Reducing Postoperative Opioid Consumption by Adding Transdermal Fentanyl Patches to Multimodal Analgesia after Breast Cancer Surgery
Received: 07-Aug-2018 / Accepted Date: 10-Aug-2018 / Published Date: 18-Aug-2018 DOI: 10.4172/2167-0846.1000326
Keywords: Breast cancer surgery; Transdermal fentanyl patch (TDF); Postoperative pain; VAS scale
Introduction
Breast cancer is by far the world's most common cancer among women and in Egypt, it represents 32% of all cancers among females [1,2]. Breast cancer surgeries are associated with moderate-to-severe pain on the first day after surgery (median score of 7 on the Numeric Rating Scale (NRS) [3]. Despite the increased awareness of pain management, postoperative pain is reported by about 80% of surgical patients [4]. Poorly controlled acute postoperative pain may result in a range of detrimental acute and chronic effects (i.e., adverse physiologic responses, delayed long-term recovery and chronic pain) [5]. With good analgesic treatment, pain intensity generally declines from moderate or severe to mild levels after the first 24-48 h after surgery [6]. Multimodal analgesia aims to get optimum effectiveness from the different agents in low dosages in order to minimize side effects from each analgesic. This important concept employs the theory that agents with different mechanisms of analgesia may have synergistic effects in treating acute pain [7]. During postoperative period, strong opioids analgesics may be used for relief of acute pain as it have high efficacy [8]. Intravenous (IV) rout of administration is the most common route used during the early postoperative period. In such cases, a multimodal drugs approach must be employed. This may include the administration of opioids, NSAIDS, and other adjuvant if needed in order to optimize acute pain control in the immediate postoperative period as a bridge till the patient start oral medications [9,10].
Fentanyl is a synthetic opioid with low molecular weight that has high potency analgesic effect specially if through intravenous route and its analgesic potency is 50 to 100 times more than morphine. Due to its small molecule weight and high lipid solubility, fentanyl is a good choice for transdermal use [11]. The advantages of patch are; better flexibility, skin conformability, and produces linear fentanyl dose kinetics with negligible dose loading [12], and according to the pharmaceutical company is indicated for management of persistent moderate to severe pain [13]. Stress, whatever physical or emotional like pain, activates neurons that secrete corticotropin-releasing hormone, which results in higher plasma cortisol levels. Prolactin is also released in response to stressor stimuli, although its exact role in the response to stress is not known [14-16]. The aim of this study was to examine the efficacy and safety of adding Transdermal fentanyl patch (TDF) to multimodal analgesia in controlling acute postoperative pain after modified radical mastectomy if applied 12 hours prior surgery.
Patients and Methods
This randomized, blinded, study was conducted after approval of local ethics committee of South Egypt Cancer Institute, Assiut University, Assiut-Egypt, and registered at www.clinicaltrials.gov at no.: “NCT03051503”. After obtaining written informed consent, Sixty four adult female patients (21-70 years old) ASA II with cancer breast, were scheduled for elective modified radical mastectomy.
Exclusion criteria were ASA III, VI, history of drug abuse, emergency, extremes of ages, pregnancy and mentally retarded patient, who cannot use PCA pump nor how to evaluate their own pain level.
Two days before surgery, preoperative data were collected including; demographic data, medical history, physical examination and results of routine laboratory investigations. One day before surgery, all enrolled patients were taught how to use Visual Analog Scale (VAS) to evaluate their own pain level. VAS scored from 0-10 (0=no pain and 10=worst pain ever) also how to use the patient controlled analgesia (PCA) device (Abbott Pain Management Provider. S. No: 96450292. Abbott Laboratory, North Chicago. IL: 60064, USA)®. All Patients were classified randomly into two groups (32 patients each). We used opaque sealed envelopes which contain computer generated schedule as a method of randomization, and the envelopes were sequentially numbered and opened before application of anesthetic plan. Premedication drugs given to all patients includes; midazolam 0.05 mg/kg and ranitidine 50 mg.
