Relationship between Cardiac Toxicities and Radiation Dose in Early-Stage Non-Small Cell Lung Cancer Patients Treated with Stereotactic Body Radiotherapy for Tumors Adjacent to the Heart
Received: 22-Mar-2023 / Manuscript No. IJM-23-92516 / Editor assigned: 27-Mar-2023 / PreQC No. IJM-23-92516(PQ) / Reviewed: 10-Apr-2023 / QC No. IJM-23-92516 / Revised: 24-Apr-2023 / Manuscript No. IJM-23-92516(R) / Published Date: 22-May-2023
Abstract
Background and purposes: Stereotactic Body Radiotherapy (SBRT) is the main treatment modality for earlystage NSCLC patients who cannot undergo the operation because of its excellent local control as well as Overall Survival (OS) rates. However, in patients with centrally located tumors, the radiation induced side effects to surrounding normal tissues can limit the treatment outcomes. This study’s goal is to examine the correlations of SBRT with cardiac toxicities and the OS rate in early-stage non-small cell lung cancer patients whose tumors are close to the heart following irradiation.
Methods: Forty-five patients who underwent SBRT with tumors close to the heart (<2 cm) were included. We reviewed dose-volume histograms and selected heart dosimetric parameters, such as maximum and mean dose, percentages of tumor volume receiving >5 Gy, >10 Gy and >20 Gy. The correlation between cardiac toxicities following SBRT, the OS rate, and cardiac dose metrics, clinical factors were assessed by multiple variate analyses using the Cox’s regression model.
Results: The 3-year overall survival rate was 45% with a median follow-up time was 22 months. The following heart dose volume histogram metrics were recorded (median, range): maximum dose (14.2 Gy, 0-79.4 Gy), mean dose (2 Gy, 0-24.7 Gy) and percentage volumes receiving >5 Gy (9.5%, 0%-100%), >10 Gy (0.3%, 0%-95.5%), and >20 Gy (0%, 0%-51.7%). Sixteen (35.5%) patients had a preexisting cardiovascular history, most commonly ischemia or hypertension. Two patients (4.5%) developed grade ≥ 3 cardiac toxicities, one of whom with a history of cardiomegaly died because of heart failure. There was a statistically significant increase in cardiac abnormalities after stereotactic body radiotherapy. We identified no direct relationship between overall survival or cardiac toxicities and any cardiac dose-volume histogram parameters. However, preexisting heart disease was found as a predictor for overall survival (p-value=0.04)
Conclusion: SBRT is safe for treating early-stage NSCLC patients with tumors close to the heart. However, it might increase the risk of cardiac abnormalities among patients with preexisting heart conditions, and this should be carefully considered when implementing stereotactic body radiotherapy.
Keywords
Stereotactic Body Radiotherapy (SBRT); Cardiac toxicities; Non-Small Cell Lung Cancer (NSCLC); Histogram; COVID-19
Abbreviation
ECOG-PS: Eastern Cooperative Oncology Group-Performance Status; maxSUV: Maximum Standardized Uptake Value; SBRT: Stereotactic Body Radiotherapy; Adeno: Adenocarcinoma; SCC: Squamous Cell Carcinoma; Gy: Gray; No. fraction: Number of fraction; BED10 (Gy): Biologically Equivalent Doses are calculated at α/β=10 Gy; EQD2 (Gy): Equivalent Dose in 2-Gy fractions; OS: Overall Survival; LC: Local Control; DVH: Dose Volume Histogram; BED 3 (Gy): Biologically Equivalent Doses are calculated with α/β=3 Gy; EQD2 (Gy): Equivalent Dose in 2-Gy fractions. GTV: Gross Tumor Volume; PTV: Planning Target Volume; SBRT: Stereotactic Body Radiotherapy; ECG: Electrocardiograph; ECOG-PS: Eastern Cooperative Oncology Group-Performance Status; SBRT: Stereotactic Body Radiotherapy; Gy: Gray; max Dose: Maximum heart dose; mean Dose: Mean heart Dose
Introduction
Stereotactic Body Radiotherapy (SBRT) is a principal treatment option for patients diagnosed with early-stage Non-Small Cell Lung Cancer (NSCLC) who cannot suffer from surgical resection. This technique is considered equally to surgery due to its excellent effectiveness on local control and overall survival. SBRT delivers a very high radiation dose in three to five fractions, which results in the biologically effective dose of 100 Gy or more to the tumor with high accuracy and conformality [1].
