supply high P for nodulation and N-fixation [21]. Vivas et al. [22] showed that the dual inoculation of mycorrhizal fungus and bacterium A substantially reduced Cd concentration in shoot of Trifolium regardless of the Cd level in soil, also the microbial treatment increased more shoot and root biomass.
BC and AMF in combination affect nutrient cycling and may therefore also influence the specialization of HMs and reduce metal phytoavailability by changing soil microbial activity and community structure [23]. Liu et al. [24] found that AM inoculation and biochar applications decreased Cd mobilization through their pronounced effect on soil alkalinization, promoted plant growth and reduced Cd uptake. Combined application of Rh and BC assumed to cause a significant improvement in plant growth as mentioned by Muhammad et al. [25]. Alfalfa was chosen for this study because it is the oldest and the most important forage crop that grown worldwide. It is one of the most important crops used for livestock feeding in Egypt, but there is a gap between the demand and the consumption of green forages. All authors showed the combination between Rh and AMF, BC and AMF, BC and Rh however, little information is available regarding the effect of combination between the three amendments BC, AMF and Rh on Cd immobilization and uptake by plants. Here, we predicted that combination between AMF and Rh inoculants with BC application would reduce Cd phytoavailability, stimulate soil microbial activity and improve alfalfa growth under elevated soil Cd concentrations, and have a synergistic effect by lowering Cd uptake by alfalfa roots. So, our ultimate aim was to (1) emphasize the efficiency of BC, AMF and Rh treatments to alleviate the toxic effects of Cd (2) to develop a friendly phytoremediation approach in the environment, in order to properly manage Cd-contaminated soil (3) to quantify the effect of BC, AMF and Rh treatments on Cd uptake by alfalfa (4) to highlight the effect of these amendments on alfalfa growth and yield under Cd stress.
Materials And Methods
Plant material
Medicago sativa L. seeds were obtained from Legumes Crops Department; Field Crops Research Institute; Agricultural Research Centre, Giza, Egypt.
Isolation of AMF and Rh from metal polluted areas: Samples of wild plants with their roots and rhizosphere soil region were collected from different localities polluted with HMs (such as Cd, Pb and Cu) in El-Menoufeya governorate, Egypt. Rhizosphere soil was differentiated from the bulk soil according to the method of Buddrus et al. [26]. Isolation, characterization of the mycorrhizal spores and Rhizobium meloliti from roots of a metal polluted alfalfa were detected according to Daft et al. and Date et al. [27,28].
Mycorrhizal inoculums: The rhizosphere of soil mass was gently removed from the root system of each plant (250 g), suspended in 1 liter tap water, then sieved using wet-sieving and decanting technique [29]. Seven sieves (400, 270, 250, 200, 150, 80 and 75 μm-mesh size) were used for extraction of mycorrhiza spores. The 250, 150 and 75 μm fraction were transferred into a glass bottle and diluted with water to give (between 20 to 50 spores/ml). The number of spores was estimated by spreading certain volume of mycorrhiza spore suspension onto a gridded filter paper or Petri-dish which was divided into squares from its base and then counted by using a binuclear microscope (30-50 x) according to Daft et al. [27] The morphological characteristics of the extracted mycorrhiza spores were determined according to the key that prepared by Trappe [30]. The AMF spores were identified as Glomus, Gigaspora, Acaulospora and Enterophospora sp.
The bacterial isolation was carried out following the conventional procedure: 1 g of homogeneised rhizosphere soil was suspended in 100 ml of sterile water (dilution 102) and 1 ml of this suspension was serially diluted to reach dilutions 104 to 107. A drop of this suspension was transferred to an agar plate with a flamed loop. The drop of this bacterial suspension was streaked into the agar plate in a way that progressively dilutes the suspension. The used growth medium was yeast extract mannitol agar medium [31], 0.5 g MgSO4. 7H2O, 0.2 g NaCl, 0.1 g Mannitol, 10 g yeast extract, were dissolved in 1000 ml distilled water at pH 7. The plates were incubated in an inverted position at about 28°C and checked for growth typical of rhizobia along the streak lines. The plates were left in the incubator until the colonies developed; that usually took about 3-5 days. Well isolated single colonies were picked and re streaked into fresh plates to obtain pure culture of the pre-sumptive strain of rhizobia. It is possible that more than one "typical" colony type may appear on a plate streaked from a single nodule and each of these types were taken onto pure culture and held for characterization [28]. The tested isolates were kindly identified at the Rhizobia Unit, Microbiology Department, Soils, Water and Environment Institute (SWERI), Agricultural Research Centre (ARC), Giza, Egypt, according to the morphological, characteristics and microscopically examination of the nodules.
A greenhouse experiment was conducted in a botanical garden of the Agriculture Research Center farm, Giza, Egypt during the winter season (November, 2015).
Soil preparation: soil was collected from the surface soil (0-15 cm) layer of an agricultural field at the Agricultural Research Centre (ARC), Giza, Egypt. The collected soil was air-dried at room temperature, and then sieved through 2 mm sieve, discernible plant litter; large debris and biological remnants were removed during sieving. Then the soil was sterilized in an oven for at least 30 minutes, or when soil temp reaches 82°C. 5 Kg of loamy-sand soil were filled in wide mouthed glazed pots 7 Kg capacity. The physical and chemical properties of the soil were determined according to Cottenie et al. [32].
Biochar (BC) that used in the pot experiment was produced from rice husk stems. Rice husk stems were cut into pieces (10-20 cm) and after drying, the biomass was pyrolyzed at 300°C for 2 hrs, followed by quenching and subsequent drying in an oven at 105°C. BC was then crushed in a 24-blade variable-speed rotator mill according to Mohammad et al. [33] and mixed thoroughly in soil before filling the pots at a rate of 20 Kg-1 7 days before sowing [34]. BC was analyzed for inorganic elements and HMs content.
Fertilization: during soil preparation, P was added as super phosphate at a rate of 200 Kg/acre. N was added as urea at a rate of 15 Kg/acre at 15, 30 and 45 days from sowing. K was added as potassium sulphate at a rate of 50 Kg/acre in three equal doses as nitrogen. After 60 days from sowing, N and K were applied at the same doses.
Seed coating with AMF and/or Rh: before planting, alfalfa seeds were surface sterilized with 2.5% sodium hypochlorite solution for one minute, washed several times with distilled water, then wetted with 40% Arabic gum solution as an adhesive agent and coated with 1 g of rhizobia and/or mycorrhiza spores [34]. Seeds were allowed to air dry in the shade for 30 min and sown immediately.
Layout of pot experiment: The pots were divided into eight groups. Each group consisted of six replicates.
• Served as Control+full NPK (fertilizer).
• Inoculated with Rhizobia (Rh)+(1/2 N of recommended dose+full PK).
• Inoculated with (Arbuscular Mycorrhizal Fungi, (AMF)+(1/3 P of recommended dose+full NK).
• Treated with BC+(full NPK)
• Rh+AMF+(1/2 N+1/3 P+K)
• BC+Rh+(1/2 N+PK)
• BC+AMF+(1/3 P+NK)
• BC+AMF+Rh+(1/3 P+1/2 N+K).