After shifting the patient to the operative theatre, basic monitoring was attached and peripheral venous line was established then an infusion of lactated ringer solution started.
Control group (No.=32)
Modified radical mastectomy was performed under standard general anaesthesia and postoperative analgesia was provided through intravenous patient controlled analgesia (PCA) for 48 hours postoperatively.
TDE group (No.=32)
Modified radical mastectomy was done under standard general anaesthesia and additionally Transdermal fentanyl patch 50 μg/h was applied 12 hours prior to the surgery by the duty anaesthesiologist who just placed the patch and fixed with plaster to prevent displacement and also labelled it with date and time of affixing. Postoperative analgesia was provided through PCA for 48 hours postoperatively
Standard general anaesthesia
Pre-oxygenation for 3 minutes, then intravenous propofol 1.5- 2 mg/kg induced with fentanyl 2 μg/kg administered over min. Tracheal intubation was facilitated by neuromuscular blocking agent (cisatracurium 0.15 mg/kg). All patients received ketorolac (30 mg) and Paracetamol (1 gm), I.V. after induction as pre-emptive analgesia.
Anesthesia maintenance was done by sevoflurane 1-1.5 MAC, cisatracurium 0.03 mg/kg given when indicated. All patients were mechanically ventilated aiming to maintain ETCO2 between 35-40 mmHg. The inspired oxygen fraction (FIO2) was 0.5 using oxygen-andair mixtures. At the end of surgery neuromuscular block was reversed in all patients using neostigmine 0.05 mg/kg and atropine 0.02 mg/kg and extubation was done in the operating room when patients met the following criteria: hemodynamic stability, adequate muscle strength, full consciousness, adequate ventilation breathing rate: 10 to 30 breaths/ min and PaCO2, 30 to 45 mmHg).
All patients received post-operative ketorolac 30 mg/12 hours, Paracetamol 1 gm/8 hours and, Morphine was given for rescue analgesia via PCA which was adjusted as following; 1 m / dose with lockout interval of 10 min with no background infusion. The analgesic regimen was adjusted to achieve a visual analog scale scores less than 3. Intra-operatively, patients of both groups were followed up for vital signs (heart rate, mean blood pressure) and the mean reading every one hour was recorded.
Post-operative: All patients were admitted to surgical department and beside routine follow up, the following were recorded:
• HR and MAP were recorded for 24 hours
• Visual analogue scale (0-10) where 0=no pain 10=worst pain ever -every 4 hours for 2 days- for pain measurement, and total postoperative morphine consumption was calculated.
• Ramsay Sedation Score was assessed one day postoperatively by 5 points as following 0=aware -1=drowsy -2=asleep/easily respond to verbal command -3=asleep/difficulty responding to verbal command -4 = asleep/no respond to verbal command.
• Nausea and vomiting scores 0=none, 1=mild, 2=moderate and 3=severe.
• Pruritus; Present=1 or Absent=0.
• Respiratory depression (decrease oxygen saturation ≥ 90% or respiratory rate less than 8) were recorded post-operatively.
• Cortisol and prolactin serum levels at immediately post-operative, 1 hour and 24 hours post-operative.
Statistical Analysis
The sample size was calculated using Epi Info software version 8 (CDC, 2014)®. With total morphine consumption as the primary outcome to achieve a power of 80% to detect an effect size of 0.8 in the outcome measures of interest, assuming a type I error of 0.05 and therefore, it was estimated that minimum sample size of 32 patients in each study group.
All analyses after cleaning by EXCEL® program were performed using the SPSS 21® software. Categorical data were described as number and percent (N, %), where continuous data described as mean and standard deviation (Mean, SD). Mann–Whitney test were used to compare between two groups while Chi square test was used for qualitative data. Where compare between continuous data by t-test. P was considered significant if 60.05 at confidence interval 95%.
