Research Article Open Access
Relevance of Reaction of Lead Compounds with Carboxylic acid in Lead Recovery from Secondary Sources
Vasant Kumar R1*, Jiakuan Yang2 and Seref Sonmez3
1Department of Materials Science and Metallurgy, University of Cambridge, Cambridge, CB2 3QZ, UK
2School of of Environmental Science and Engineering, Huazhong University of Science and Technology (HUST), 1037 Luoyu Road, Wuhan, Hubei, 430074, China
3Department of Materials & Metallurgical Engineering, Faculty of Chemistry and Metallurgy, Istanbul Technical University, 34436, Maslak, Istanbul, Turkey
- *Corresponding Author:
- Vasant Kumar R
Department of Materials Science and Metallurgy
University of Cambridge, Cambridge, CB2 3QZ, UK
E-mail: rvk10@cam.ac.uk
Received Date: February 28, 2013; Accepted Date: March 13, 2013; Published Date: March 20, 2013
Citation: Vasant Kumar R, Yang J, Sonmez S (2013) Relevance of Reaction of Lead Compounds with Carboxylic acid in Lead Recovery from Secondary Sources. J Powder Metall Min 2:107. doi: 10.4172/2168-9806.1000107
Copyright: © 2013 Vasant Kumar R, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Powder Metallurgy & Mining
Abstract
Lead in spent batteries is in the form of lead sulphate (PbSO4) along with lead oxides and a small proportion of metallic lead. It is recovered as lead by using energy intensive decomposition and reduction process. Dried powder from the spent lead paste and lead-alloy grid from used battery is charged in smelters either in stand alone reactors or more often with lead concentrates derived from lead sulphide ores. Decomposition of PbSO4 requires the use of relatively high temperature (typically >1000°C) using fossil fuel as the source of energy. The smelting route is associated with CO2 greenhouse gas emission, pollutants emission of lead fumes and SO2 and energy consumption of several KWh per Kg of lead thus treated.
A new process of recovering lead directly from spent battery paste as PbO precursor for making new paste has been developed. In this new environmentally sound, paste to paste process, the net-carbon, SO2 and lead dust emissions are very low towards making new batteries without the use of high temperature pyrometallurgy or the energy-intensive electrowinning processes. By reacting spent lead battery paste with organic reagents and then combusting the organic crystallites, high surface area PbO is directly available for making pastes for new batteries.
In this paper the importance of the chemical reactions between the lead compounds and the organic carboxylic acids are discussed.
Keywords
Carboxylic acid; Lead recovery; Batteries; Lighting and ignition; Crystallization
Introduction
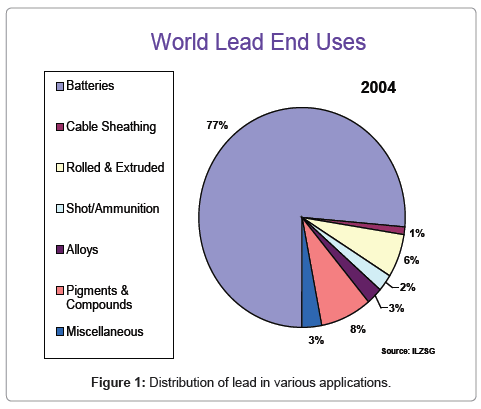
Lead acid batteries are the mainstay of secondary batteries and have found widespread industrial use as a result of low cost, simplicity of design, relative safety and good specific power density. It is estimated that over 500 million rechargeable lead acid batteries are produced each year worldwide. A large proportion of these batteries are used for starting, lighting and ignition (SLI) in automotive applications but also for traction as in milk floats and face very little competition from any economic alternatives. Of the nearly 8 million tonnes per year (mtpy) of lead produced worldwide, more than 75 % is used in batteries (Figure 1). Over half of the total lead is derived from recovering lead from used batteries with lead sulfide ores contributing the rest. It is possible to imagine that in the near future over 95 % of all lead (12 mtpy by 2015) will be used in batteries for vehicles and emergency power supply and most of this will be met mainly by recovering lead from used batteries.