Approximately equal number of alfalfa seeds was sown in each pot (50 seeds/ pot). Pots (7 Kg capacity) were regularly irrigated. The 1st three replicates from each group were irrigated with dechlorized tap water, while the other three replicates were irrigated with 1 mM CdCl2 solution (it based on the Cd concentration in some contaminated soils of Egypt), the irrigation was intermittent. After 60 days of sowing, some plants were collected for measuring: fresh and dry weight of shoots and roots; mineral content in leaves and roots; Cd content in roots, stem, and leaves; nitrogenase and dehydrogenase activity; total microbial count and mycorrhizal colonization (%). After 6 months of sowing, Cd content in seeds, translocation of Cd from roots to seeds and fresh and dry weight of the produced seeds, as well as their total content of carbohydrates and proteins were measured.
Microbial determination
For microbial determination, soil samples were kept at 4°C in plastic bags to stabilize the microbiological activity distributed during soil sampling and handling. Plate count technique was applied using potato dextrose agar medium (PDA) and nutrient agar medium [31] to enumerate total saprophytic and pathogenic fungi and bacteria count in respective order. Total actinomycetes were estimated by the standard procedure of Rolf and Bakken [35].
The percentage (%) of native AM fungi colonization in plant root tissues was determined as described by Phillips and Hayman [36]. Root pieces that contained even a single vesicle or arbuscules along with hyphae were considered infected:
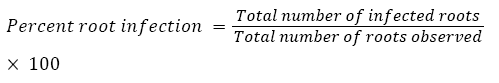
Enzymes activities
Dehydrogenase activity (μgnTPF/ gm soil) was estimated according to the methods of Skujins [37].
Nitrogenase activity in root nodules was assayed by the acetylene reduction according to Somasegaran [38].
Chemical analyses
Alfalfa seeds were extracted in 70% ethanol and diluted to volume for estimation of the total soluble proteins and carbohydrates content.
Total soluble proteins content was estimated by using Folin-phenol reagent in alkaline medium containing copper sulphate [39].
Total carbohydrates content was measured by the method described by Duboise et al. [40].
Mineral ions content was measured at Soil, Water and Environment Research Institute (S.W.E.R.I), Agricultural Research Centre, Egypt.
Nitrogen was determined using the modified Micro-Kjeldahl method [41].
Phosphorus was determined by using vanadate molybdate method [42].
Potassium was measured using flame photometer according to Megroth and Cegarra [43].
Calcium and magnesium were estimated using Inductively Coupled Spectrometry Plasma (ICP) Model Ultima2-JobinYvon.
The translocation factor (TF) for Cd within a plant was determined to show Cd translocation properties from roots to seeds [44]. It was expressed as:
TF=metal (in seeds)/metal (in roots).
Statistical analysis was carried out according to the method of complete randomized blocks design (CCRBSE. Bas) using two-way analysis of variance ANOVA followed by Duncan's Multiple Comparison Test using IBM Statistical Product and Service Solutions, SPSS Statistics for Windows, Version 21. P˂0.05 and P˂0.01 were denoted as being statistically significant for the means compared, using least significant difference (LSD at 5 and 1%) according to Snedecor and Cochran [45].
Results And Discussion
BC analysis (Table 1) showed little content of HMs in absence of Cd. Physical and chemical properties of the soil (Table 2) indicated a sandy loam (SL) structure with an exchangeable Ca, K, Mg and Na.
| pH | 8.7 |
| Temp of pyrolysis | 330-350 |
| EC(Ms/cm) | 1392 |
| TDS (ppm) | 555 |
| Si (mg/Kg) | 220 |
| Ca | 200 |
| K | 175 |
| Mg | 180 |
| Water % | 4 |
| Ash % | 51 |
| Organic matter (%) | 49 |
| Organic carbon (%) | 42.7 |
| H (mg) | 2.38 |
| N | 1 |
| S | 0.2 |
| O | 2.34 |
| Volatile Matter (%) | 2 |
| Fe (mg/L) | 8.7 |
| Al | 0.97 |
| Cu | 0.07 |
| Pb | 0 |
| Zn | 0.5 |
| Ni | 0.01 |
| Cd | 0 |
| Cr | 0.01 |
| Na | 7.3 |
| Mg | 5.12 |
Table 1: Physico-chemical properties of Biochar.
| Physical Properties | Soil texture | pH | EC* Ms/cm | SP*(%) | Cations and anions (mg/Kg) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sand (%) | Silt (%) | Clay (%) | Coarse sand (%) | Na+ | K+ | Ca++ | Mg++ | Cl- | HCO- | CO3- | SO4-- | ||||
| 37.9 | 17.2 | 9.4 | 35.5 | Sandy loams. | 8 | 3.2 | 28 | 18.5 | 0.3 | 7.7 | 3.5 | 27.7 | 0.8 | -- | 3.5 |
Table 2: Chemical and physical characteristics of the sandy loam (SL) soil sample from the agricultural field (at the Agricultural Research Centre (ARC)) used for cultivation of alfalfa plant. EC=Electrolyte conductivity, SP=Saturation percent.
Concerning to growth of alfalfa, the results indicated a deleterious impact of 1 mM Cd on fresh and dry weight of shoots and roots (Table 3). This reduction might be due to Cd uptake primarily through roots [46]. One of the vital causes of plant growth reduction under Cd-stress is the alteration of photosynthetic efficiency and consequently, a decrease in CO2 assimilation and photosynthates accumulation [47]. One of the other most important reasons behind the harmful yield of alfalfa on alfalfa growth is the diminishing of essential minerals. The results indicated that leaves and roots content of N, P, K, Ca and Mg decreased by the effect of 1 mM CdCl2 (Table 4). This might be due to competition between Cd and other minerals for binding sites or transporters; this led to mineral deficiency or imbalance and depression of alfalfa growth.
| Treatment | Cd (1 mM) | Shoot f.wt. (g) | Shoot d.wt. (g) | Root f.wt. (g) | Root d.wt. (g) |
|---|---|---|---|---|---|
| Control | -- | 16.81 | 6.75 | 5.09 | 0.73 |
| 0 | Cd | 12.3 | 3.39 | 3.93 | 0.28 |
| Rh | -- | 16.9 | 6.9 | 5.22 | 0.82 |
| Cd | 16.95 | 7.2 | 5.68 | 1.42 | |
| AMF | -- | 23.92 | 7.33 | 5.6 | 0.95 |
| Cd | 28.29 | 8.34 | 6.01 | 1.63 | |
| Biochar | -- | 30.65 | 9.15 | 6.54 | 1.83 |
| Cd | 29.09 | 8.9 | 6.36 | 1.75 | |
| Rh+AMF | -- | 24.42 | 7.51 | 5.91 | 0.98 |
| Cd | 28.57 | 8.57 | 6.08 | 1.66 | |
| BC+Rh | -- | 31.32 | 9.38 | 8.3 | 1.87 |
| Cd | 30.01 | 9.01 | 7.43 | 1.84 | |
| BC+AMF | -- | 31.77 | 10.78 | 10.2 | 2.59 |
| Cd | 31.4 | 9.96 | 9.16 | 2.23 | |
| BC+AMF+Rh | -- | 32.66 | 11.21 | 11.85 | 2.62 |
| Cd | 32.06 | 10.9 | 10.67 | 2.41 | |
| L.S.D | 5% | 0.376 | 0.838 | 0.239 | 0.216 |
| L.S.D | 1% | 0.381 | 0.848 | 0.243 | 0.218 |
Table 3: Effect of Cd (1 mM) and the treatments with BC (20 g Kg-1 soil), Rh and AMF on fresh and dry weight (g) of alfalfa shoot and root/plant (60-d-old). Each value is a mean of three replicates.