Advances in radiation delivery systems, immobilization strategies such as Deep Inspiration Breath Holding (DIBH), especially the Image-Guided Radiotherapy (IGRT) have also improved the quality of treatment and minimized radiation toxicities. However, the radiation-induced side effects of surrounding normal tissues, such as the pulmonary parenchyma and heart, remain challenging and can limit the treatment outcomes [2].
There are numerous reports regarding late cardiological complications following thoracic SBRT. Moreover, heart structural abnormalities following chest radiotherapy have been described in breast cancer or Hodgkin’s lymphoma patients. Such changes caused by microscopic histopathological damages are known as Radiation- Induced Heart Disease (RIHD) [3]. These damages may manifest in the pericardium, myocardium, valves, conduction system, or coronary arteries. The RIHD was first reported in phase III, prospective trial of Radiation Therapy Oncology Group (RTOG 0617), in which heart dose-volume parameters were proven as the main predictors for overall survival in advanced-stage NSCLC patients treated with concurrent chemoradiotherapy. This trial showed that increased volumes receiving at least 5 Gy and 30 Gy were strongly associated with a worse prognosis. Additionally, in a recent study discovered that higher radiation doses to the upper parts of the heart (superior vena cava, left atrium) were significantly related to increased non-cancer death. However, they did not clarify whether RIHD was the main reason for these deaths. They hypothesized that the radiation induced damage to the cardiac conducting system could result in abnormal manifestation in heart functional screening tests. Although the heart adverse events were observed and clearly described in many clinical trials, the impact of radiation on the heart in early-stage lung cancer patients who getting radiotherapy as their treatment is not well known, especially for SBRT. Despite strict compliance of the dose constraints in making the radiotherapy plan and technological advances in radiation delivery systems, severe toxicities have been reported and pose a concern for clinicians [4].
Because NSCLC patients at the early stage with tumors located within 2 cm of the heart are at high risk for damage when receiving SBRT, we conducted this retrospective study to investigate the impact of heart radiation dose on the overall survival and cardiovascular toxicity.
Materials and Methods
Patients
Medical records of early-stage non-small cell lung cancer patients who received definitive treatment with SBRT at the Yamanashi university hospital, from 2005 to 2012, were carefully reviewed with permission from the cancer institutional review board [5]. The selected patients were those who had the distance from the tumor to the heart within 2 centimeters, retrievable SBRT plan with the dose-volume histogram, and duration of follow-up was at least 6 months. In the observational period, patients were scheduled to come back to our institution for clinical examination and underwent chest CT scanning with contrast. Patients were indicated for heart disease screening tests (electrocardiography, cardiac ultrasonography, and serum laboratory tests) and examined by cardiologists whenever clinical or para-clinical abnormalities were observed. We also documented information on preexisting heart disease, including major cardiological risk factors (hypertension, smoking, diabetes, and hyperlipidemia) [6].
Patient outcome and cardiac toxicities
The time interval from the first day of SBRT to the last follow-up day or death was defined as overall survival, and the causes of death (cancer or non-cancer) were collected. The cancer cause was determined when the patient developed the status of progressive disease or distant metastasis at the time of death [7]. The common terminology criteria for adverse events (version 4.03) were implemented for grading cardiac toxicity. Any new heart problems that occurred after SBRT were documented as cardiac toxicity, including abnormalities in ECG, pericardial thickening or calcification, pericardial fluid, pericardial effusion, and other conditions (such as cardiac arrhythmia, coronary artery disease and heart failure) [8].
