Research Article Open Access
Relationship between the Asbestos Cumulative Exposure Index (ACEI) and the Latency Period of Asbestos Related Diseases (ARD) within an Italian Study Group of Ex-Asbestos Workers
Giovanni Maria Ferri*, Chiara Monica Guastadisegno, Graziana Intranuovo, Vito Luisi, Domenica Cavone, Brunella Licchelli, Elena Viola Buononato, Linda Macinagrossa and Raffaele Molinini
Department of Interdisciplinary Medicine (DIM), Section B. Ramazzini, Unit of Occupational Medicine, University Hospital Policlinico - John XXIII in Bari, Italy
- *Corresponding Author:
- Ferri GM
Department of Interdisciplinary Medicine (DIM)
Section B. Ramazzini, Unit of Occupational Medicine
University Hospital Policlinico - John XXIII in Bari
Piazza G. Cesare, 1170124 Bari, Italy
Tel: +39805478212
E-mail: giovannimaria.ferri@uniba.it
Received date: July 11, 2016; Accepted date: July 30, 2016; Published date: August 5, 2016
Citation: Ferri GM, Guastadisegno CM, Intranuovo G, Luisi V, Cavone D, et al. (2016) Relationship between the Asbestos Cumulative Exposure Index (ACEI) and the Latency Period of Asbestos Related Diseases (ARD) within an Italian Study Group of Ex-Asbestos Workers. Occup Med Health Aff 4:243. doi: 10.4172/2329-6879.1000243
Copyright: © 2016 Ferri GM, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Occupational Medicine & Health Affairs
Abstract
Objectives: The association between asbestos exposure, measured by mean of Asbestos Cumulative Exposure Index (ACEI) and the latency period of non-malignant asbestos-related diseases (ARD) diagnosed according to the American Thoracic Society (ATS) criteria was studied.
Methods: 306 exposed asbestos workers in Bari, Italy, were included in a health surveillance program. By means of a standardized questionnaire we assessed asbestos exposure through ACEI. Latency period of Asbestos Related Diseases (ARD) was also assessed.
Results: We found a significant inverse correlation between latency and ACEI increasing with ARD severity. ACEI and 30-35 years of age at time of first exposure were inversely associated with the latency period. The risk of ARD increased from baseline to the 2nd follow-up and among subjects exposed for the first time before 1960.
Conclusions: The most important factors that caused a reduction in the latency period were the year of first exposure and the ACEI score while smoking habits did not show to play a significant role.
Keywords
Asbestos; Ex-exposed workers; Asbestos cumulative exposure index; Asbestos related diseases; Latency period; Health surveillance program
Introduction
Asbestos is a set of silicate minerals with microcrystalline structure and fibrous morphology, which gives it interesting physical properties, especially thermal resistance and sound absorption [1]. It was plenty used in the industries of shipbuilding, building, railways and in asbestos-cement production [1]. Moreover, its ability to be woven with other materials such as cotton or hemp or nylon allowed multiple applications in sectors as diverse as textiles, electricity, petrochemicals, glass and even sugar [2]. There was massive growth in asbestos use from 1877 to 1967 when its public health consequences were discovered, because of a spatial distribution of deaths due to unexpected sources of asbestos exposure [3]. In the early 1990s, many countries banned various industrial uses of asbestos or imposed restrictions because of its cancerous effects [4,5]. The EPA (Environmental Protection Agency) and the IARC (International Agency for Research on Cancer) classify Asbestos as a group 1 carcinogen agent [1,6,7]. Asbestos related diseases (ARD) include pleural diseases (i.e., pleural plaques that involve parietal pleura and diffuse pleural thickening that involves mainly visceral pleura), asbestosis and asbestos related cancers ARC) [8].
Diffuse pleural thickening involves visceral pleura and includes asbestos related pleural effusion, blunted cost phrenic angle, crow’s feet of pleural-parenchymal fibrous strands and rounded atelectasis; higher exposure level may be required respect to the pleural plaques, whereas bilateral non-symmetric pleural plaques are exposures to asbestos indicators [9].
Asbestosis, a pneumoconiosis causing interstitial lung fibrosis, usually becomes evident after an appreciably extended latency period. The duration and intensity of exposure influence the occurrence of parenchymal pulmonary fibrosis [10]. Moreover low environmental asbestos exposure leads only to an extremely low risk and smoking effects should be considered in the evaluation of early asbestosis [1,9]. The major malignancies associated with asbestos are lung cancer and mesothelioma, with additional cancer risk reported for other sites [1,8,11]. Cancer risk increases with cumulative asbestos exposure, with an increased risk even at low levels of exposure; the joint effect of asbestos and smoking is supra additive, which may depend in part on the presence of asbestosis [12]. No safe threshold for asbestos exposure were established for lung cancer and mesothelioma [12]. In 1986, the American Thoracic Society established criteria for the diagnosis of non-malignant asbestos related disease with updates in 2001 and in 2004 [9,11,13]. Both malignant and non-malignant asbestos related diseases are associated with a long latency period.
About ARC a minimum of 10 years from the first exposure is required to attribute the mesothelioma to asbestos exposure, though in most cases the latency interval is longer (30-40 years) [9] and a minimum lag-time of 10 years from the first asbestos exposure is required to attribute the lung cancer to asbestos [14,15].