Results
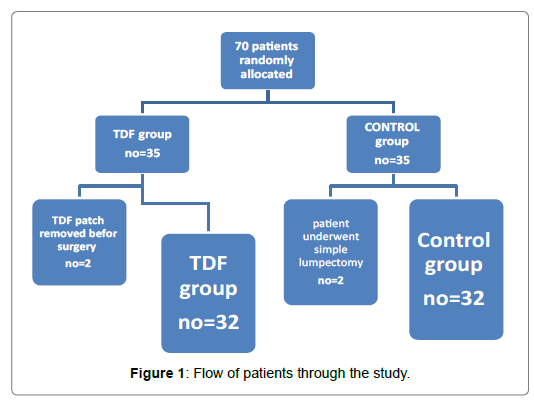
In this study; seventy adult female patients enrolled to do modified radical mastectomy and randomly classified into 2 groups (35 patients in each group), sixty four patients of them were finally analysed, the TDF Group (n=32) and control Group (n=32).
Figure 1 illustrates the flow of the patients through the study. The demographic data of the patients were similar between groups (Table 1).
| Control group (NO.=32) |
TDF group (NO.=32) |
P. value | ||||
|---|---|---|---|---|---|---|
| Age (years) | ||||||
| Range | 31-69 | 28-70 | 0.184 | |||
| Mean ± SD | 54.3 ± 6.1 | 57 ± 9.14 | ||||
| Weight (kg) | ||||||
| Range | 60-86 | 54-84 | 0.259 | |||
| Mean ± SD | 70.5 ±7.35 | 68.13 ± 8.68 | ||||
| Height (cm) | ||||||
| Range | 150-180 | 152-178 | 0.055 | |||
| Mean ± SD | 167.13 ± 6.7 | 163.77 ± 6.61 | ||||
| BMI (kg /m2) | ||||||
| Range | 18.73-33.3 | 17.92-34.96 | 0.794 | |||
| Mean ± SD | 25.37 ± 3.46 | 25.5 ± 3.7 | ||||
| Site of surgery | ||||||
| Right | 17 | 14 | 0.844 | |||
| Left | 15 | 18 | ||||
| Duration of anesthesia (minutes) | ||||||
| Range | 121-169 | 115-177 | 0.738 | |||
| Mean ± SD | 148.13 ± 6.7 | 139.77 ± 7.61 | ||||
Data are expressed as mean ± SD, TDF=Trans dermal fentanyl group. BMI=body mass index. P. value<0.05 considered statistically significant.
Table 1: Demographic data of both groups.
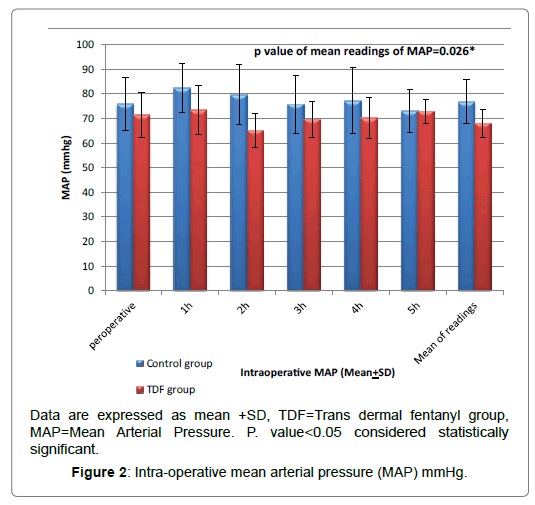
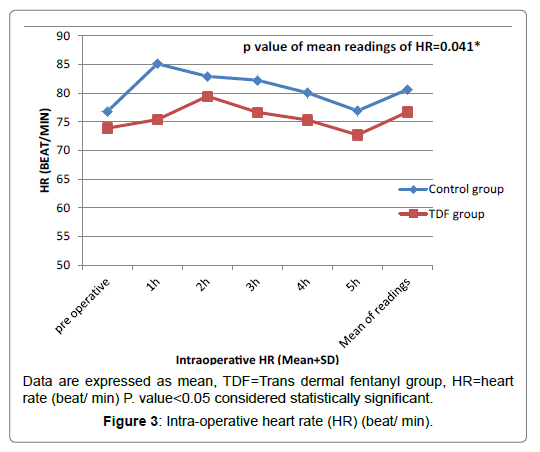
Regarding intra operative MAP and heart rate, there was statistical significant reduction in patients of TDF group in comparison to control group patients at intra operative specially 1st and 2nd hours (P ≤ 0.05), but the baseline readings there were no significant differences (P>0.05) as shown in the Figures 2 and 3.