Treatment methods
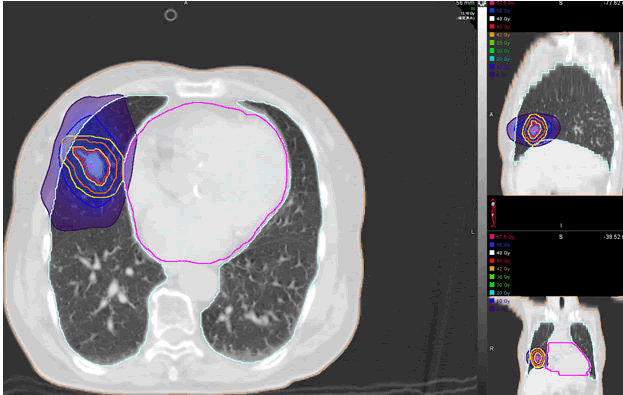
All patients were administered 45 Gy-50 Gy in 4 or 5 fractions. SBRT was performed using our linac system, which is a combination of a linear accelerator with an on-rail CT scanner using a common couch. To verify the reproducibility of the tumor position during the SBRT period, we applied the DIBH method to patients by training them the self-breath-holding technique at inspiration with our selfdeveloped device called Abches [9]. Thoracic CT scanning under DIBH was performed for all patients, and the CT images obtained were put into the treatment planning system. The treatment plans were generated and evaluated by the treatment core team (the attending physician and the medical physicist) and the senior radiation oncologist. The final approved plan was then delivered to the patient. After completing the routine QA process, patients were started treatment within 7 days from the simulation day. An example of a typical SBRT plan for a patient whose tumor near to the heart is illustrated (Figure 2). The heart dose constraints were done based on the criteria of the American Association of Physicists in Medicine (AAMP report Task Group 101) (Figure 1) [10].
DVH retrieval
For our current study, we used patient backup data and reviewed the DVHs and selected the cardiac dosimetric parameters, which includes the maximum dose (max dose), mean dose, and volumetric percentages receiving at least 5 Gy, 10 Gy and 20 Gy (V5, V10, and V20, respectively) [11]. We reviewed the tumor and heart delineations for all patients and edited if necessary based on the RTOG guidelines. The prescribed doses and heart doses were documented and changed into the Biological Equivalent Dose (BED) and 2-Gy fractionation Equivalent Dose (EQD2) using α/β=3 for the heart and 10 for the tumor using a linear-quadratic model [12].
Statistical analysis
Patients’ baseline characteristics, treatment information, outcome, cardiac toxicity, and cardiac DVH dose parameters were reported by descriptive statistics. Estimation of overall survival probability for whole patients was done with Kaplan-Meier method. Differences in cardiac toxicity before and after SBRT were assessed by Chi’s square test with the p-value<0.05 was considered as statistically significance. The correlations of heart dose metrics (max dose, mean dose, V5, V10, and V20) and clinical features (age, sex, comorbidity, performance status, operability, max-SUV, and histology) with OS and cardiac abnormalities were investigated using Spearman’s correlation coefficient, and multivariate analysis was performed using Cox’s regression model. The SPSS version 20 (IBM Corporation, Armonk, NY, USA) was used to do all statistical calculations [13].
Results
Patient clinical characteristics and outcomes
Table 1 summarize the baseline characteristics and outcomes (overall survival) of the entire cohort [14].
| Factors (N=45) | Median (range) or frequency |
|---|---|
| Clinical | |
| Age (years old) | 80.3 (46-89) |
| Gender (Female/Male) | 17/28 |
| Preexisting heart disease (yes/no) | 16/29 |
| ECOG-PS (0/1/2) | 15/7/23 |
| Operability (Operable/Inoperable) | Oct-35 |
| Stage (1a/1b/2a) | 12/13/20 |
| Tumor volume (cc) | 13.4 (1.6-55.2) |
| Distance (cm) from tumor to nearest heart substructure | |
| Whole heart | 1.2 (0-2.0) |
| Left ventricle | 0.9 (0-1.8) |
| Right ventricle | 1.4 (0.1-1.8) |
| Left atrium | 1.5 (0.5-1.9) |
| Right atrium | 1.2 (0-2.0) |
| Ascending aorta | 0.4 (0-1.5) |
| Left pulmonary artery | 1.6 (1.5-1.7) |
| Superior vena cava | 1.2 (0-1.9) |
| Histology (Adeno/SCC/others/no biopsy) | 27/10/3/5 |
| maxSUV | 5.7 (1.2-19.4) |
| SBRT treatment | |
| Prescription points (isocenter/ITV D95/PTV edge) | 18/12/15 |
| Algorithm (Clarkson/convolution/superposition) | 3/9/33 |
| Dose/fraction (Gy) | 12 (6-15) |
| Total dose (Gy) | 52 (49-70) |
| BED10 | 105 (80-150) |
| EQD2 (Gy) | 88 (66.7-125) |
| SBRT technique (3D/ IMRT/ DCAT) | 18/10/17 |
| Outcomes (Overall survival) | |
| Follow-up time (months) | 22.0 (3.8-106.7) |
| Survival (no event/event) | 26/19 |
| 3-year estimate (%) | 45 (%) |
| Cause of death (heart disease/non-heart disease) | 1/18 |
Table 1: Patient clinical and treatment characteristics.