About latency time of non-malignant ARD, pleural plaques develop 20-30 years after first exposure, benign pleural effusion after 10-20 years [9]. The latency for development of diffuse pleural thickening is variable and could depend from a relationship with the extent of asbestos exposure [16] and is approximately 30 years following exposure [17].
Since 1992, in Italy, is in force the Italian law 257/1992 which banned further mining, production and trade of asbestos and asbestoscontaining goods. The Italian legislation provides that health surveillance of workers previously exposed to asbestos should be continued even after the cessation of exposure to asbestos (Legislative Decree n°277/91). The law makes no reference to the frequency and the limit of extension in time of the clinical examination..
The aims of this study were to study the relationship of nonmalignant ARD latency period with asbestos exposure, assessed by means of an asbestos cumulative exposure index (ACEI), and with other potential factors of latency time reduction.
Materials and Methods
In this study ARD were defined as non-malignant asbestos related diseases (unilateral or bilateral pleural plaques, diffuse pleural thickening and asbestosis) and ACR were defined as asbestos related cancers (mesothelioma and lung cancer). Latency time was defined as the time from first exposure to diagnosis [18].
A group of 306 exposed asbestos workers was selected from subjects submitted to medical examinations from 2000 to 2011 in the Occupational Medicine Unit of the Hospital “Azienda Ospedaliera Universitaria Policlinico-Giovanni XXIII” in the town of Bari, Italy.
Inclusion criteria were a documented occupational asbestos exposure and at least two medical examinations from 2000 to 2011 excluding the first access. Participation was voluntary. All participants received information about the study and gave their written informed consent.
Clinical assessment
During the first medical access the health history of the recruited subjects was carefully collected, with particular attention to previous respiratory diseases. The subjects were also submitted to a clinical examination and health checks with blood and urine analysis were carried out. Functional breathing test (Global Spirometry and DLCO - Diffuse Lung Carbon Monoxide) and full size chest X-ray were also performed. The diagnosis of ARDs was carried out according to the American Thoracic Society criteria [11].
Diagnostic criteria for asbestosis included a history 25 fibers/ml years exposure to asbestos, the presence of bilateral fine and inspiratory crackles on auscultation and the presence of sub-pleural interstitial opacities on chest radiograph, in the absence of other causes of interstitial pulmonary fibrosis.
Criteria for the diagnosis of diffuse pleural thickening (DPT) were involvement of >25% of the chest wall on plain chest radiograph, 8 cm × 5 cm × 3 cm [19] in total on chest computer tomography, and/or the presence of Blesovsky’s syndrome [20].
Pleural plaques were diagnosed according to their presence on chest radiograph or computer tomography scan and included calcified and non-calcified circumscribed pleural thickening [19].
It was carried out a thoracic Computed Tomography scan (CT) when a RX reading was suspected for pleural or lung disease or whether osteopontin and mesothelin values were abnormal.
A CT negative or showing alterations of non-malignant pleural disease or asbestosis led to the conclusion of the diagnostic protocol and they were activated the legal obligations procedures.
The patients were sent to further diagnostic analysis in the Thoracic Surgery Unit if, by means of CT, they were suspected neoplastic changes in the pleura or lung.
An individual risk profile was defined for each worker related to exposure assessment results (ACEI) and medical examination outcomes, it was defined also in relation to other risk factors (i.e., tobacco smoking habits, neoplastic family history, radiation exposure) that allowed to program the health surveillance.
Annual periodic medical examinations were extended to workers so identified with high risk profile related with the scale of exposure intensity (ACEI) [21,22] and with non-malignant asbestos-related diseases, while exposure to low-medium risk profile and in absence of ARD periodic medical examination was proposed over three years.
Exposure assessment
During the first medical access also the history of every single work task was carefully collected. Domestic exposure, lifestyle habits and hobbies were also collected to find any other asbestos exposure sources for the risk assessment according with literature data and exposure definition criteria (Italian national mesothelioma register ReNaM) [23].
During the second medical examination (i.e., the first follow up) the exposure characterization was carried out through the administration of a standardized questionnaire developed and validated by Magnani [21,22]. It included lifelong occupational history with specific sections for various industrial sectors (Table 1), detailed description of parental asbestos related occupations, residential history, including address and description of dwelling and their neighborhood. The questionnaire included also demographic characteristics, radiation treatments and tobacco consumption.