Post-operative heart rate and MAP were reduced significantly in patients of TDF group compared to control group patients in the early postoperative period (P>0.01) as shown in Table 2.
| Control Group | TDF Group | P. value | |||
|---|---|---|---|---|---|
| Range | Mean ± SD | Range | Mean ± SD | ||
| HR (bpm) 0.5 h |
60-106 | 72.13 ± 9.61 | 65-108 | 80.33 ± 9.97 | 0.002** |
| 1 h | 60-110 | 70.6 ± 10.13 | 65-110 | 78 ± 10.65 | 0.019* |
| 2 h | 60-95 | 75.27 ± 8.2 | 63-106 | 79.8 ± 11.77 | 0.089 |
| 4 h | 60-99 | 78.37 ± 10.42 | 65-103 | 82.53 ± 8.91 | 0.101 |
| 8 h | 69-103 | 81.53 ± 8.01 | 60-100 | 82.8 ± 9.76 | 0.586 |
| 12 h | 66-108 | 82.3 ± 9.1 | 70-97 | 83 ± 7.76 | 0.751 |
| 24 h | 71-98 | 83.07 ± 6.73 | 69-100 | 82.67 ± 7.49 | 0.449 |
| MAP (mmhg) | |||||
| 0.5 h | 70-100 | 87.33 ± 7.96 | 70-95 | 80 ± 7.8 | 0.001** |
| 1 h | 70-110 | 85.5 ± 11.17 | 70-90 | 71.83 ± 7.48 | 0.006** |
| 2 h | 70-110 | 87.5 ± 9.63 | 70-100 | 84.83 ± 7.13 | 0.228 |
| 4 h | 80-110 | 89.17 ± 9.01 | 70-100 | 86.67 ± 9.86 | 0.309 |
| 8 h | 70-105 | 90.5 ± 8.74 | 70-110 | 91 ± 7.81 | 0.816 |
| 12 h | 70-110 | 88.33 ± 8.24 | 70-115 | 90.67 ± 10.06 | 0.330 |
| 24 h | 75-100 | 86.5 ± 6.97 | 70-105 | 91.83 ± 7.6 | 0.140 |
Data expressed as (Mean ± SD) and range TDF=Trans dermal fentanyl group, MAP=Mean Arterial Pressure. HR=heart rate, bpm=beats/ min. P. value<0.05 considered statistically significant.
Table 2: Postoperative heart rate (beats/ min) and MAP (mmhg).
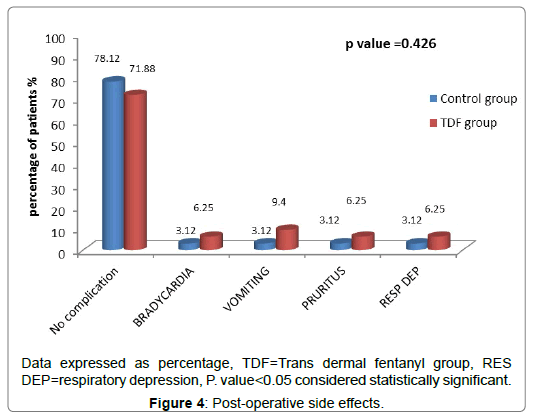
And, there were significant decreased in both VAS scores (p<0.05) and the total amount of postoperative morphine consumption (7.43 ± 4.39) in TDF group in comparison to control group (13.47 ± 4.73) as shown in Tables 3 and 4 without significant change in side effects (Figure 4), except sedation scores, which was statistically increased but clinically not effective, in early post-operative hours (Table 5).