| Treatments | Cd (1 mM) | N | P | K | Ca | Mg | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Roots | Leaves | Roots | Leaves | Roots | Leaves | Roots | Leaves | Roots | Leaves | ||
| Control | 0 | 2.53 | 3.65 | 0.12 | 0.162 | 1.9 | 2.05 | 2.16 | 2.34 | 0.31 | 0.42 |
| 0 | Cd | 1.22 | 1.47 | 0.1 | 0.135 | 1.01 | 1.15 | 0.18 | 0.38 | 0.27 | 0.36 |
| Rh | -- | 2.57 | 3.69 | 0.12 | 0.148 | 2.11 | 2.18 | 2.34 | 2.53 | 0.32 | 0.43 |
| Cd | 2.87 | 4 | 0.14 | 0.162 | 2.48 | 2.56 | 2.46 | 2.65 | 0.34 | 0.46 | |
| AMF | -- | 2.54 | 3.66 | 0.13 | 0.175 | 2.2 | 2.77 | 2.51 | 2.7 | 0.33 | 0.44 |
| Cd | 2.78 | 3.91 | 0.14 | 0.189 | 2.51 | 2.59 | 2.58 | 2.77 | 0.36 | 0.49 | |
| Biochar | -- | 2.83 | 3.96 | 0.2 | 0.27 | 2.62 | 2.71 | 2.64 | 2.84 | 0.37 | 0.5 |
| Cd | 2.79 | 3.92 | 0.19 | 0.256 | 2.54 | 2.63 | 2.74 | 2.94 | 0.36 | 0.49 | |
| Rh+AMF | -- | 2.74 | 3.87 | 0.18 | 0.243 | 2.56 | 2.65 | 2.56 | 2.75 | 0.34 | 0.46 |
| Cd | 2.9 | 4.04 | 0.2 | 0.27 | 2.57 | 2.66 | 2.73 | 2.93 | 0.36 | 0.49 | |
| BC+Rh | -- | 2.91 | 4.05 | 0.22 | 0.297 | 2.71 | 2.8 | 2.72 | 2.91 | 0.38 | 0.51 |
| Cd | 2.88 | 4.02 | 0.2 | 0.27 | 2.66 | 2.75 | 2.71 | 2.91 | 0.37 | 0.5 | |
| BC+AMF | -- | 2.96 | 4.09 | 0.23 | 0.311 | 2.76 | 2.85 | 2.77 | 2.97 | 0.4 | 0.54 |
| Cd | 2.92 | 4.06 | 0.21 | 0.284 | 2.73 | 2.83 | 2.75 | 2.95 | 0.38 | 0.51 | |
| BC+AMF+Rh | -- | 3.12 | 4.26 | 0.26 | 0.351 | 2.93 | 3.51 | 2.88 | 3.08 | 0.43 | 0.58 |
| Cd | 3.01 | 4.15 | 0.24 | 0.324 | 3.02 | 3.33 | 2.84 | 3.04 | 0.4 | 0.54 | |
Table 4: Effect of Cd (1 mM) and the treatment with BC (20 g Kg-1 soil), Rh and AMF on the mineral ions content (N, P, K, Ca and Mg) mg L-1 of air dried leaf and root tissue of alfalfa (60 day old from sowing).
In addition, the decrease of micronutrients content may be due to the interference of Cd with their moving pathway from roots to seeds. The reduction in K+ uptake in leaves and roots under Cd stress might be due to the antagonism between Cd2+ and K+ ions. Decreasing of Mg and N content under Cd stress had a negative effect on photosynthesis, consequently plant growth. When plants absorb high amount of Cd, the metal causes oxidative injury to the plasma membrane [48] and reduces N uptake, thus led to disturb in the nitrogen metabolism under Cd stress that affect plant growth.
With regard to the effect of Cd on soil microbe's activity in response to CdCl2, the results indicated that the total microbial count and mycorrhizal colonization % under Cd stress significantly decreased compared to control (Table 5). This might be due to Cd which had a negative effect on soil nutrient cycling and soil fertility, so it affects both the microbial metabolic processes and microbial count. Liu et al. [24] reported that Root AM colonization rates were obviously decreased by Cd stress. Giller et al. [49] reported that HMs pollution reduces microbial diversity in the soil because microorganisms that are sensitive to HMs toxicity will abruptly decline in abundance or even become extinct; changes in microbial community structure may seriously affect the ability of soil microbes to degrade organic matter which leads to decreased soil fertility. Nitrogenase activity in the nodules was negatively affected by Cd stress (Table 6). This result was supported by Wani et al. [50] who added that the sucrose synthase and malate level decreased under Cd stress, which results in the decrease of the activity of nitrogenase enzyme. The present study also indicated that the dehydrogenase activity was inhibited by 1 mM CdCl2, (Table 6). This deleterious effect on dehydrogenase may also be generated by binding of Cd to the cysteinyl and histidel groups of enzymatic proteins [51]. Rhizosphere dehydrogenase plays an important role in the biological oxidation of soil organic matter and can be considered a good measure of the change of microbial oxidative activity under environmental pollutions. Moreover, Cd could affect the enzyme activity through several ways including: altering the affinity of enzyme to its substrate, denaturing of the enzyme protein, and influencing the synthesis of enzyme [52]. Cd could alter enzyme activity by binding to its functional groups (sulfhydryl, carboxyl, imidazole, etc.) and/or by displacing other metal ions associated with it, resulting in the complex mechanisms of enzyme in response to Cd stress [53].