| Asbestos related diseases | ||||||||
|---|---|---|---|---|---|---|---|---|
| Total | No | Yes | ||||||
| VARIABLES | n | (%) | n | (%) | n | (%) | Z | p value |
| Age (Years) | ||||||||
| < 50 | 5 | 1.63 | 4 | 3.75 | 1 | 0.50 | 0.14 | 0.56 |
| 51-60 | 47 | 15.36 | 25 | 22.90 | 22 | 11.16 | 1.08 | 0.13 |
| 61-70 | 140 | 45.75 | 61 | 55.90 | 79 | 40.10 | 1.88 | 0.06 |
| >70 | 114 | 37.25 | 19 | 17.45 | 95 | 48.20 | -2.49 | 0.01* |
| First Exposure Age (Years) | ||||||||
| <25 | 197 | 64.38 | 71 | 65.13 | 126 | 63.95 | 0.14 | 0.44 |
| 25-29 | 71 | 23.20 | 24 | 22.01 | 47 | 23.85 | -0.10 | 0.46 |
| 30-34 | 20 | 6.54 | 7 | 6.42 | 13 | 6.59 | -0.02 | 0.49 |
| >=35 | 18 | 5.88 | 7 | 5.88 | 11 | 5.58 | 0.08 | 0.46 |
| Cumulative exposure index | ||||||||
| Low-medium | 227 | 74.18 | 99 | 90.8 | 128 | 64.97 | 4.17 | 0.00* |
| High | 79 | 25.82 | 10 | 9.20 | 69 | 35.03 | -1.64 | 0.05* |
| Latency | ||||||||
| Low | 146 | 73.98 | 0 | 0 | 146 | 74.1 | - | - |
| High | 51 | 26.02 | 0 | 0 | 51 | 25.9 | - | - |
| Smoking habits | ||||||||
| Non smoker | 106 | 34.98 | 40 | 36.7 | 66 | 34.02 | 0.34 | 0.37 |
| Ex-smoker | 123 | 40.59 | 37 | 33.94 | 86 | 44.33 | -1.03 | 0.15 |
| Smoker | 74 | 24.42 | 32 | 29.36 | 42 | 21.65 | 0.89 | 0.18 |
| Not reported | 3 | 0.98 | 0 | 0 | 3 | 1.5 | ||
| Ventilation deficit | ||||||||
| None | 235 | 76.8 | 85 | 77.98 | 150 | 76.16 | 0.35 | 0.36 |
| Obstructive | 29 | 9.48 | 15 | 13.76 | 14 | 7.1 | 0.59 | 0.27 |
| Restrictive | 33 | 10.78 | 6 | 5.5 | 27 | 13.7 | -0.55 | 0.28 |
| Mixed | 9 | 2.94 | 3 | 2.76 | 6 | 3.04 | -0.09 | 0.46 |
| Diffusion deficit | ||||||||
| None | 158 | 76.69 | 46 | 82.15 | 112 | 74.67 | 1.01 | 0.15 |
| Light | 28 | 13.59 | 6 | 10.71 | 22 | 14.67 | -0.25 | 0.40 |
| Moderate | 12 | 5.83 | 4 | 7.14 | 8 | 5.33 | 0.13 | 0.45 |
| High | 8 | 3.89 | 0 | 0 | 8 | 5.33 | - | - |
| Industrial category | ||||||||
| Cement-asbestos industry | 62 | 20.26 | 9 | 8.25 | 53 | 26.9 | -1.21 | 0.11 |
| Constructions | 4 | 1.31 | 0 | 0 | 4 | 2.03 | - | - |
| Food industry | 3 | 0.98 | 0 | 0 | 3 | 1.52 | - | - |
| Rubber industry | 1 | 0.33 | 1 | 0.91 | 0 | 0 | - | - |
| Steel industry | 62 | 20.26 | 25 | 22.94 | 37 | 18.8 | 0.51 | 0.6 |
| Navalindustry | 25 | 8.17 | 12 | 11 | 13 | 6.6 | -0.14 | 0.89 |
| Chemical -petrochemical industry | 3 | 0.98 | 1 | 0.91 | 2 | 1.01 | - | - |
| Energy industry | 3 | 0.98 | 1 | 0.91 | 2 | 1.01 | - | - |
| Railway | 94 | 30.72 | 50 | 45.9 | 44 | 22.33 | 0.27 | 0.7 |
| Military , Naval transport | 25 | 8.16 | 2 | 1.83 | 23 | 11.67 | -0.45 | 0.32 |
| Miscellaneous (domestic exposure, trade ,health system, land transport) |
24 | 7.84 | 8 | 7.33 | 16 | 8.12 | - | - |
| Total | 306 | 100.00 | 109 | 100.00 | 197 | 100.00 | ||
| * p≤ 0.05;ACEI = Asbestos Cumulative Exposure Index | ||||||||
Table 1: Baseline description of the study group.
Magnani questionnaire, specific for job sectors, provided an estimate of occupational exposure with a procedure "stepwise”: in the first time they were considered the used materials, their fibers content and their crispness; then they were considered the performed duties, specified in terms of mechanical stress applied to materials through the instruments used directly by the worker.
Finally the factors that modulate the exposure were considered, such as speed of emission of the particles, surface of the source, presence of suction systems with local and other sources in the same environment work, size and physical characteristics of the premises [21, 22].
The intensity of exposure (I) was expressed through median concentration of asbestos fibers, obtained by a panel of industrial hygienist according to literature data in the following semi quantitative scale: I=0.0135 ff /ml median concentration in extremely clean industrial practices; I=0.135 ff/ml median concentration in well protected industrial practices (good confinement or presence intake during directs contact); I=1.35 ff/ml median concentration in early or unprotected work activities (without confinement or aspiration or control systems) without powerful sources; I=13.5 ff/ml median concentration in early or unprotected work activities without any containment or control systems with massive sources; I=135 ff/ml median concentration of unprotected work activities with massive sources, with high-speed dust emission, without any confinement or control system.