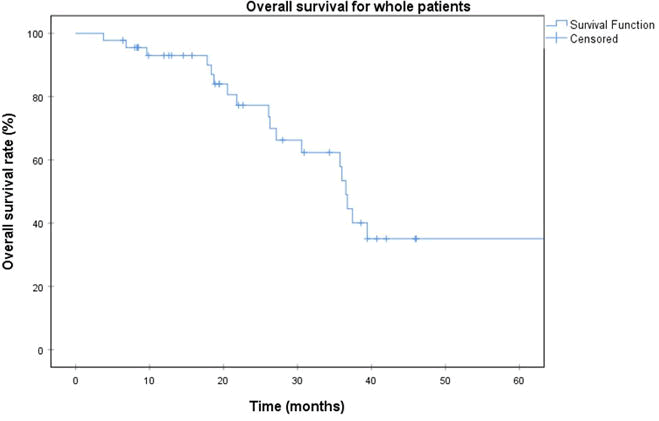
We included 45 patients, 28 men and 17 women, with a median age of 80.3 years (range, 46-89 years). The median tumor volume was 13.4 cm3 (range, 1.6 cm3-55.2 cm3). Sixteen patients had preexisting cardiovascular conditions, mainly hypertension and coronary artery disease. Half of the patients had a performance status index of 0 or 1. The median follow-up was 22 months (range, 3.8-106.7 months). The 3-year survival rate for all patients was 45% (Figure 2) [15].
Heart dose distribution and toxicity
Table 2 reports the cardiac dose-volume histogram parameters with doses converted to the BED and EQD2 to account for different fractionations (α/β was set as 3 Gy in the linear-quadratic model). In BED3 Gy, the median max dose was 71.8 Gy (range, 0 Gy-324.7 Gy), and it was 43.1 Gy (range, 0 Gy-194.8 Gy) in EQD2. The median mean dose in BED3Gy was 7.4 Gy (range, 0 Gy-108.2 Gy), and it was 4.4 Gy (range, 0 Gy-64.9 Gy) in EQD2. The median (range) V5, V10, and V20 were 9.5% (0%-100%), 0.3% (0%-95.5%), and 0% (0%-51.7%), respectively. We did not find any relationship between cardiac dose parameters and OS rate or cardiac toxicity (Table 2).
| Parameter | Median (range) |
|---|---|
| max dose (Gy) | 14.2 (0-79.4) |
| max dose in BED3 (Gy) | 71.8 (0-324.7) |
| max dose in EQD2 (Gy) | 43.1 (0-194.8) |
| mean dose (Gy) | 2 (0-24.7) |
| mean dose in BED2 (Gy) | 7.4 (0-108.2) |
| mean dose in EQD2 (Gy) | 4.4 (0-64.9) |
| V5 (%) | 9.5 (0-100) |
| V10 (%) | 0.3 (0-95.5) |
| V20 (%) | 0 (0-51.7) |
Note: V5, V10, V20: % of volume receiving >5 Gy, >10 Gy and >20 Gy respectively.
Table 2: Heart DVH parameters.