Lead enjoys the reputation for being the leader material in recycling rate, defined in terms of % of lead recycled with respect to the amount of lead available for recycling. Recycling rates of over 90% are achieved in many parts of the world. It is a misnomer to categorise this as recycling, as Pb in used batteries is mainly in the form of lead sulphate (PbSO4). It is recovered as lead by using energy intensive decomposition and reduction process.
Dry paste (Table 1) from used battery is charged in smelters either in stand alone reactors or more often with lead concentrates derived from lead sulphide ores. Decomposition of PbSO4 requires the use of relatively high temperature using coal or coke as the source of energy. Thus the smelting route is associated with CO2 greenhouse gas emission, criteria pollutants emission of lead, NOx and SO2 at an energy usage of 2 to 10 KWh per Kg of lead. Treatment of gases for harmful emission is expensive and legally binding and continues to get stricter. More advanced smelting technologies such as Isasmelt use Fe or soda to fix the S in the furnace by forming FeS-PbS matte or a slag containing Na2SO4. Disposal of hazardous matter or leachable slag is also expensive and harmful to the environment. Shipping spent batteries to other distant locations will not be tenable in the long run.
| Material | Wt % |
| Lead sulphate | 55-65 |
| Lead dioxide | 15-40 |
| Lead monoxide | 5-25 |
| Metallic lead | 1-5 |
| Carbon black, plastics, fibres, other sulphates | 1-4 |
Table 1: Range of Compositions from a dry lead battery waste.
Several research methods have been advanced to overcome some of the problems, especially with sulphur encountered in the smelting route. A common factor is the use of NaOH (aq) or Na2CO3 (aq) solutions to fix S as soluble Na2SO4(aq) which can be crystallized out as a saleable by-product. The insoluble PbCO3 or Pb(OH)2 collected as sludge or filter cake is then routed to the smelter. Unfortunately it has not been possible to fully avoid the retention of some S in the sludge/ filter cake and the associated SO2 emission.
In order to evade smelting altogether, some of the new processes have dissolved the lead sludge/filter-cake in powerful acids such as HCl, H2SiF6 or HBF4 in order to recover lead by electrowinning which is capital intensive and is often suitable only for large scale operations entailing large movement of hazardous raw materials. It is also energy intensive at 5-12 KWh per Kg of lead and also polluting, if the purchased electricity for such a process is derived from fossil fuel power plants.
Once lead is recovered it is then re-oxidised to PbO granules for making pastes with H2SO4(aq) for applying to lead grids in the manufacture of battery electrodes. A lead battery is a very versatile system for use in vehicles for starting, lighting and ignition (SLI). The energy density of a lead battery is 50 Wh calculated with one Kg of Pb as the base. This amount is 2-3 orders of magnitude lower than the energy required to reproduce that lead by the current recovery methods. Thus less than 0.5% of the energy is made available. It is true that batteries are convenient sources of energy; but these calculations show that with respect to energy it is unsustainable. But not recovering lead from used batteries is not an option!
In this paper, it is intended to demonstrate the development of a sustainable automotive lead battery such that the energy required to recover lead directly as the battery precursor is of the same order of magnitude as the energy available from a new lead battery. It is quite clear that despite the excellent success of the recycling infrastructure for the lead battery, there is a need for a simpler and a more benign route in the face of tougher legislations and cost competitiveness. It is attractive to invent a cost effective process that does not involve smelting or electro winning. Most importantly, despite stringent legislations, the successful record for lead recycling must be maintained. There has been a recent tendency to ship a significant proportion of lead wastes across continents to sustain recycling in order to minimise any local environmental costs. This practice will be banned under Basel Convention forbidding large scale movements of toxic wastes. An environmentally sustainable process that can be operated on small scale as well as in large plants is in great demand.