For each intensity of exposure value (I) we also defined a job coefficient (C), expressed in the following scale: Job coefficient 3 corresponded to I=0.00135 ff/ml; Job coefficient 4 corresponded to I=0.135 ff/ml; Job coefficient 5 corresponded to I=1,35 ff/ml; Job coefficient 6 corresponded to I=13,5 ff/ml; Job coefficient 7 corresponded to I=135 ff/ml.
So the Asbestos Cumulative Exposure Index (ACEI) was obtained through the relationship ACEI = A × B × C [A= duration of exposure, the calculation of the years of exposure; B=% exposure/die (min 0.1 - max 1), the quantitative estimate of the percentage of working time spent at that concentration; C= job coefficient, the semi-quantitative estimation of the concentration or intensity of exposure (I)].
The percentage of daily exposure to asbestos was expressed using values between 10% and 100% of the duration of full shift.
If workers had done more work activities with asbestos exposure they were calculated the different ACEI in the same way. So total ACEI was calculated according to this formula: ACEI tot = ACEI1+ACEI2+ACEI3… +ACEIn.
All the values ≤ 3.75 were defined low-medium ACEI values and all the values > 3,75 were defined high ACEI values. This cut off (3.75) was the value corresponding to the 66th percentile of the ACEI cumulative frequency distribution.
Statistical methods
A natural log transformation of the ACEI was carried out because of the asymmetrical distribution of the variable. This transformation was carried out to enable us the use of a parametric approach. Pearson and Bonferroni statistics were used to study correlations and linear regression between normally distributed variables. The Spearman coefficient was used for non-parametric correlations. The Armitage trend test was used to compare different proportions. A univariate analysis of all studied factors was used to evaluate the presence of confounders or effect modifiers. A multivariate analysis by means of an unconditional logistic regression model was also carried out to obtain adjusted estimates. To apply such a multiple regression model a dichotomy of the latency period (medium-low/high) was carried out using as cut off the value corresponding to 66th percentile of the distribution (36 years).
Results
The main characteristics of the group with ARD (Pleural Plaques, Asbestosis, Asbestos Related Cancer) and without ARD were compared. A significant higher frequency of ARD was observed among subjects over 70 years of age. No significant difference was observed for the age at first exposure. The subjects having a high ACEI showed a significant higher occurrence of ARD. A low latency period (≤36 years) was observed in 74.11% of the 197 subjects with ARD and high latency periods in the remaining 25.89%. There were no significant differences in ARD frequency between smokers, ex-smokers and non-smokers and for different levels of ventilatory deficit. For diffusion deficit, while there were no differences between none, light and moderate, all subjects with high diffusion deficit had ARD. No significant differences related to the industries where the subjects were employed were found (Table 1).
A significant increase of ARD frequencies from baseline through the first and second follow-up was observed (χ2 of trend =13.74; p≤0.01) and it was mainly due to the onset of Asbestosis (χ2 of trend =17.59; p ≤ 0.01) (Table 2).
| Baseline | First follow up | Second follow up | Armitage test | |
|---|---|---|---|---|
| n (%) | n (%) | n (%) | χ2 of trend | |
| No ARD(Healty subjects) | 110(35.95) | 99 (32.35) | 67 (21.90) | |
| UPP(Unilateral Pleural Plaques) | 8 (02.61) | 8 (02.61) | 11 (03.59) | 2.03 |
| BPP(Bilateral Pleural Plaques) | 127 (41.50) | 133 (43.16) | 131 (42.81) | 6.61* |
| ASB(Asbestosis and Pleural Plaques) | 59 (19.28) | 63 (20.59) | 94 (30.72) | 17.59*** |
| ARC(Asbestos Related Cancer) | 2 (00.65) | 3 (00.98) | 3 (00.98) | 0.26 |
| Total ARD(Total Asbestos related diseases) | 196 (64.05) | 207 (67.64) | 239 (78.10) | 13.74*** |
| * p≤0.05;** p≤0.025;*** p≤0.01 | ||||
Table 2: Frequency distribution of ARD by different time follow-up.
A significant increase of the ACEI (natural log) means resulted associated to the increase of the ARD severity: the highest means of ACEI (natural log) was observed for Asbestosis (Table 3).
| ACEI (Natural log of ff/ml ) | |||
|---|---|---|---|
| N | Mean | SE | |
| ARC (Asbestos Related Cancer) | 2 | 0.690 | 1.37 |
| UPP (Unilateral Pleural Plaques) | 9 | 1.560 | 1.83 |
| BPP (Bilateral Pleural Plaques) | 127 | 3.100 | 1.77 |
| ASB (Asbestosis and Pleural Plaques) | 59 | 5.760 | 2.02 |
| Total ARD(Asbestos Related Diseases) | 197 | 3.800 | 2.28 |
| Trend testZ = 9.00 p≤0.01 | |||
Table 3: Distribution of ACEI ( AsbestosComulative Exposure Index )means by different ARD.