| Post-operative VAS | Control group | TDF group | P. value | ||
|---|---|---|---|---|---|
| Range | Mean ± SD | Range | Mean ± SD | ||
| VAS at 0 h | 1 - 3 | 2.1 ± 1 | 1 - 2 | 1.8 ± 0.9 | 0.009* |
| VAS at 4 h | 1 - 4 | 2.4 ± 0.9 | 1 - 2 | 1.9 ± 0.5 | 0.005* |
| VAS at 8 h | 1 - 4 | 2 ± 0.5 | 1 - 3 | 2.4 ± 0.5 | 0.019 |
| VAS at 12 h | 2 - 3 | 3 ± 0.8 | 1 - 3 | 2.4 ± 0.8 | 0.016 |
| VAS at 16 h | 1 - 3 | 2.5 ± 0.8 | 1 - 3 | 2.4 ± 1.1 | 0.577 |
| VAS at 20 h | 1 - 4 | 2.5 ± 0.9 | 1 - 3 | 2.3 ± 0.7 | 0.527 |
| VAS at 24 h | 2 - 4 | 3.2 ± 1 | 2 - 3 | 2.7 ± 0.9 | 0.158 |
| VAS at 32 h | 2 - 4 | 3.1 ± 0.8 | 1 - 3 | 2.7 ± 1.1 | 0.177 |
| VAS at 40 h | 1 - 4 | 2.5 ± 0.9 | 1 - 3 | 2.3 ± 0.7 | 0.527 |
| VAS at 48 h | 1 - 3 | 2.4 + 0.6 | 1 - 3 | 2.6 ± 0.9 | 0.319 |
Data expressed as (Mean ± SD) and range TDF=Trans dermal fentanyl group, P. value<0.05 considered statistically significant.
Table 3: Post-operative VAS scores.
| Control Group | TDF Group | P. value | |||
|---|---|---|---|---|---|
| Range | Mean ± SD | Range | Mean ± SD | ||
| Total P.O. morphine consumption (mg) | 0-16 | 9.47 ± 2.73 | 0-9 | 4.43 ± 2.39 | 0.001** |
Data expressed as (Mean ± SD) and range TDF=Trans dermal fentanyl group, P. value<0.05 considered statistically significant.
Table 4: Total post-operative morphine consumption.
| Post-operative sedation score |
Control group | TDF group | P. value | ||
|---|---|---|---|---|---|
| Range | Mean | Range | Mean | ||
| 0 h | (1-2) | 2 | (1-3) | 2 | 0.09* |
| 4 h | (1-1) | 1 | (1-2) | 2 | 0.00* |
| 8 h | (1-1) | 1 | (1-2) | 2 | 0.956 |
| 12 h | (1-1) | 1 | (1-2) | 1 | 0.743 |
| 16 h | (1-1) | 1 | (1-2) | 1 | 0.548 |
| 20 h | (1-1) | 1 | (1-2) | 1 | 0.910 |
| 24 h | (1-1) | 1 | (1-2) | 1 | 0.956 |
Data expressed as (Mean and range). TDF=Trans dermal fentanyl group, P. value<0.05 considered statistically significant.
Table 5: Post-operative sedation score.
Finally, levels of prolactin and cortisol hormones were significantly decreased in TDF group indicating less stress and better pain control as shown in Tables 6 and 7.
| Prolactin level | Control group | TDF group | P. value |
|---|---|---|---|
| Immediately | |||
| Range | 3-140 | 3-140 | 0.386 |
| Mean ± SD | 38 ± 42.8 | 42.4 ± 41.4 | |
| After 1 h | |||
| Range | 4-156 | 4-125 | <0.001** |
| Mean ± SD | 79.9 ± 50.1 | 31.6 ± 25.7 | |
| After 24 h | |||
| Range | 4-140 | 3-105 | 0.009** |
| Mean ± SD | 65.8 ± 42.1 | 28.3 ± 22.1 |
Data expressed as (Mean ± SD) and range TDF=Trans dermal fentanyl group, P. value<0.05 considered statistically significant.