Lead batteries are critical in automotive starting, lighting and ignition (SLI) applications, stand-by power applications (telecommunications, computer network etc., and will be further driven by the more widespread usage of renewables such as wind and solar) and motive applications (vans, forklifts, carts, e-bikes, etc). Developments in e-bikes from a low base of less than 0.5 MTPY in 2001 to more than 40MTPY in 2010, has paved the way for using lead acid batteries in EVs. Advance testing of lead batteries for hybrid electrical vehicles (HEV) is in progress in many regions of the world for micro-, mild- and medium HEV applications driven by the need to cut down the CO2 emissions below 160-120 g/Km at an acceptable economic cost. Lead batteries are capable of meeting this performance/cost criteria as evidenced by the demonstration work carried out by the Advanced Lead-Acid Battery Consortium.
Kumar et al. [1] have developed a novel technology for recovering lead from spent lead acid battery pastes via a hydrometallurgical process. With this novel technology, nano-structural leady oxide with 200~500 nm can be obtained through leaching–calcination proc.
A brief description of the new process
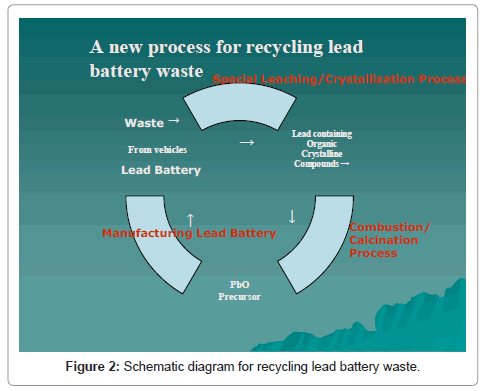
In brief, the method comprises of dissolving in a leachingprecipitation process, the lead-bearing active components of battery paste in an aqueous solution of carboxylic acid and precipitating a lead bearing organic precursor. The lead organic precursor is converted by combustion-calcination to lead monoxide containing an amount of metallic lead as desired for direct battery paste preparation. The organics embodied in the precursor serves as the fuel by combustion to aid calcination. Any residual lead sulphate that escapes fixing in the solution during leaching is used beneficially, as it can be recovered in the calcined lead product in the form of binder for the preparation of the precursor for making new lead acid batteries. A simplified flow sheet is shown in Figure 2 [1-3].
Leaching-crystallization
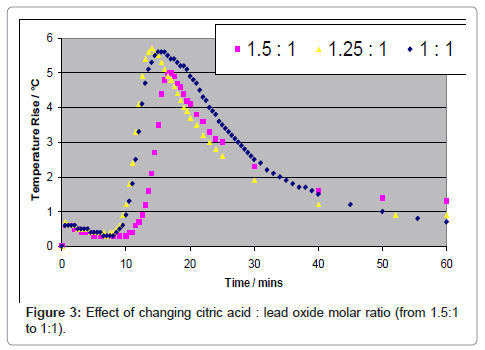
Many forms of carboxylic acids and related compounds are routinely used in food industry and in domestic use, making the process of leaching very innocuous. Unlike other hydrometallurgical processes currently under development, lead is not fully dissolved in the leachate for electrowinning, but is only transient towards crystallization [4-8]. For example if acetic acid is used as the leaching agent, since lead acetate is soluble in the aqueous solution, lead can be fully leached. However, if citric acid (C6H8O7.H2O) is used as the reagent, since lead citrate has limited solubility in an aqueous solution, lead can be dissolved but is readily crystallized. Typical results are shown by considering PbO as the reactant for varying molar ratios of citric acid to PbO. The leaching process is exothermic, accompanied by an increase in temperature while during crystallization the solution is cooled back to the starting temperature as shown in Figure 3.
The rate of reaction increased with increasing concentration of the acid solution, up to a concentration of approximately 40 % w/w. Concentrating the solution further did not increase the reaction rate, it also stopped the reaction going to completion. The yields, as calculated from weighing the recovered crystallites at optimum conditions were good at around 98%.