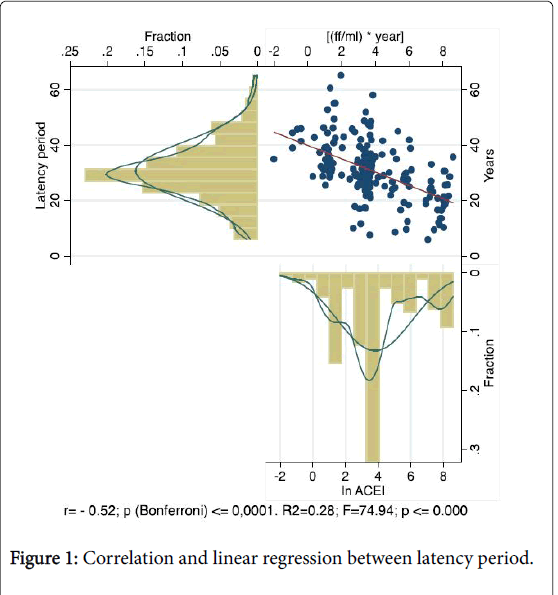
A significant inverse correlation (r=-0.52; p≤0.0001) and a significant inverse linear regression [R2 =-0.28; F=74.94; p≤0.00] were observed between ACEI and latency period among subjects with ARD (Figure 1).
This inverse correlation increased with the severity of ARD with the highest observed value among subjects with Asbestosis and Bilateral Pleural Plaques (r=-0.53; p≤0.000) (Table 4).
| Non smokers | Ex Smokers | Smokers | Total | ||
|---|---|---|---|---|---|
| Monolateral Pleural Plaques (MPP) | n | 3 | 5 | - | 8 |
| r | 0.94 | -0.78 | - | -0.39 | |
| p | 0.210 | 0.110 | - | 0.33 | |
| n | 39 | 56 | 29 | 124 | |
| Bilateral Pleural Plaques (BPP) | r | -0.49 | -0.45 | -0.20 | -0.47 |
| p | 0.001 | 0.004 | 0.290 | 0.000 | |
| n | 23 | 24 | 12 | 59 | |
| Asbestosis and Bilateral Pleural Plaques (ASB) | r | -0.59 | -0.38 | -0.79 | -0.53 |
| p | 0.002 | 0.060 | 0.001 | 0.000 | |
| n | 106 | 123 | 74 | 303 | |
| Total ARD | r | -0.56 | -0.46 | -0.57 | -0.52 |
| p | 0.000 | 0.000 | 0.001 | 0.000 | |
| ACEI: Asbestos Cumulative Exposure Index; ARD:Asbestos Related Diseases | |||||
Table 4: Correlations between latency and in ACEI by smoking habits and ARD.
In smokers with Bilateral Pleural Plaques there was a reduction of this inverse correlation while among smokers affected by Asbestosis with Bilateral Pleural Plaques the inverse correlation was stronger (Table 5).
| Number of obs = 193 LR chi2(6) = 14.04 Prob> chi2 = 0.03 Pseudo R2 = 0.06 Log likelihood = -101.23 | ||||||
| Low-medium latency | Odds Ratio | Std. Err. | z | P>z | 95% Conf. | Interval |
| ACEI (ff/ml /year) | ||||||
| Medium-low | 1.00 | |||||
| High | 3.1 | 1.29 | 2.71 | 0.01 | 1.37 | 7.01 |
| Age at first exposure (Years) | ||||||
| <25 | 1.00 | |||||
| 25-30 | 1.16 | 0.47 | 0.37 | 0.71 | 0.52 | 2.59 |
| 30-35 | 3.76 | 4.04 | 1.23 | 0.22 | 0.46 | 30.87 |
| >=35 | 0.42 | 0.28 | -1.32 | 0.19 | 0.11 | 1.53 |
| Smoking habits | ||||||
| Non smokers | 1.00 | |||||
| Ex smokers | 1.53 | 0.62 | 1.04 | 0.30 | 0.69 | 3.38 |
| Smokers | 1.19 | 0.56 | 0.37 | 0.71 | 0.48 | 2.98 |
| _cons | 1.89 | 0.61 | 1.97 | 0.05 | 1.00 | 3.55 |
| ACEI: Asbestos Cumulative Exposure Index | ||||||
Table 5: Factors of low medium latency period (=36 years).
The multivariate analysis related to the study of the medium-low (≤36 years) latency factors (age at first exposure, exposure cumulative index, smoking habits) showed that medium-low latency was associated to high ACEI [OR=3.10 (1.37-7.01)], highly but not significantly associated to the class of age of first exposure from 30 to 35 years [OR=3.76 (0.46-30.87)], while smoking [OR=1.19 (0.48-2.98)] wasn’t significantly associated (Table 5).
The baseline risk of ARD increased from medium ACEI (33th to 66th percentile) [OR=4.25 (2.23-8.13)] to high ACEI (over the 66th percentile) [OR=10.00 (4.49-20.60)] with a significant trend and it was independent by smoking habits, age at first exposure to asbestos and year of first exposure.
Smoking habit showed an increasing but not significant risk trend from baseline to second follow-up. A significant independent risk of ARD was also associated to the year of first exposure ≤ 1960 [OR=25.93 (6.40-104.20)] and from 1961 to 1975 (OR=3.55 [1.75-7.23]).