There were no acute cardiac events within 3 months after SBRT. However, two patients developed grade ≥ 3 cardiac adverse events during the follow-up period. Both patients had preexisting heart conditions. One of these patients was still alive with coronary artery disease (myocardial infarction) that required intervention. The other patient had a medical history of cardiomegaly and died of congestive heart failure 3 years after SBRT. The heart DVH statistics of these two patients are reported in detail in Table 3.
| Patient | Final status | Time to occurrence toxicity (months) | Heart toxicity | Prescribed dose (Gy) | Max-dose (Gy) | Mean dose (Gy) | V5Gy (%) | V10Gy (%) | V20Gy (%) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | died | 9.2 | Congestive Heart failure | 55 | 49.1 | 7.7 | 49.3 | 38.1 | 9.6 |
| 2 | alive | 9.7 | Pericardial Effusion | 48 | 1.2 | 0.2 | 0 | 0 | 0 |
Table 3: Dosimetric detail of 2 cases with grade ≥ 3 heart toxicity.
We did observe a significant elevation in cardiac toxicity after SBRT (p<0.05), including abnormalities in ECG, pericardial thickening or calcification, pericardial fluid, and pleural effusion (Table 4). However, we did not observe any correlation between heart DVH metrics and post-SBRT toxicity.
| Cardiac toxicity | Pre-SBRT (%) | Post-SBRT (%) | Chi’s square test |
|---|---|---|---|
| Grade ≥ 3 | 0 | 4.5 | <0.05 |
| Abnormality in ECG | 15.6 | 42.2 | <0.05 |
| Pericardial thickening or calcification | 71.1 | 75.6 | <0.05 |
| Pericardial fluid | 62.2 | 68.9 | <0.05 |
| Pleural effusion | 6.7 | 31.1 | <0.05 |
Table 4: Heart toxicity.
Table 5 shows the results of the multivariate analysis with Cox’s regression model examining potential predictive factors for OS. None of the heart dosimetric parameters were found to be significantly predictive of OS. However, preexisting cardiac conditions were significantly correlated with OS, with a hazard ratio of 3.55 (95% confidence interval, 1.06-11.85, p=0.04) (Table 5).
| Factor | Overall survival | ||
|---|---|---|---|
| Hazard ratio | 95% CI | P-value | |
| Age at SBRT | 1.06 | 0.98-1.14 | 0.133 |
| Gender | 0.24 | 0.03-2.01 | 0.187 |
| Preexisting heart disease | 3.55 | 1.06-11.86 | 0.04 |
| ECOG-PS | 2.13 | 0.69-6.65 | 0.191 |
| Operability | 1.91 | 0.32-11.42 | 0.477 |
| Histology | 1.15 | 0.52-2.55 | 0.722 |
| Gross tumor volume | 1.05 | 0.97-1.12 | 0.22 |
| Max dose | 1.02 | 0.98-1.06 | 0.256 |
| Mean dose | 0.94 | 0.83-1.06 | 0.296 |
| V5Gy (%) | 0.98 | 0.93-1.03 | 0.394 |
| V10Gy (%) | 1.16 | 0.94-1.42 | 0.174 |
| V20Gy (%) | 0.2 | 0.03-1.16 | 0.073 |
Note: V5Gy, V10Gy, V20Gy: % of volume receiving >5 Gy, >10 Gy and >20 Gy respectively.
Table 5: Multi-variate analysis for OS (Cox’s regression).
We did not identify any factors (clinical or heart dose parameters) that were associated with post-SBRT toxicity by univariate or multivariate analyses.
Discussion
Cardiac toxicity following stereotactic body radiotherapy in the treatment of early-stage NSCLC remains a concern among the community of clinical radiation oncologists. There is published evidence for the association between cardiac dose distribution and cardiovascular complications or mortality in advanced stage NSCLC patients receiving concurrent chemoradiation. Similar findings have been observed in patients undergoing lung SBRT. Other authors stated that irradiating heart substructures with high doses may increase the development of heart toxicity. However, whether this kind of relationship truly exists in early-stage NSCLC is unclear. Furthermore, these correlations are not strong enough to change the current clinical recommendations, because physicians are not able to determine which patients are not at risk nor which part of the heart is the most vulnerable to radiation. So, early detection of patients who are at high risk for cardiac toxicities can bring benefits during follow-up, potentially improving patient outcomes. Our objective is to evaluate the associations of heart dose distribution with cardiac events and overall survival rate in patients with tumors that are proximate to the heart. Unfortunately, no correlation between heart dosimetry and OS or cardiac toxicity was found in this study. However, our analysis showed that there was an increase in heart adverse events after SBRT. Moreover, we also identified that preexisting heart conditions could be associated with elevated mortality. Our result may help clinicians to comprehensive the heart toxicity after SBRT for patients with earlystage NSCLC.