| Treatments | Cd (1 mM) | Total colony no./plate | Actino-Mycetes | Frequency (%) | Fungi | Frequency (%) | Bacteria | Frequency (%) | Colonization (%) |
|---|---|---|---|---|---|---|---|---|---|
| Control | 0 | 92 | 3 | 3.26 | 2 | 2.18 | 87 | 94.56 | 50 |
| 0 | Cd | 28 | 0 | 0 | 1 | 3.57 | 27 | 96.43 | 20 |
| Rh | -- | 94 | 2 | 2.13 | 3 | 3.19 | 89 | 94.68 | 65 |
| Cd | 98 | - | 0 | - | 0 | 98 | 100 | 70 | |
| AMF | -- | 95 | 3 | 3.16 | 57 | 60 | 35 | 36.84 | 68 |
| Cd | 98 | - | 0 | 87 | 88.77 | 11 | 11.23 | 75 | |
| Biochar | -- | 104 | 4 | 3.85 | 56 | 53.84 | 44 | 42.31 | 80 |
| Cd | 100 | 3 | 3 | 4 | 4 | 93 | 93 | 84 | |
| Rh+AMF | -- | 99 | 1 | 1.01 | 3 | 3.03 | 95 | 95.96 | 75 |
| Cd | 104 | 1 | 0.96 | 2 | 1.92 | 101 | 97.12 | 84 | |
| BC+Rh | -- | 115 | 5 | 4.35 | 2 | 1.74 | 108 | 93.91 | 79 |
| Cd | 107 | 5 | 4.67 | 2 | 1.87 | 100 | 93.46 | 87 | |
| BC+AMF | -- | 121 | - | 0 | - | 0 | 121 | 100 | 89 |
| Cd | 118 | - | 0 | 3 | 2.54 | 115 | 97.46 | 90 | |
| BC+AMF+Rh | -- | 168 | 8 | 4.76 | 10 | 5.95 | 150 | 89..28 | 94 |
| Cd | 157 | - | 0 | 2 | 1.27 | 155 | 98.73 | 92 |
Table 5: Effect of Cd (1 mM) and the treatment with BC (20 g kg-1 soil), Rh and AMF on the total microbial count (CFU × 106/g d. soil) and colonization of myccorhiza (%) in alfalfa roots (60-d-old).
| Treatments | Cd (1 mM) | Nitrogenase | Dehydrogenase |
|---|---|---|---|
| Control | 0 | 0.13 | 5.44 |
| 0 | Cd | 0.1 | 1.6 |
| Rh | -- | 0.313 | 5.443 |
| Cd | 0.731 | 2.78 | |
| AMF | -- | 0.276 | 5.565 |
| Cd | 0.755 | 2.95 | |
| Biochar | -- | 1.1 | 6.11 |
| Cd | 0.91 | 4.28 | |
| Rh+AMF | -- | 0.831 | 5.73 |
| Cd | 0.851 | 2.99 | |
| BC+Rh | -- | 1.31 | 6.355 |
| Cd | 1.289 | 5.48 | |
| BC+AMF | -- | 1.47 | 6.756 |
| Cd | 1.35 | 5.966 | |
| BC+AMF+Rh | -- | 1.52 | 7.56 |
| Cd | 1.456 | 7.1 | |
| L.S.D | 5% | 0.081 | 0.046 |
| L.S.D | 1% | 0.028 | 0.095 |
Table 6: Effect of Cd (1 mM) and the treatment with BC (20 g kg-1 soil), Rh and AMF on the activity of nitrogenase (μ mole C2H2 g-1 nodule/plant) of alfalfa nodules and dehydrogenase enzymes (μg TPF g-1 dry soil/ day) in soil rhizosphere (60-d-old). Each value is a mean of three replicates.
The results showed the variation in accumulation of Cd in root, stem, leaves and seeds and its translocation from roots to seeds (Table 7). The highest content of Cd was in roots of alfalfa under Cd stress as it close to the source so accumulate more Cd. The roots accumulated higher amount of Cd compared to shoots as the plants increase their root biomass to cope the Cd stress [54]. This was in a harmony with Wang et al. [55] who showed that alfalfa had well capability to bioaccumulate Cd than Pb and Zn; hence the TF of Cd from alfalfa root to seeds was low and decreased with increasing Cd content in the soil. Uptake of Cd in roots mainly depends on root structure and root activities [56].
| Treatments | Cd (1 mM) | Roots | Stem | Leaves | Seed | TF Root to Seeds |
|---|---|---|---|---|---|---|
| Control | 0 | 42.8 | 22.7 | 16.5 | 1.2 | 0.028 |
| 0 | Cd | 169.48 | 72 | 27.86 | 18.88 | 0.111 |
| Rh | -- | 40.1 | 30 | 14.67 | 0.2 | 0.005 |
| Cd | 112 | 68 | 17.35 | 0.73 | 0.006 | |
| AMF | -- | 38 | 23.5 | 13.79 | 0.25 | 0.006 |
| Cd | 92.2 | 51.48 | 15.5 | 1.01 | 0.011 | |
| BC | -- | 26 | 15 | 11.37 | 0 | 0 |
| Cd | 46 | 21.6 | 12.72 | 0 | 0 | |
| Rh+AMF | -- | 33 | 22.76 | 13.07 | 0.17 | 0.005 |
| Cd | 84.2 | 43.8 | 14.26 | 0.97 | 0.011 | |
| BC+Rh | -- | 31 | 21 | 12.36 | 0 | 0 |
| Cd | 57 | 22.8 | 13.89 | 0 | 0 | |
| BC+AMF | -- | 28 | 18 | 11.16 | 0 | 0 |
| Cd | 37 | 23 | 12.08 | 0 | 0 | |
| BC+AMF+Rh | -- | 22 | 18 | 11 | 0 | 0 |
| Cd | 34 | 19.5 | 11.3 | 0 | 0 |
Table 7: Effect of Cd (1 mM) and the treatment with BC (20 g kg-1 soil), Rh and AMF on Cd content (mg g-1d. wt.) in alfalfa roots, stems, leaves (60 day old) and seeds (6 months from sowing). Each value is a mean of three replicates.
The results of the present study indicated an adverse effect of Cd on the alfalfa yield (seed fresh and dry weight) (Table 8). This might be attributed to Cd accumulation with long term and its translocation from root, stem, leaves to seeds. This conclusion was recorded by Rizwan et al. [57] who stated that Cd is toxic to plants and caused reduction in growth, yield and quality of plants. The dry matter and yield of many higher plants such as pea, wheat, rapeseed and maize have been reported to decrease under multiple HMs stresses [58]. Reduction in yield is a typical index of sensitivity of plants to various stresses, as it represents the cumulative effects of damaged or inhibited physiological functions. This result is in agreement with Agrawal and Mishra [59] who showed that the exposure of pea (Pisum sativum L) to Cd stress caused significant reduction in yield. After long-term of Cd treatment, the reduction of Ca accumulation during seed development was correlated with a decrease in seed fresh weight, suggesting the deleterious effect of Cd on seed development.
| Treatments | Cd (1 mM) | Fresh weight (g) | Dry weight (g) | Total carbohydrates | Total proteins |
|---|---|---|---|---|---|
| Control | 0.0 | 1.683 | 0.52 | 228.07 | 176.34 |
| 0.0 | Cd | 0.942 | 0.03 | 218 | 168.3 |
| Rh | -- | 2.267 | 0.69 | 224.9 | 180.4 |
| Cd | 2.306 | 0.712 | 226.3 | 182.6 | |
| AMF | -- | 2.356 | 0.73 | 307.4 | 171.34 |
| Cd | 2.53 | 0.781 | 319.5 | 173 | |
| BC | -- | 2.572 | 0.84 | 367.5 | 188.4 |
| Cd | 2.564 | 0.827 | 365.4 | 187.65 | |
| Rh+AMF | -- | 2.47 | 0.742 | 332.8 | 181.65 |
| Cd | 2.533 | 0.782 | 335.5 | 183.9 | |
| BC+Rh | -- | 2.605 | 0.855 | 377.8 | 190.25 |
| Cd | 2.597 | 0.849 | 377.5 | 189.6 | |
| BC+AMF | -- | 2.767 | 0.865 | 385.7 | 198.6 |
| Cd | 2.759 | 0.861 | 385.6 | 197.8 | |
| BC+AMF+Rh | -- | 2.84 | 0.877 | 390.4 | 205.25 |
| Cd | 2.836 | 0.875 | 390 | 203.5 | |
| L.S.D | 5% | 0.026 | 0.044 | 0.964 | 1.383 |
| L.S.D | 1% | 0.027 | 0.044 | 0.977 | 1.4 |
Table 8: Effect of Cd (1 mM) and the treatment with BC (20 g Kg-1 soil), Rh and AMF on the yield of alfalfa [fresh and dry weight (g) seeds/ plant], carbohydrates and protein content (mg g-1d. wt.) of the produced seeds (6 months from sowing). Each value is a mean of three replicates.