A similar but decreasing association was found during the 1th and 2nd follow-up (Table 6).
| Baseline | 1th Follow-up | 2th Follow-up | |
|---|---|---|---|
| ARD | OR (90% CI) | OR (90% CI) | OR (90% CI) |
| ACEI | |||
| Low | 1.00 | 1.00 | 1.00 |
| Medium | 4.25 (2.23-8.13) | 4.83 (2.51-9.26) | 3.70 (1.84-7.39) |
| High | 10.00 (4.49-20.6) | 13.60 (6.24-29.6) | 12.96 (4.97-33.7) |
| 1th EXPOSURE AGE | |||
| >35 years | 1.00 | 1.00 | 1.00 |
| 35-30years | 0.83 (0.18-3.87) | 0.78 (0.15-3.80) | 0.78 (0.15-3.98) |
| 30-25years | 1.31 (0.38-4.62) | 0.94 (0.25-3.40) | 1.18 (0.31-4.41) |
| <25years | 0.63 (0.19-2.06) | 0.60 (0.17-2.02) | 1.06 (0.30-3.67) |
| SMOKING STATUS | |||
| No | 1.00 | 1.00 | 1.00 |
| Yes | 1.37 (0.75-2.48) | 1.39 (0.74-2.56) | 1.50 (0.77.2.89) |
| YEAR OF 1th EXPOSURE | |||
| >1975 | 1.00 | 1.00 | 1.00 |
| 1961-1975 | 3.55 (1.75-7.23) | 3.41 (1.65-7.00) | 3.44 (1.65-7.13) |
| <=1960 | 25.93 (6.4-104.2) | 18.49 (4.54-75.2) | 12.70 (2.56-62.9) |
| ACEI: Asbestos Cumulative Exposure Index; ARD: Asbestos Related Diseases. | |||
Table 6: Distribution of ARD risks by different period of time and factors.
Discussion
Management of Asbestos Related Diseases (ARD) are currently an increasing concern for the planners of occupational and public health policies.
Many countries are now experiencing epidemics of ARDs that are a consequence of occupational exposures happened in the period 1960s-1980s. ARD are generally characterized by a long latency period. It is likely that asbestos-related mortality and morbidity will continue to increase in the next years [24,25].
The follow-up of workers occupationally exposed to asbestos has individual positive effects and social positive effects. The individual positive effects are related to the early diagnosis of asbestos diseases by means of medical screening programs and to the notification of occupational disease for compensation. The social positive effects are linked to the assessment of an epidemiological surveillance finalized to the evaluation of the impact of cohort’s follow-up in terms of public health benefits [26,27].
In our study the assessment of occupational exposure was obtained using an Asbestos Cumulative Exposure Index (ACEI). Data showed high frequencies of ARD in almost all the subjects who had worked in industries with a recognized exposure to asbestos [28,29].
The observed significant increasing trend of the ACEI (natural log) means with severity of ARD (Table 3) was in agreement with the widely demonstrated dose response relationship for asbestosis onset [30] while the low ACEI among subject with Asbestos Related Cancer (ARC) was consistent with the no threshold theory [31,32].
The observed highest frequencies of ARD among subjects exposed for the first time before the 1960 were consistent with the results of other experiences in Netherland [33], Finland [34], Israel [10] and Italy [26,35].
Latency times of single ARD were associated with the ACEI. The length of lag time between exposure and the onset of Asbestos Related Diseases (ARD) it can vary from many decades to few years. Asbestosis can occur shortly after exposure if the exposure is very high, while Pleural Plaques (PP) requires a long latency period. Previous experiences reported the occurrence of PP associated to the ACEI adjusted for a latency period of about 15 years [36].
In our study the multivariate analysis showed that latency was significantly associated with the Asbestos Cumulative Exposure Index (ACEI). The low-medium latency (<36 years) showed a high, but not significant, association with the age at first exposure of 30-35 years. This relationship could be explained by an individual lower immune answer due to constitutional and aging factors facilitating the occurrence of Asbestos Related Diseases (ARD).
The response of the respiratory system against inhaled agents could be modified by changes of respiratory functions and physical and biochemical substances occurring during the normal aging process [37]. It was epidemiologically demonstrated that in humans, the elderly, compared to the general population, are more susceptible to the effects of high levels of environmental ubiquitous particulates. For elderly subjects (50-70 years old), carriers of non-specific bronchial hyper responsiveness and with high total immunoglobulin E, there is an increased susceptibility to the effects of urban pollution [38]. Also smoking accelerates aging of the small airway epithelium [39]. Our results may be due also to the inclusion in this age class of the 35% of asbestos cement workers and 40% of railway rolling stock workers.
In our study, the latency of non-malignant ARD, in contrast with ARC, did not seem to be associated to smoking as indicated in other studies [40].
A limitation of our study was the lack of data about smoking habits in terms of pack/years. In smokers with bilateral pleural plaques there was a paradoxical reduction of the negative correlation between ACEI and latency. In patients with asbestosis, smoking seemed to increase the reduction of the latency period. We didn’t have an explanation of this finding and we can only speculate about the role of the efficiency reduction of mucus-ciliary clearance among smokers. An experimental study reported a marked increase of the number of fibres in the lung macrophages of animals exposed to smoke [41]. In smokers’ airways, contrary to non-smokers, inhaled particles don’t penetrate deep into the bronchial tree, but are deposited centrally. The lung and nasal debris mixed with saliva are often swallowed or expectorated. This phase is not influenced by the presence of lung disease but in certain circumstances the effects of smoking in the development of bronchitis could accelerate this stage. The presence among the smokers of a “smoking associated fibrosis” could be partially responsible for the reduction of the inverse correlation between ACEI and latency among subjects with Bilateral Pleural Plaques, with an increase of the latency period, because this fibrosis, involving the alveolar walls, could slow down the asbestos fibres movements [42].