Previously published studies showing an association of heart dosimetric parameters with radiation-induced cardiac events and reduced OS in patients with breast cancer, lymphoma, or advanced stage NSCLC experienced standard fractionated radiotherapy. All the patients in our study were at early-stage and treated with SBRT, which is fundamentally different from conventional radiotherapy techniques. In SBRT, a high dose is delivered precisely and conformally to the tumor while maintaining a very low dose to the surrounding tissues. However, in the case of tumors located near the heart, the amount of radiation delivered to the entire heart or to small regions of the heart can be quite high. This could lead to the occurrence of cardiac adverse events, but the data supporting this hypothesis are insufficient. The lack of correlation between heart dose and patient outcome or cardiac toxicity that we observed is similar to the results of other studies. However, our results highlight the need to pay more attention to patients with tumors close to the heart and previous heart conditions, as they may be at increased risk. These patients require careful followup because of the increased potential for developing cardiac events.
Limitations of our study can be pointed out. Firstly, because of a retrospective study, much information was lost during their follow-up. Identifying the exact cause for heart events and the influence of radiation overdose on survival requires further prospective studies. Secondly, the severity of preexisting heart disease or other comorbidities was not fully assessed. In older patients, these factors could affect the quality of SBRT and reduce OS. Finally, although there are reports that radiation dose to the heart substructures may correlate with non-cancer morbidity [14], we did not investigate in more detail the amount of radiation to the cardiac substructures (such as left and right atrium, left ventricle, coronary artery, aorta, and others).
Conclusion
SBRT is still a safe treatment modality for early-stage NSCLC patients with tumor close to the heart. However, patients who have preexisting cardiovascular conditions, might significantly develop the risk of cardiac adverse events after SBRT. Radiation doses to the heart and its substructures show large variability. Cardiac events occurred more frequently in patients with a history of heart problems. At present, the effect of radiation dose on cardiac toxicity is unclear in patients undergoing SBRT for early-stage lung cancer. Longer followup and a larger cohort are needed to assess for late cardiac sequelae.
Acknowledgment
The authors are thankful to all members of the department of radiology, Yamanashi university hospital for providing their data and especially to Ms. Y Watanabe and R Mochizuki for their help in submitting our manuscript.
Funding
None.
Conflict of Interest
There are not any conflicts of interest between the authors.
Ethical Statement
The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
References
- Schonewolf CA, Heskel M, Doucette A, Singhal S, Frick MA, et al. (2019) Five-year long-term outcomes of stereotactic body radiation therapy for operable versus medically inoperable stage i non-small-cell lung cancer: Analysis by operability, fractionation regimen, tumor size, and tumor location. Clin Lung Cancer 20: e63-e71.
[Crossref] [Google Scholar] [PubMed]
- Kann BH, Verma V, Stahl JM, Ross R, Dosoretz AP, e al. (2019) Multi-institutional analysis of stereotactic body radiation therapy for operable early-stage non-small cell lung carcinoma. Radiotherap Oncol 134: 44-49.
[Crossref] [Google Scholar] [PubMed]
- Onishi H, Shirato H, Nagata Y, Hiraoka M, Fujino M, et al. (2011) Stereotactic Body Radiotherapy (SBRT) for operable stage I non-small-cell lung cancer: Can SBRT be comparable to surgery? Int J Radiat Oncol Biol Phys 81: 1352-1358.
[Crossref] [Google Scholar] [PubMed]
- Speirs CK, DeWees TA, Rehman S, Molotievschi A, Velez MA, e al. (2017) Heart dose is an independent dosimetric predictor of overall survival in locally advanced non-small cell lung cancer. J Thoracic Oncol 12: 293-301.