Table 6: Prolactin levels in the studied groups (ng/ml).
| Cortisol level | Control group | TDF group | P. value |
|---|---|---|---|
| Immediately | |||
| Range | 80-700 | 60-800 | 0.765 |
| Mean ± SD | 289.2 ± 206.3 | 277 ± 203.4 | |
| After 1 h | |||
| Range | 140-1100 | 90-800 | 0.001** |
| Mean ± SD | 433.7 ± 236.6 | 298.5 ± 65.0 | |
| After 24 h | |||
| Range | 100-950 | 80-710 | 0.047* |
| Mean ± SD | 305.2 ± 187.5 | 257.3 ± 163.2 |
Data expressed as (Mean ± SD) and range. TDF=Trans dermal fentanyl group, P. value<0.05 considered statistically significant.
Table 7: Cortisol levels in the studied groups (micg/dl).
Discussion
Surgery remains the first choice in the treatment of solid neoplastic tumours including breast tumours [17]. And according to McCaffery et al. who showed that, over 50% of surgical patients experienced inadequate pain relief following surgery with negative physiological and psychological consequences [18]. Fentanyl has the following advantages; high lipophilicity, has a short duration of action with lower incidence of side effects, and less risk of respiratory depression make it good choice for acute pain management [7]. The transdermal fentanyl patch (TDF) is a skin-patch opioid that releases dose-dependent fentanyl into the bloodstream in a steady manner [19]. It provides a stable plasma level similar to intravenous (IV) use. TDF has a slow onset and this is why it is commonly used for chronic pain management [20]. This clinical trial showed that patients of TDF group had better analgesia during first hours after surgeries in comparison to control group with good control of both intra and post-operative HR and MAP and tolerated side effects except sedation scores which were significantly high. The total doses of post-operative morphine consumption were significantly lower in the TDF than in the control group.
Recently, patient-controlled analgesia (PCA) systems have been established as a standard treatment for moderate to severe postoperative pain [21], instead of traditionally intramuscular (IM) or intravenous (IV) boluses of opioid analgesics and up to 30% of patients given IM or IV analgesia report severe post-operative pain, reducing to around 10% in patients treated post operatively with PCA systems. Also it may lead to analgesic overdose and toxicity beside these methods are invasive, requiring a needle for administration, introducing the risks of needle-stick injury and infection to the patient and hospital personnel [22]. Our study investigated the effectiveness of incorporating the application of low dose transdermal fentanyl patch into the multimodal analgesia model and its effectiveness in achieving maximum patient comfort with minimum complications. Sebel et al. described transdermal fentanyl patch for first time in 1987 [23], and in fact fentanyl was the first opioid commercially available for transdermal administration. The Food and Drug Administration (FDA) has granted limited approval of its use for patients complaining of chronic cancer pain [24]. Regarding transdermal fentanyl use in the management of acute pain like this study, Gourlay et al. [25], approved that the efficacy of transdermal fentanyl in the treatment of acute pain following abdominal surgery. Also, Lehmann et al. [26], who concluded that patients who received transdermal fentanyl required significantly less additional fentanyl and reported less pain than patients in the placebo group following major urological operations.