Finally this study highlights the presence of ARD in exposed workers even long time after the end of exposure and also confirmed the relevance of the use of an appropriate cumulative exposure index in health surveillance of subjects with past high asbestos exposure [43]. The association between ARD and year of first exposure was here confirmed and also a significant trend was observed (Table 6).
Our multivariate analysis showed that the reduction of the latency period is associated to a high ACEI, that the age of first exposure (30-35 years) could be likely a factor, instead smoking did not play, in this experience, an important role. Among the smokers is only evident a significant high inverse correlation between ACEI and ARD (Table 4).
The factors determining the length of the latency period are still not yet completely understood. Explanation of these factors that contribute to reducing the latency of the disease would be very interesting and further studies are needed.
The study of the asbestos cumulative exposure index and latency is useful for a better understanding of the cause/effect relationship in asbestos related disease but it could furthermore provide a legal support for the notification and complaint of occupational asbestos related diseases.
Acknowledgment
We thank all departmental nurses and technicians for their support in patients management.
Conflict of Interest
None.
References
- IARC Working Group (2012) Evaluation of Carcinogenic Risks to humans: Arsenic, metals, fibres, and dusts. IARC Monogr Eval Carcinog Risks Hum 100: 11-465.
- Marinaccio A, Binazzi A, Cauzillo G, Chellini E, De Zotti R, et al. (2007) Epidemiological surveillance of malignant mesothelioma cases in italy: incidence and asbestos exposure figures by the italian mesothelioma registry (ReNaM). Epidemiol Prev 31: 23-26.
- Marinaccio A, Scarselli A, Binazzi A, Altavista P, Belli S, et al. (2008) Asbestos related diseases in Italy: an integrated approach to identifie unexpected professional or environmental exposure risks at municipal level. Int Arch Occup Environ Health 81: 993-1001.
- Kazan-Allen L (2015) International ban asbestos secretariat (Ibas): Chronology of national asbestos bans. London: ibas.
- Kameda T, Takahashi K, Kim R, Jiang Y, Movahed M, et al. (2014) Asbestos: use, bans and disease burden in Europe. Bull Word Health Organ 92: 790-797.
- Berman DW, Crump KS (2008) Update of potency factors for asbestos- related lung cancer and mesothelioma. Crit Rev Toxicol 38: 1-47.
- International Agency for Research on Cancer (1987) IARC monograph on the evaluation of carcinogenic risks to human. Lyone IARC S7: 106-111.
- Leong SL, Zainudin R, Kazan-Allen L, Robinson BW (2015) Asbestos in Asia. Respirology 20: 548-555.
- Consensus report (1997) Asbestos, asbestosis, and cancer: the Helsinki criteria for diagnosis and attribution. Scand J Work Environ Health 23: 311-316.
- Bar-Shai A, Tiran B, Topilsky M, Greif J, Fomin I, et al. (2012) Continued progression of asbestos-related respiratory disease after more than 15 years of non-exposure. Isr Med Assoc J 14: 560-565.
- American Thoracic Society (2004) Diagnosis and initial management of nonmalignant diseases related to asbestos. Am J Respir Crit Care Med 170: 691-715.
- Markowitz S (2015) Asbestos-related lung cancer and malignant mesothelioma of the pleura: selected current issues. Semin Respir Crit Care Med 36: 334-346.
- American Thoracic Society (1987) Standardization of spirometry-1987 update. Am Rev Respir Dis 136: 1285-1298.
- Ollier M, Chamoux A, Naughton G, Pereira B, Dutheil F (2014) Chest CT scan screening for lung cancer in asbestos occupational exposure: a systematic review and meta-analysis. Chest 145: 1339-1346.
- Bourgault MH, Gagné M, Valcke M (2014) Lung cancer and mesothelioma risk assessment for a population environmentally exposed to asbestos. Int J Hyg Environ Health 217: 340-346.
- Jeebun V, Stenton SC (2012) The presentation and natural history of asbestos induced diffuse pleural thickening. Occup Med (Lond) 62: 266-268.
- Broaddus VC, Everitt JL, Black B, Kane AB (2011) Non neoplastic and neoplastic pleural endpoints following fiber exposure. J Toxicol Environ Health B Crit Rev 14: 153-178.
- Frost G (2013) The latency period of mesothelioma among a cohort of British asbestos workers (1978-2005). Br J Cancer 109: 1965-1973.
- Park EK, Yates D, Wilson DJ (2014) Lung function profiles among individuals with nonmalignant asbestos-related disorders. Saf Health Work 5: 234-237.
- Miles SE, Sandrini A, Johnson AR, Yates DH (2008) Clinical consequences of asbestos related diffuse pleural thickening: a review. J Occup Med Toxicol 3: 20.
- Magnani C, Agudo A, González CA, Andrion A, Calleja A, et al. (2000) Multicentric study on malignant pleural mesotelioma and non-occupational exposure to asbestos. Br J Cancer 83: 104-111.
- Mastrangelo G, Ballarin MN, Bellini E, Bicciato F, Zannol F, et al. (2009) Asbestos exposure and benign asbestos diseases in 772 formerly exposed workers: dose-response relationship. Am J Ind Med 52: 596-602.