The results indicated a decrease in total carbohydrates and proteins content in the produced seeds under Cd stress. The content and rate of transport of photosynthates and amino acids from leaves to be stored in the produced seeds might be affected by the rate of enzymes activity involved in these metabolic processes, which were negatively affected by the presence of Cd ions. Sun et al. [60] attributed this effect to inhibition of photosynthate transport after prolonged Cd exposure from source leaves to other organs. Devi et al. [61] reported that Cd may be interfering with the enzymatic reactions related to the cycles of carbohydrate metabolism. The present study evaluated the effectiveness of BC application, AMF and Rh inoculation in spurring Cd immobilization, reducing Cd uptake and promoting plant growth. The results showed that BC application reduced the Cd concentration and its total uptake in alfalfa plants. Rh and/or AMF inoculants significantly enhanced alfalfa growth and biomass in both control and Cd-stressed plants. Symbiosis between AMF and Rh with the plant was more effective. This efficiency could be attributed to the isolation of AMF and Rh microbial strains from contaminated soils with HMs that are considered more tolerant to metals adsorption and have developed resistance according to Teng et al. [62]. Cd immobilization by different rhizosphere bacteria involves excretion of organic substances and viscous slime outside the bacterial cell for multiple reasons, i.e., adhesion to surfaces, protection and retention of water, etc. [63]. Wei et al. [64] observed that mainly carboxyl and phosphate groups were responsible for Cd2+ binding ability of extracellular polymeric substances secreted by Pseudomonas putida. Potential mechanisms underlying improved plant tolerance to metals as a result of AMF inoculation can be possible due to: (i) restriction of metals by compounds secreted by the fungus (ii) precipitation in polyphosphate granules in the soil (iii) metal adsorption to chitin in the cell wall (iv) chelating of metals inside the fungus (v) changes in rhizosphere pH (vi) the regulation of gene expression under stress conditions [65]. AMF and associated Rh appear to have several specific strategies to reduce Cd toxicity and its translocation to upper plant parts. These strategies should include (1) AMF or Rh is resistant to Cd, (2) capable of binding free Cd2+, (3) actively colonizing root surface and/or rhizosphere, (4) possess some plant growth promoting rhizobium (PGPR) traits. Moreover, the results indicated that AMF and Rh increased uptake of essential elements for growth such as Mg, Ca, N, P and K. This might be attributed to their ability to adapt and alleviate the harmful effect of Cd. Abeer et al. [66] reported that AMF inoculation not only reduced the deleterious effect of Cd stress by reducing its uptake, but significantly increased the uptake of other important mineral elements like potassium (K), calcium (Ca), manganese (Mn) and magnesium (Mg). In addition, increased Mg content in AMF treated plants has a direct effect on the Chlorophyll synthesis and photosynthesis, consequently increased the production of photosynthates, and the transported carbohydrates into the nodules, so increased nodulation and the activity of nitrogenase. Saleh et al. [67] showed that N-fixing bacteria (NFB) may synergistically interact with AMF. In this side, Rabie and Almadini [68] showed that dual inoculation with AM fungi and NFB can support both needs for N and P and increase the growth of host plant. NFB provide fixed nitrogen not only to the plant, but also to the fungus. Moreover, NFB can also assist in mobilizing nutrients from the soil and improving the growth of infected plants. Cohn et al. [69] stated that Rhizobium sp. can produce compounds that induce flavonoid production in nearby plants (legumes) that may ultimately increase root colonization of plant roots by AMF.
With respect to application of BC, the results indicated that BC successfully ameliorated Cd stress and enhanced growth and yield of alfalfa, particularly with Rh and AMF. This effect could be attributed to BC efficiency in Cd adsorption. Several mechanisms may give BC this ability; first, BC has a porous structure, large surface area, and high surface charge density, so BC has a strong ability to adsorb inorganic pollutants [70]. Secondly, BC generally has alkaline properties that can influence Cd2+ hydrolysis. Raising pH will drive more Cd to its less mobile ionic form Novak et al. [71]. Accordingly, Liu et al. [24] found that BC increased pH, while contributing to Cd immobilization. Finally, BC has the ability to enhance plant growth because it derived from crop straw that might contain valuable macronutrients (especially N and P) and some metal ions, e.g., Ca2+ and Mg2+ [72]. These properties would promote plant growth either directly, by helping overcome nutrient limitation under stress [73], or indirectly, by improving the physico-chemical properties of the soil [74]. In addition, Zheng et al. [75] stated that BC application in Cd-contaminated soils increased the soil water holding capacity, available concentrations of P and K and yield of lettuce plant, thus the highest production of grain and straw yields might also be due to the supply of nutrients to the plants with BC application. Additionally, BC acts as soil amendment to improve carbon sequestration and crop yields while valorizing crop residues and stabilizing soil contaminants [76].
The results showed that the response of alfalfa to inoculation with Rh and AMF in soil amended with BC was the most prominent. The existence of beneficial microorganisms in the rhizosphere area increased dehydrogenase activity and mycorrhizal infection. The highest microbial population was obtained by the three treatments (BC, Rh and AMF) after 60 days from sowing. This might be attributed to the role of these microorganisms (AMF and Rh) in improving soil fertility and plant development, i.e., nitrogen fixation, releasing of certain nutrient elements, e.g., P, Fe, Zn, Mn and K, in order to contribute with some plant growth substances [77]. As the isolated Rh and AMF were HMs tolerant and seemed to alleviate the toxic action of Cd where the morphological features of these plants seemed very healthy which was reflected on the plant growth. This was attributed to the external mycelium of AMF provides a wider exploration of soil volumes by spreading beyond the root exploration zone [78], thus providing access to greater volume of HMs that also stored in the mycorrhizal structures in the root and in the spores [79]. BC amendments can increase AMF % root colonization in plant roots [80] grown in acidic soils [81]. Liu et al. [24] reported that the colonization of maize roots was higher in the combined soil amendment with AMF inoculation and BC than that in the AMF inoculation alone, which also indicated a protective effect of BC against Cd toxicity to AMF colonization. Moreover, BC changes soil nutrient availability, alters activity of other micro-organisms, detoxifies allelochemicals or alters plant-AMF signaling processes and serves as a refuge from hyphal grazers [82]. Addition of BC increases pH which may have some stimulatory effects on AMF abundance, because BC is a reservoir of both signaling and inhibitory compounds (allelochemicals) [83].