[Crossref] [Google Scholar] [PubMed]
- Dess RT, Sun Y, Matuszak MM, Sun G, Soni PD, et al. (2017) Cardiac events after radiation therapy: Combined analysis of prospective multicenter trials for locally advanced non-small-cell lung cancer. J Clin Oncol 35: 1395.
[Crossref] [Google Scholar] [PubMed]
- Tembhekar AR, Wright CL, Daly ME (2017) Cardiac dose and survival after stereotactic body radiotherapy for early-stage non-small-cell lung cancer. Clin Lung Cancer 18: 293-298.
[Crossref] [Google Scholar] [PubMed]
- Reshko LB, Kalman NS, Hugo GD, Weiss E (2018) Cardiac radiation dose distribution, cardiac events and mortality in early-stage lung cancer treated with Stereotactic Body Radiation Therapy (SBRT). J Thoracic Disease 10: 2346.
[Crossref] [Google Scholar] [PubMed]
- Anderson JD, Hu J, Li J, Schild SE, Fatyga M (2021) Impact of cardiac dose on overall survival in lung Stereotactic Body Radiotherapy (SBRT) compared to conventionally fractionated radiotherapy for Locally Advanced Non-Small Cell Lung Cancer (LA-NSCLC). J Cancer Therap 12: 409.
[Crossref] [Google Scholar] [PubMed]
- Darby SC, Ewertz M, McGale P, Bennet AM, Blom-Goldman U, et al. (2013) Risk of ischemic heart disease in women after radiotherapy for breast cancer. New England J Med 368: 987-998.
[Crossref] [Google Scholar] [PubMed]
- van den Bogaard VA, Ta BD, van der Schaaf A, Bouma AB, Middag AM, et al. (2017) Validation and modification of a prediction model for acute cardiac events in patients with breast cancer treated with radiotherapy based on three-dimensional dose distributions to cardiac substructures. J Clin Oncol 35: 1171.
[Crossref] [Google Scholar] [PubMed]
- van Nimwegen FA, Schaapveld M, Cutter DJ, Janus CP, Krol AD, et al. (2015) Radiation dose-response relationship for risk of coronary heart disease in survivors of Hodgkin lymphoma. J Clin Oncol 34.
[Crossref] [Google Scholar] [PubMed]
- Bradley JD, Rebecca P, Ritsuko K, Gregory M, George B, e al. (2015) Standard-dose versus high-dose conformal radiotherapy with concurrent and consolidation carboplatin plus paclitaxel with or without cetuximab for patients with stage IIIA or IIIB non-small-cell lung cancer (RTOG 0617): A randomised, two-by-two factorial phase 3 study. Lancet Oncol 16: 187-199.
- Stam B, Peulen H, Guckenberger M, Mantel F, Hope A, et al. (2017) Dose to heart substructures is associated with non-cancer death after SBRT in stage I-II NSCLC patients. Radiothera Oncol 123: 370-375.
[Crossref] [Google Scholar] [PubMed]
- Onishi H, Kuriyama K, Komiyama T, Tanaka S, Sano N, et al. (2003) A new irradiation system for lung cancer combining linear accelerator, computed tomography, patient self-breath-holding, and patient-directed beam-control without respiratory monitoring devices. Int J Radiat Oncol Biol Phys 56: 14-20.
[Crossref] [Google Scholar] [PubMed]
- Onishi H, Kawakami H, Marino K, Komiyama T, Kuriyama K, et al. (2010) A simple respiratory indicator for irradiation during voluntary breath holding: A one-touch device without electronic materials. Radiology 255: 917-923.
[Crossref] [Google Scholar] [PubMed]
Citation: Nguyen NN, Vu HN , Nguyen TT, Tran QV, Nguyen VC, et al. (2023) Relationship between Cardiac Toxicities and Radiation Dose in Early-Stage Non-Small Cell Lung Cancer Patients Treated with Stereotactic Body Radiotherapy for Tumors Adjacent to the Heart. Int J Inflam Cancer Integr Ther 10: 230.
Copyright: © 2023 Nguyen NN, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Usage
- Total views: 946
- [From(publication date): 0-2023 - Feb 02, 2025]
- Breakdown by view type
- HTML page views: 861
- PDF downloads: 85