- Nesti M, Adamoli S, Ammirabile F, Ascoli V, Barbieri PG, et al. (2003) Linee Guida Per La Rilevazione E La Definizione Dei Casi Di Mesotelioma Maligno E La Trasmissione Delle Informazioni All’ispesl Da Parte Dei Centri Operativi Regionali. Ispesl Istituto Superiore Per La Prevenzione E La Sicurezza Del Lavoro Dipartimento Di Medicina Del Lavoro Laboratorio Di Epidemiologia E Statistica Sanitaria Occupazionale.
- Marsili D, Terracini B, Santana VS, Ramos-Bonilla JP, Pasetto R, et al. (2016) Prevention of asbestos-related disease in countries Currently Using asbestos. Int J Environ Res Public Health 13: E494.
- Vainio H, Oksa P, Tuomi T, Vehmas T, Wolff H (2016) Helsinki Criteria update 2014: asbestos continues to be a challenge for disease prevention and attribution. Epidemiol Prev 40: 15-19.
- Mastrangelo G, Marangi G, Ballarin MN, Bellini E, De Marzo N, et al. (2013) Post-occupational health surveillance of asbestos workers. Med Lav 104: 351-358.
- Prazakova S, Thomas PS, Sandrini A, Yates DH (2014) Asbestos and the lung in the 21st century: an update. Clin Respir J 8: 1-10.
- Silvestri S, Barbieri PG, Cavariani F (2010) Selezione A- Catalogo dell’uso dell’amianto in comparti produttivi, macchinari impianti ISPESL III Rapporo ReNaM.
- Silvestri S, Costantini AS (2003) Rischio amianto:esposizioni pregresse ed esposizioni attuali. G Ital Med Lav Erg 25: 398-401.
- Cvitanovic S, Znaor L, Konsa T, Ivancevic Z, Peric I, et al. (2003) Malignant and non-malignant asbestos related pleural and lung disease: 10 years follow up study. Croat Med J 44: 618-625.
- Cohen BL (2006) Test of the linear-no thresholod theory: rationale for procedures. Dose Response 3: 369-390.
- Hoshi M, Morimura K, Wanibuchi H, Wei M, Okochi E, et al. (2004) No-observed effect levels for carcinogenicity and for in vivo mutagenicity of a genotoxic carcinogen. Toxicol Sci 81: 273-279.
- Budorf A, Dahhan M, Swuste P (2003) Occupational characteristics of cases with asbestos-related diseases in The Netherlands. Ann Occup Hyg 47: 485-492.
- Koskinen K, Rinne JP, Zitting A, Tossavainen A, Kivekäs J, et al. (1996) Screening for asbestos-induced diseases in Finland. Am J Ind Med 30: 241-251.
- Coviello V, Carbonara M, Bisceglia L, Di Pierri C, Ferri GM, et al. (2002) Mortality in a cohort of asbestos cement workers in Bari. Epidemiol Prev 26: 65-70.
- Jarvholm B (1992) Pleural plaques and exposure to asbestos: a mathematical model. Int J Epidemiol 21: 1180-1184.
- Pfau JC, Serve KM, Noonan CW (2014) Autoimmunity and asbestos exposure. Autoimmune Dis 2014: 782045.
- Boezen HM, Vonk JM, van der Zee SC, Gerritsen J, Hoek G, et al. (2005) Susceptibility to air pollution in elderly males and females. Eur Respir J 25: 1018-1024.
- Walters MS, De BP, Salit J, Buro-Auriemma LJ, Wilson T, et al. (2014) Smoking accelerates aging of the small airway epithelium. Respir Res 15: 94.
- Seljkoff IJ, Hammond EC (1979) Asbestos and smoking. JAMA 242: 458-459.
- Churg A, Tron V, Wright JL (1987) Effects of cigarette smoke exposure on retention of asbestos fibers various morphologic compartments of the guinea pig lung. Am J Pathol 129: 385-393.
- Bledsoe JR, Christiani DC, Kradin RL (2014) Smoking-associated fibrosis and pulmonary asbestosis. Int J Chron Obstruct Pulmon Dis 10: 31-37.
- Roelofs C (2015) Latency attention deficit: Asbestos abatement workers need us to investigate. Am J Ind Med 58: 1231-1234.
Relevant Topics
- Child Health Education
- Construction Safety
- Dental Health Education
- Holistic Health Education
- Industrial Hygiene
- Nursing Health Education
- Occupational and Environmental Medicine
- Occupational Dermatitis
- Occupational Disorders
- Occupational Exposures
- Occupational Medicine
- Occupational Physical Therapy
- Occupational Rehabilitation
- Occupational Standards
- Occupational Therapist Practice
- Occupational Therapy
- Occupational Therapy Devices & Market Analysis
- Occupational Toxicology
- Oral Health Education
- Paediatric Occupational Therapy
- Perinatal Mental Health
- Pleural Mesothelioma
- Recreation Therapy
- Sensory Integration Therapy
- Workplace Safety & Stress
- Workplace Safety Culture
Recommended Journals
Article Tools
Article Usage
- Total views: 12301
- [From(publication date):
August-2016 - Dec 04, 2024] - Breakdown by view type
- HTML page views : 11519
- PDF downloads : 782