The symbiosis between microorganisms and plants has been employed for the elimination of environmental contaminants to achieve high effectiveness and ecological sustainability [62]. The mechanisms involved in the metal resistance rhizobia might be attributed to: (i) changes in the metal efflux of microbial cell membranes; (ii) intracellular chelation due to the production of metallothione in proteins [84]; and (iii) the transformation of heavy metals to their less toxic oxidized forms through microbial metabolism [85]. Moreover, the metabolism of rhizobia also increases metal bioavailability in the soil through alterations in the soil pH, resulting in the release of chelators (i.e., siderophores) and organic acids capable of enhancing the complexation of metals and their mobility [86]. Microbial volatilization is another preferred method of metal bioremoval (i.e., selenium and mercury) in many rhizosphere bacteria [87]. The mixture (BC, AMF and Rh) was the most effective treatment. Inoculation with Rh and AMF to soil that amended with BC remediated the harmful effects of Cd and decreased its content and translocation from roots, stems to leaves. Interaction between BC, Rh and AMF sharply decreased Cd content and accumulation in leaves to be 11.30 compared to 27.86 mg g-1 d. wt. under Cd stress. Rh and AMF exhibited a grateful decrease in Cd content in seeds to become 0.73 and 1.01, respectively compared to 18.88 mg g-1 d. wt. under Cd stress. Translocation factor (TF) of Cd from root to seed sharply decreased with the individual treatment of Rh or AMF, moreover BC individually or in mixture with Rh and AMF alleviated the Cd impact, since no Cd ions were detected in seeds of alfalfa. As a result of this positive effect, photosynthate transport was increased from leaves to seeds, and led to the accumulation of total carbohydrates with the mixture of BC, AMF and Rh.
Conclusion
Addition of BC, Rh and AMF to the HMs-polluted soil significantly decreased the uptake of HMs, increased the HMs immobilization and inhibited their toxicity on the plant growth and the rhizosphere microbes. These treatments led to improving the soil properties and fertility, consequently the growth of the cultivated plants and the rhizosphere microbes.
Recommendation
Mixing of BC with the soil to amend the heavy metals-polluted soils. Application of BC with Rh and AMF together to achieve the highest efficiency for Cd alleviation and increasing the soil fertility. BC treatment is easy to apply, cheap and safe on plant, soil and environment. This would maintain soil fertility and plant, as well as animal and human health.
References
- Fu F1, Wang Q (2011) Removal of heavy metal ions from wastewaters. J Environ Manage 92: 407-418.
- Ibraheem AS, Seleem AA, El-Sayed MF, Hamad BH (2016) Single or combined cadmium and aluminium intoxication of mice liver and kidney with possible effect of zinc. J Basic App Zool 77: 91-101.
- Van DE, Baker A, Reeves AJM, Pollard RDS, Schat AJH (2013) Hyperaccumulators of metal and metalloid trace elements: facts and fiction. Plant Soil 362: 319-334.
- Liu L, Zhang Q, Hu LL, Tang JJ, Xu L, et al. (2012) Legumes can increase cadmium contamination in neighboring crops. PLoS One 7:Â e42944.
- DalCorso G, Silvia F, Antonella F (2010) Regulatory networks of cadmium stress in plants. Plant Signal Behav 5: 663-667.
- Machado MD, Soares HM, Soares EV (2010) Removal of chromium, copper, and nickel from an electroplating effluent using a flocculent brewer’s yeast strain of Saccharomyces cerevisiae. Water, Air, & Soil Pollution 212: 199-204.
- Rizwan M, Meunier JD, Davidian JC, Pokrovsky OS, Bovet N, et al. (2016) Silicon alleviates Cd stress of wheat seedlings (Triticumturgidum L. cv. Claudio) grown in hydroponics. Environ Sci Pollut Res 23: 1414-1427.
- Liu L, Wang YF, Yan XW, Li JW, Jiao NY, et al. (2017) Biochar amendments increase the yield advantage of legume-based inter cropping systems over monoculture. AgriEcosyst Environ 237: 16-23.
- Rajapaksha AU, Chen SS, Tsang DCW, Zhang M, Vithanage M, et al. (2016) Engineered/designer biochar for contaminant removal/immobilization from soil and water: potential and implication of biochar modification. Chemosphere 148: 276-291.
- Rehman MZU, Rizwan M, Ali S, Fatima N, Yousaf B, et al. (2016) Contrasting effects of biochar, compost and farm manure on alleviation of nickel toxicity in maize (Zea mays L.) in relation to plant growth, photosynthesis and metal uptake. Ecotox Environ Saf 133: 218-225.
- Yousaf B, Liu G, Wang R, Rehman MZ, Rizwan MS, et al. (2016) Investigating the potential influence of biochar and traditional organic amendments on the bioavailability and transfer of Cd in the soil–plant system. Environ Earth Sci 75: 1-10.
- Tahir A, Muhammad R, Shafaqat A, Muhammad ZR, Muhammad FQ, et al. (2017) Effect of biochar on cadmium bioavailability and uptake in wheat (Triticumaestivum L.) grown in a soil with aged contamination. Ecotoxicology and Environmental Safety 140: 37-47.
- Smith SE (2008) Read DJ Mycorrhizal symbiosis. 3rd edn, Academic Press, London.
- SolÃsdomÃnguez FA, ValentÃnvargas A, Chorover J, Maier RM (2011) Effect of arbuscular mycorrhizal fungi on plant biomass and the rhizosphere microbial community structure of mesquite grown in acidic lead/zinc mine tailings. Sci Total Environ 409: 1009-1016.
- Wu SL, Zhang X, Chen BD, Wu ZX, Li T, et al. (2016) Chromium immobilization by extraradical mycelium of arbuscular mycorrhiza contributes to plant chromium tolerance. Environ Exp Bot 122: 10-18.
- Ogar A, Sobczyk Å, Turnau K (2015) Effect of combined microbes on plant tolerance to Zn-Pb contaminations. Environ Sci Pollut R 22: 19142-19156.
- Yang YR, Liang Y, Ghosh A, Song YY, Chen H, et al. (2015) Assessment of arbuscular mycorrhizal fungi status and heavy metal accumulation characteristics of tree species in a lead-zinc mine area: potential applications for phytoremediation. Environ Sci Pollut Res 22: 13179-13193.
- Seth CS (2012) A review on mechanisms of plant tolerance and role of transgenic plants in environmental clean-up. Bot Rev 78: 32-62.
- Ã…gren GI, Wetterstedt JÃ…M, Billberger MFK (2012) Nutrient limitation on terrestrial plant growth - modeling the interaction between nitrogen and phosphorus. New Phytol 194: 953-960.
- Franzini VI, Azcon R, Mendes FL, Aroca R (2009) Interactions between Glomus species and Rhizobium affect the nutritional physiology of drought-stressed legume hosts. J Plant Physiol 167: 614-619.