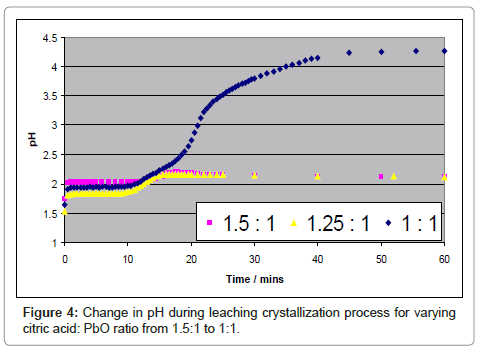
The corresponding pH changes also indicate that citric acid is being consumed. At stoichiometric citric acid: PbO ratio, the pH increases from 1.6 to 4.2 in 50-60 minutes suggesting that Pb to citrate in the crystallized product is in the 1:1 molar ratio. Measurement of the solubility of the lead product suggests that a major factor lowering the yield is lead remaining in solution. In fact, values of yield calculated from ICP data of remaining lead in solution suggests that the recoveries are over 99 % with PbO as the starting material.
Thus the leaching reaction can be expressed as:
PbO(s)+C6H8O7H2O(1) →Pb(C6H6O7 )H2O(S)+H2O(1) (1)
A SEM picture of the organic crystallite is shown in figure 4. The crystalline nature can be clearly seen and is also confirmed by x-ray diffraction data as shown in figure 5 and compares well with the literature data for lead citrate (A and B) [9]. PbO2 is a very difficult compound to dissolve in aqueous media without the help of a reducing agent such as H2O2. PbO2, once reduced in-situ with H2O2 in the solution, can then be leached by C6H8O7.H2O which then self-crystallizes to Pb(C6H6O7). H2O. The lead citrate crystal is identical in both XRD data (see XRD pattern C in figure 5) and in the SEM morphology (as in figure 6) to that made from PbO. The chemical reaction can be represented as:
PbO2+C6H8O7.H2O+H2O2 →Pb(C6H6O7 ).H2O+O2+2H2O (2)
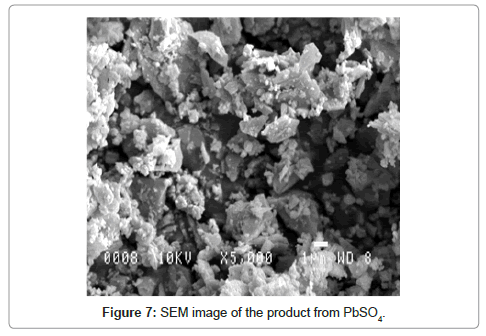
The majority fraction in the spent paste is made up of PbSO4, which is the most difficult compound to leach into the solution. We have shown that is it possible to leach PbSO4, and crystallize lead citrate from the solution by using a sulphate fixing agent such as sodium citrate (C6H5Na3O7.2H2O) or NaOH along with citric acid (C6H8O7. H2O). However the morphology is different with PbSO4 as the starting material as shown in figure 7. The crystals are smaller and less plate-like. It is suggested that rate of leaching can determine the morphology of the lead citrate crystals. PbO and PbO2 dissolve faster than PbSO4. The XRD pattern of lead citrate from PbSO4, did not match the lead (II) citrate ([Pb(C6H6O7)]·H2O) in CSD (Patterns a to C in figure 5) or any other known data, the chemical formula of precursor III can be described as [Pb(C6H5O7)m]·nH2O, where the values of m and n were deduced experimentally to be 2 and 3 respectively using thermal analysis and described in the next section. Thus the reaction can be described as follows:
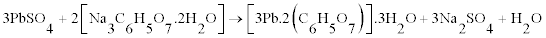 (3)
(3)
Combustion-calcination
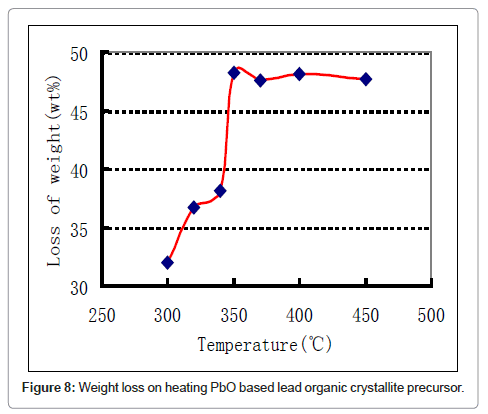
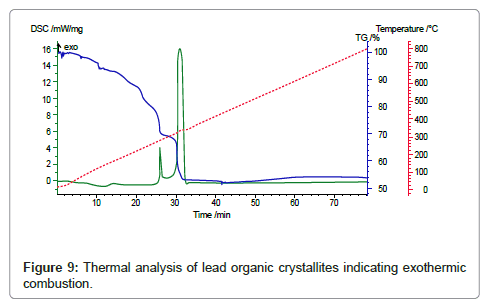
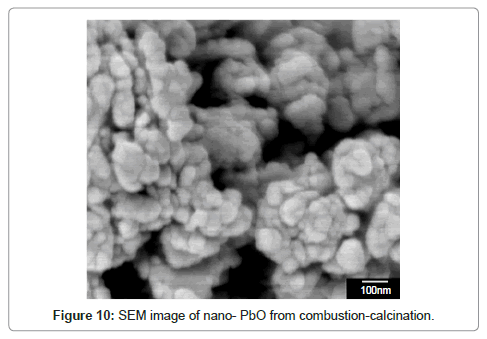
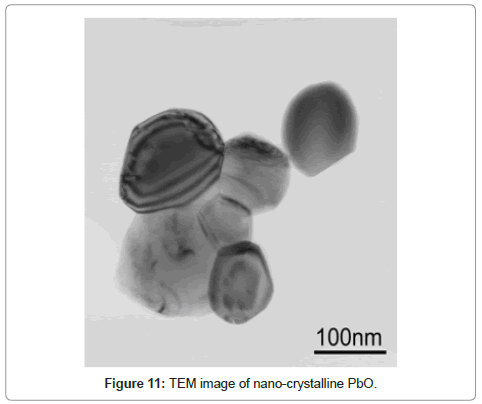
In the subsequent step the lead organic precursor is subject to heating for combusting and removing the organics embodied in the crystals. A typical data for heating the precursor from PbO starting material is shown in figure 8. A wt. loss of 48 % is achieved at around 350oC and there is only subsequent minor change on further heating. Using thermal analysis, under differential scanning calorimetry (DSC) and thermo-gravimetric analysis (TGA), it is confirmed that an exothermic reaction accompanied by ~ 48 % weight loss takes place at ~ 350°C (Figure 8). With PbSO4 as the starting material, the wt change after combustion is 38% in the 170-350°C range, thus the stoichiometry was confirmed as [3Pb.2(C6H5O7)]·3H2O in contrast with the citrates from PbO and PbO2, where the corresponding wt change was 48% in accordance with the stoichiometry ([Pb(C6H6O7)]·H2O. These thermal data correspond to combustion of the organics liberating a large quantity of heat that can be used for melting and refining the lead-alloy grid from a spent battery. The combustion reaction is accompanied by the liberation of lead in the form of PbO/Pb, the degree of oxidation is dependent upon the operating conditions. Another novel feature of the new process is the ability to produce the PbO product in a nano-crystalline form as shown in the SEM and TEM images in figure 9-11 respectively.
As much of the energy required is met directly by the combustion of organics used in the process, the overall energy requirement is estimated to be in the order of 250 Wh per Kg of Pb equivalent, based on thermal analysis data. This potential new process is not only significantly more sustainable from energy and materials point of view, but also has lower environmental impact due to significantly lower emissions and discharge. This is an example of a work carried out to make use of battery sustainable with respect to materials and energy. Under conventional approach, consideration has only been given to sustainability of materials and not of energy in the recovery of electroactive components of a battery.