According to Van Bastelaere et al. [27], transdermal fentanyl patches were convenient and its use is easy because each patch can be left in place for 3 days with stable plasma fentanyl concentrations. For these reasons, he selected the transdermal fentanyl patch for his study of post-operative pain management following total knee arthroplasty (TKA). In agreement with us, Minville et al. [28] who found that a 50 μg/hr TDF, placed 10 h preoperatively, was very effective in decreasing both the pain severity and the consumption of rescue morphine in patients undergoing total hip arthroplasty for for 24 hours postoperative. Also, Abrisham et al. [29], reported that two 25 μg/hr TDFs (which equal 50 μg/hr TDF), which were placed simultaneously on the lateral chest wall approximately 12 hours preoperatively, resulted in effective pain relief and decreased the total post-operative morphine consumption during the first 72 hours after total knee arthroplasty (TKA) surgery without adverse side effects. In post-operative cancer patients, Osipova et al. studied the effect of preoperative TDF in thoracoabdominal oncological surgery for prevention and treatment of acute post-operative pain syndromes, and concluded that TDF prevent early post-operative acute opioid tolerance and hyperalgesia. Increasing the benefit of TDF use in multimodal analgesia with NSAID and this confirm our findings [30].Regarding side effects of TDF, many studies have concluded that; we should pay attention to the side effects associated with the use of the transdermal fentanyl patch specially in opioid naive patients. [31]. Sandler et al. [32], who reported that a TDF 75 μg/hr when used in patients undergoing abdominal hysterectomy, was associated with a high incidence of respiratory depression, requiring intensive monitoring and oxygen supplementation. Also Van Bastelaere et al. [27], who noticed the efficacy of transdermal fentanyl for postoperative pain relief following orthopaedic surgery, but reported that intense respiratory depression was sometimes seen in some patients.
According to Apfel et al. [33], the incidence of post-operative nausea and vomiting (PONV) is high when he used TDF 75 μg/hr in his study. This is mainly explianed by administration of emetogenic stimuli, such as volatile anaesthetics and high dose fentanyl (75 μg/hr). So we used in our study TDF patch in concentration of 50 μg/hr instead of 75 μg/ hr to decrease PONV. We preferred to put TDF patch 12 hours before surgery to get steady plasma level in Sevarino et al found significantly better analgesia with 50 μg/hr fentanyl patches as compared to the 25 μg/hr patch, but the patch was place only one hour before the start of surgery and was the sole analgesic used. This was why we used the 50 μg/hr fentanyl patch and found it to provide adequate pain relief [34]. A prospective multi-center randomized controlled trial comparing the fentanyl transdermal to IV PCA morphine in 650 patients demonstrated identical patient global assessment of pain control, with over 80% of patients reporting good or excellent pain relief in both treatment arms following major abdominal or orthopedic surgery, with similar pain intensity scores and side-effects reported in both groups [35]. Similar satisfaction and pain intensity score reporting was repeated in further active-comparator [36,37].
Several lines of evidence suggest that stress is characterized by increased levels of cortisol and inhibits NK cell activity. In fact, these are the cells most susceptible to the effects of cortisol, and their activity is considered to be a reliable indicator of the cell immunity suppression caused by stress. Based on these lines of evidence, we were interested in determining whether the presence of post mastectomy pain is associated with increased these stress hormones as an objective tool of assessment. So decrease in the levels of these hormones as in our study confirm the fact of better pain control in TDF group [38,39].
Conclusion
Applying Transdermal fentanyl patch (TDF) 50 μg/hr, 12 hours prior surgery as a part of multimodal analgesia to control acute postoperative pain after modified radical mastectomy was associated with less stress response, better pain control and decreased total amount of postoperative morphine consumption.
References
- Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, et al. (2015) Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 136: e359-386.
- Ibrahim AS, Khaled HM, Mikhail NN, Baraka H, Kamel H (2014) Cancer incidence in Egypt: Results of the national population-based cancer registry program. J Cancer Epidemiol 2014: 1-18.
- Apfelbaum JL, Chen C, Mehta SS, Gan TJ (2003) Postoperative pain experience: Results from a national survey suggest postoperative pain continues to be undermanaged. Anesth Analg 97: 534-540.