- Muleta D (2010) Legume Responses to Arbuscular Mycorrhizal Fungi Inoculation in Sustainable Agriculture. Microbes for Legume Improvement, Springer, Cham, Netherlands, pp: 293-323.
- Vivas A, Voros I, Biro B, Campos E, Barea JM, et al. (2003) Symbiotic efficiency of autochthonous arbuscular mycorrhizal fungus (G. mosseae) and Brevibacillus sp. isolated from cadmium polluted soil under increasing cadmium levels. Environmental Pollution 126: 179-189.
- Hammer EC, Balogh-Brunstad Z, Jakobsen I, Olsson PA, Stipp SLS, et al. (2014) A mycorrhizal fungus grows on biochar and captures phosphorus from its surfaces. Soil BiolBiochem 77:Â 252-260.
- Liu L, Jiwei L, Feixue Y, Xinwei Y, Fayuan W, et al. (2018) Effects of arbuscular mycorrhizal inoculation and biochar amendment on maize growth, cadmium uptake and soil cadmium speciation in Cd-contaminated soil. Chemosphere 194: 495-503.
- Muhammad TA, Muhammad S, Hafiz NA, Zahir Z (2015) Synergistic Effect of Rhizobia and Biochar on Growth and Physiology of Maize. Agronomy Journal 107: 2327-2334.
- Buddrus SK, Schmid M, Schreiner K, Welzl G, Hartmann A, et al. (2010) Root colonization by Pseudomonas sp. DSMZ 13134 and impact on the indigenous rhizosphere bacterial community of barley. MicrobEcol 60: 381-393.
- Daft MJ, Hogarth BG (1983) Competitive interactions amongst four species of Glomus on maize and onion. Trans Br MycolSoc 80: 339-345.
- Date RA, Halliday J (1987) Collection, isolation, cultivation and maintenance of rhizobia. In: Symbiotic Nitrogen Fixation Technology, Marcel Dekker Inc, New York, USA.
- Trappe JM (1982) Synoptic keys to the genera and species of zygomycetous mycorrhizal fungi. Phytopathology 72: 1102-1108.
- Difco M (1985) Dehydrated Culture Media and Reagents for Microbiology. Laboratories incorporated Detroit. Michigan, USA, pp: 621.
- Cottenie A, Verloo M, Kiekens L, Velghe G, Camerlynck R, et al. (1982) Chemical analysis of plant and soils. Laboratory of Analytical and Agrochemistry. State University of Ghent, Belgium, pp: 100-129.
- Mohammad IA, Adel RA, Usman AH, El-Naggar AA, Aly HMI, et al. (2015) Conocarpus biochar as a soil amendment for reducing heavy metal availability and uptake by maize plants. Saudi J Biol Sci 22: 503-511.
- Massoud ON (2005) Microbiological and chemical evaluation of compost and its application in organic farming. Ph D. Thesis, Department of Botany, Faculty of Sience, ElMenoufia University, Egypt.
- Rolf AO, Bakken LR (1987) Viability of soil bacteria: optimization of plate counting technique and comparison between total counts and plate counts within different size groups. Microbial Ecology 13: 59-74.
- Phillips JM, Hayman DS (1970) Improved procedures for clearing roots and staining parasitic and vesicular-arbuscular mycorrhizal fungi for rapid assessment of infection. Trans Brit MycolSoc 55: 158-161.
- Skujins J (1976) Extracellular enzymes in soil–CRC. Crit Rev Microbiol 4: 383-421.
- Somasegaran P, Hoben HJ (1994) Hand book For Rhizobia. Springer-verlag, New York, USA.
- Lowry OH, Rosebrough NS, Farrand AL, Randall RJ (1951) Protein measurement with Folin phenol reagent. J BiolChem 193: 263-275.
- Duboise MKA, Gilles JK, Hamilton PA, Rebers Fred S, et al. (1956) Colorimetric Method for Determination of Sugars and Related Substances. Anal Chem 28: 350-356.
- AOAC (1980) Official Methods of Analysis of the Association of Official Analytical Chemists. Anal ChemAmerChemSoc 52: 148.
- Megroth SP, Cegarra J (1992) Chemical extractability of heavy metals during and after long–term applications of sewage sludge to soil. J Soil Sci 43: 313-321.
- Stoltz E, Greger M (2002) Accumulation properties of As, Cd, Cu, Pb and Zn by four wetland plant species growing on submerged mine tailings. Environmental and Experimental Botany 47: 271-280.
- Snedecor GW, Cochran WG (1980) Statistical Methods. 6th Edn, State University Press, Ames, Iowa, USA.
- Shreya T, Shruti B, Neerja S (2017) Some Morphological and Biochemical Changes in Gram Seedlings Under Cadmium Stress. J BioremediatBiodegrad 8: 403.
- Iannone MF, MarÃa D G, MarÃa PB (2015) Cadmium induces different biochemical responses in wild type and catalase-deficient tobacco plants. Environmental and Experimental Botany 109:Â 201-211.
- Gill SS, Tuteja N (2010) Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant PhysiolBiochem 48: 909-930.
- Giller K, Witter E, McGrath S (1998) Toxicity of heavy metals to micro-organisms and microbial processes in agricultural soils: a review. Soil Biology and Biochemistry 30: 1389-1414.
- Wani AS, Inayatullah T, Syed SA, Riyaz AD, Shaziya N, et al. (2017) Efficacy of 24-epibrassinolide in improving the nitrogen metabolism and antioxidant system in chickpea cultivars under cadmium and/or NaCl stress. Scientia Horticulturae 225: 48-55.
- Moreno JL, Hernandez T, Garcia C (1999) Effects of cadmium-contaminated sewage sludge compost on dynamics of organic matter and microbial activity in an arid soil. BiolFertil Soils 28: 230-237.
- Huang Q, Shindo H (2000) Effects of copper on the activity and kinetics of free and immobilized acid phosphatase. Soil BiolBiochem 32: 1885-1892.
- Tan X, Ziquan W, Guannan L, Wenxiang H, Gehong W, et al. (2017) Kinetics of soil dehydrogenase in response to exogenous Cd toxicity. Journal of hazardous materials 329: 299-309.
- Rizwan M, Ali S, Adrees M, Rizvi H, Rehman MZ, et al. (2016) Cadmium stress in rice: toxic effects, tolerance mechanisms and management: a critical review. Environ Sci Pollut Res 23: 17859-17879.
- Wang S, Wang F, Gao S (2015) Foliar application with nano-silicon alleviates Cd toxicity in rice seedlings. Environ Sci Pollut Res Int 22: 2837-2845.
- Stritsis C, Steingrobe B, Claassen N (2014) Cadmium dynamics in the rhizosphere and Cd uptake of different plant species evaluated by a mechanistic model. International Journal of Phytoremediation 16: 1104-1118.
- Rizwan M, Ali S, Abbas T, Zia-ur-Rehman M, Hannan F, et al (2016) Cadmium minimization in wheat: a critical review. Ecotoxicol Environ Saf 130: 43-53.