The spent aqueous solution containing Na2SO4 can be subjected to the documented process for crystallizing Na2SO4 by evaporation / crystallization / centrifugation as a useful by-product for the chemical industry [10].
Use of mixed carboxylic acids
Since citric acid is relatively expensive reagent it is important to minimize the consumption of this reagent. It is advantageous to produce [3Pb.2(C6H5O7)]·3H2O rather than ([Pb(C6H6O7)]·H2O due to the more favorable chelating Pb: citrate ratio in the former. It is also advantageous to increase the rate of reaction. A mixture of acetic acid, citric acid, H2O2 and NaOH can effectively improve the rates of reaction for leaching and crystallization while decreasing the consumption of citric acid. Results from this work have been recently submitted.
Conclusions
Reactions relevant to recycling spent lead acid batteries for directly recovering lead oxide precursor in a “paste to paste” process for making new batteries are described. Leaching is carried out using organic reagents which are derived from biological sources. The potential for recovering PbO using combustion-calcinations is described. Energy demand for calcination and for melting and refining the grid alloys can be met by the organics in a net low-carbon process.
References
- Kumar RV, Sonmez S, Kotzeva VP Lead Recycling, Patent PCT/GB2007/ 004222; WO2008/056125.
- Sonmez S, Kumar RV (2009) Leaching of waste battery paste components. Part 1: Lead citrate synthesis from PbO and PbO2. Hydromet 95: 53-60.
- Sonmez S, Kumar RV (2009) Leaching of waste battery paste components. Part 2: Leaching and desulphurisation of PbSO4 by citric acid and sodium citrate solution. Hydrometallurgy 95: 82-86.
- Yang JK, Kumar RV, Singh DP (2012) Combustion synthesis of PbO from lead carboxylate precursors relevant to developing a new method for recovering components from spent lead–acid batteries. J Chem Technol Biotech 87: 1480-1488.
- Yang JK, Zhu XF, Kumar RV (2011) Ethylene glycol-mediated synthesis of PbO nanocrystal from PbSO4: A major component of lead paste in spent lead acid battery. Mater Chem Phys 131: 336-342.
- Li L, Zhu X, Yang D, Gao L, Liu J, et al. (2012) Preparation and characterization of nano-structured lead oxide from spent lead acid battery paste. Journal of Hazardous Materials 203: 274-282.
- Zhu X, Li L, Sun X, Yang D, Gao L, et al. (2012) Preparation of basic lead oxide from spent lead acid battery paste via chemical conversion. Hydrometallurgy 117: 24-31.
- Kumar RV, Sarasonki T (2010) A review of Materials & Chemistry for Secondary Batteries, Chapter 3, 53- 81, in “High Density Energy Lithium Batteries”; KE Aifantis, SA Hackney and RV Kumar(Editors), Wiley-VCH Verlag.
- Kourgiantakis M, Matzapetakis M, Raptopoulou CP, Terzis A, Salifoglou A (2000) Lead-citrate chemistry synthesis, spectroscopic and structural studies of a novel lead(II)-citrate aqueous complex. Inorg Chim Act 297: 134-138.
- Prengaman RD, Morgan C, Hine E, Homer P, Griffin GM (2001) US Patent, No: 6177056.
Relevant Topics
- Additive Manufacturing
- Coal Mining
- Colloid Chemistry
- Composite Materials Fabrication
- Compressive Strength
- Extractive Metallurgy
- Fracture Toughness
- Geological Materials
- Hydrometallurgy
- Industrial Engineering
- Materials Chemistry
- Materials Processing and Manufacturing
- Metal Casting Technology
- Metallic Materials
- Metallurgical Engineering
- Metallurgy
- Mineral Processing
- Nanomaterial
- Resource Extraction
- Rock Mechanics
- Surface Mining
Recommended Journals
Article Tools
Article Usage
- Total views: 16312
- [From(publication date):
March-2013 - Apr 04, 2025] - Breakdown by view type
- HTML page views : 11631
- PDF downloads : 4681