- Sharma RK, Agrawal M (2005) Biological effects of heavy metals: an overview. J Environ Biol 26: 301-313.
- Agrawal SB, Mishra S (2009) Effects of supplemental ultraviolet-Band cadmium on growth, antioxidant sand yield of Pisum sativum L. Ecotoxicol Environ Saf 72: 610-618.
- Sun SC, Wang HX, Li QR (1985) Preliminary studies on physiological changes and injury mechanism in aquatic vascular plants treated with cadmium. Acta PhotophysiologicaSinica 11: 113-121.
- Devi R, Munjral N, Gupta Anil K, Kaur N (2007) Cadmium induced changes in carbohydrate status and enzymes of carbohydrate metabolism, glycolysis and pentose phosphate pathway in pea. Environ Expt Bot 61: 167-174.
- Teng Y, Wang X, Li L, Li Z, LuoY, et al. (2015) Rhizobia and their bio-partners as novel drivers for functional remediation in contaminated soils. Frontiers in plant science 6: 1-11.
- Sayyed RZ, Jamadar DD, Patel PR (2011) Production of exo-polysaccharide by Rhizobium sp. Ind J Microbiol 51: 294-300.
- Wei X, Fang L, Cai P (2011) Influence of extracellular polymeric substances (EPS) on Cd adsorption by bacteria. Environ Pollut 59: 1369-1374.
- Malekzadeh E, Alikhani HA, Savaghebi-Firoozabadi GR, Zarei M (2011) Influence of arbuscular mycorrhizal fungi and an improving growth bacterium on Cd uptake and maize growth in Cd-polluted soils. Spanish Journal of Agricultural Research 9: 1213-1223.
- Abeer H, Abd_Allah EF, Alqarawi AA, Dilfuza E (2016) Bioremediation of adverse impact of cadmium toxicity on Cassia italica Mill by arbuscular mycorrhizal fungi. Saudi Journal of Biological Sciences 23: 39-47.
- Saleh M, Saleh A (2006) Increased heavy metal tolerance of cowpea plants by dual inoculation of an arbuscular mycorrhizal fungi and nitrogen-fixer Rhizobium bacterium. African Journal of Biotechnology 5: 133-142.
- Rabie GH, Almadini AM (2005) Role of bioinoculants in development of salt-tolerance of Viciafaba plants under salinity stress. African Journal of Biotechnology 4: 210-222.
- Cohn J, Day RB, Stacey G (1998) Legume nodule organogenesis. Trends in Plant Science 3: 105-110.
- Xu X, Cao X, Zhao L (2013) Comparison of rice husk-and dairy manure-derived biochars for simultaneously removing heavy metals from aqueous solutions: role of mineral components in biochars. Chemosphere 92:Â 955-961.
- Novak JM, Lima IM, Xing B, Gaskin JW, Steiner C, et al. (2009) Charcaterization of designer biochar produced at different temperatures and their effects on a loamy sand. Annals of Environmental Science 3: 195-206.
- Woolf D, Amonette JE, Street-Perrott FA, Lehmann J, Joseph S, et al. (2010) Sustainable biochar to mitigate global climate change. Nature Communications 1: 56.
- Abbas T, Rizwan M, Ali S, Adrees M, Rehman MZ, et al. (2017) Effect of biochar on alleviation of cadmium toxicity in wheat (Triticumaestivum L.) grown on Cd-contaminated saline soil. Environ Sci Pollut Res pp: 1-13.
- Igalavithana AD, Ok YS, Niazi NK, Rizwan M, Al-Wabel MI, et al. (2017) Effect of corn residue biochar on the hydraulic properties of sandy loam soil. Sustain 9: 1-10.
- Zheng R, Sun G, Li C, Reid BJ, Xie Z, et al. (2017) Mitigating cadmium accumulation in greenhouse lettuce production using biochar. Environ Sci Pollut Res 24: 6532-6542.
- Zhang G, Bi X, Li L, Chan LY, Li M, et al. (2013) Mixing state of individual submicron carbon–containing particles during spring and fall seasons in urban Guangzhou, China: A case study. Atmospheric Chemistry and Physics 13: 4723-4735.
- Morsy EM, Abdel-Kawi KA, Khalil MNA (2009) Efficiency of Trichoderma viride and Bacillus subtilis as biocontrol agents against Fusarium solani on tomato plants. Egyptian Journal of Phytopathology 37: 47-57.
- Malcova R, Vosátka M, Gryndler M (2003) Effects of inoculation with Glomus intraradices on lead uptake by Zea mays L. and Agrostiscapillaris L. Appl Soil Ecol 23: 55-67.
- Chen B, Christie P, Li L (2001) A modified glass bead compartment cultivation system for studies on nutrient and trace metal uptake by arbuscular mycorrhiza. Chemosphere 42: 185-192.
- Elmer WH, Pignatello JJ (2011) Effect of biochar amendment on arbuscular mycorrhizae and Fusarium crown and root rot of asparagus in replant soils. Plant Dis 95: 960-966.
- Yamato M, Okimori Y, Wibowo IF, Anshiori S, Ogawa M, et al. (2006) Effects of the application of charred bark of Acacia mangium on the yield of maize, cowpea and peanut, and soil chemical properties in South Sumatra, Indonesia. Soil Sci Plant Nutr 52: 489-495.
- Warnock DD, Lehmann J, Kuyper TW, Rillig MC (2007) Mycorrhizal responses to biochar in soil e concepts and mechanisms. Plant and Soil 300: 9-20.
- Massoud ON, Hanaa AA, Ebtsam MM (2016) Response of Wheat (Triticumasativum) Yield to some Plant Growth Promoting Rhizobacteria (PGPR) and its Impact on Storage Period. Middle East Journal of Applied Sciences 6: 379-387.
- Nies DH (1995) The cobalt, zinc, and cadmium efflux system CzcABC from Alcaligeneseutrophus functions as a cation-proton antiporter in Escherichia coli. J Bacteriol 177: 2707-2712.
- Nies DH (2003) Efflux-mediated heavy metal resistance in prokaryotes. FEMS Microbiol Rev 27: 313-339.
- Schalk IJ, Hannauer M, Braud A (2011) New roles for bacterial siderophores in metal transport and tolerance. Environ Microbiol 13: 2844-2854.
- Zhang W, Chen L, Liu D (2012) Characterization of a marine-isolated mercury-resistant Pseudomonas putida strain SP1 and its potential application in marine mercury reduction. ApplMicrobiolBiotechnol 93: 1305-1314.
Citation: Osama NM, Ibrahim MZ, Ghazi SM, Shedeed ZA, Doaa MN (2018) Remediation of Cadmium Toxicity on Alfalfa (Medicago sativa L.) Using Biochar as a Bioadsorbent, Rhizobium meliloti and Arbuscular Mycorrhizal Fungi as Biofertilizers. J Bioremediat Biodegrad 9: 429. DOI: 10.4172/2155-6199.1000429
Copyright: © 2018 Osama NM, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricteduse, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 6141
- [From(publication date): 0-2018 - Nov 15, 2025]
- Breakdown by view type
- HTML page views: 5043
- PDF downloads: 1098