- Oderda G (2012) Challenges in the management of acute postsurgical pain. Pharmacotherapy 32: S6-S11.
- Kehlet H, Dahl JB (1993) The value of multimodal or balanced analgesia in the postoperative pain treatment. Anesth Analg 77: 1048-1056.
- Vadivelu N, Mitra S, Narayan D (2010) Recent advances in postoperative pain management. Yale J Biol Med 83: 11-25.
- Dolin SJ, Cashman JN, Bland JM (2002) Effectiveness of acute postoperative pain management: I. Evidence from published data. Br J Anaesth 89: 409-423.
- Harding G, Vallow S, Leidy NK, Olson W, Hewitt DJ, et al. (2007) Ease of care with patient controlled analgesia systems: questionnaire development and validation. J Adv Nurs 59: 530-541.
- Kornick CA, Santiago-Palma J, Khojainova N, Primavera LH, Payne R, et al. (2001) A safe and effective method for converting cancer patients from intravenous to transdermal fentanyl. Cancer 92: 3056-3061.
- Chelly JE, Grass J, Houseman TW, Minkowitz H, Pue A (2004) The safety and efficacy of a fentanyl patient-controlled transdermal system for acute postoperative analgesia: A multicenter, placebo-controlled trial. Anesth Analg 98: 427-33.
- Grond S, Hall J, Spacek A, Hoppenbrouwers M, Richarz U, et al. (2007) Iontophoretic transdermal system using fentanyl compared with patient-controlled intravenous analgesia using morphine for postoperative pain management. Br J Anaesth 98: 806-815.
- Park JH, Kim JH, Yun SC, Roh SW, Rhim SC, et al. (2011) Evaluation of efficacy and safety of fentanyl transdermal patch (Durogesic®D-TRANS) in chronic pain. Acta Neurochir 153: 181-190.
- Chrousos GP, Elenkov IJ (2000) Interactions of the endocrine and immune systems. In: DeGroot LJ, Jameson JL (Editors), Endocrinology. Academic Press, New York, pp 571-586.
- Handa RJ, Weiser MJ (2014) Gonadal steroid hormones and the hypothalamo-pituitary-adrenal axis. Front Neuroendocrinol 35: 197-220.
- Slattery DA, Neumann ID (2008) No stress please! Mechanisms of stress hyporesponsiveness of the maternal brain. J Physiol 586: 377-385.
- Bolin ED, Harvey NR, Wilson SH (2015) Regional Anesthesia for breast surgery: Techniques and benefits. Curr Anesthesiol Rep 5: 217-224.
- McCaffery M, Ferrell BR (1997) Nurses’ knowledge of pain assessment and management: How much progress have we made? J Pain Symptom Manage 14: 175-188.
- Minkowitz HS, Gruschkus SK, Shah M, Raju A (2014) Adverse drug events among patients receiving postsurgical opioids in a large health system: risk factors and outcomes. Am J Health Syst Pharm 71: 1556-1565.
- Davis MP (2006) Management of cancer pain: Focus on new opioid analgesic formulations. Am J Cancer, 5: 171-82.
- Harding G, Vallow S, Leidy NK, Olson w, Hewitt DJ, et al. (2007) Ease of care with patient controlled analgesia systems: Questionnaire development and validation. J Adv Nurs, 59: 530-541.
- Chelly JE, Ben-David B, Williams BA, Kentor ML (2003) Anesthesia and postoperative analgesia: outcomes following orthopedic surgery. Orthopedics 26 (8 Suppl): s 865-s 871.
- Sebel PS, Barrett CW, Kirk CJ, Heykants J (1987) Transdermal absorption of fentanyl and sufentanil in man. Eur J Clin Pharmacol 32: 529-531.
- Gupta H, Babu RJ (2013)Transdermal delivery: Product and patent update. Recent Pat Drug Deliv Formul. 7: 184-205.
- Gourlay GK, Kowalski SR, Plummer JL, Cherry DA, Szekely SM, et al. (1990)The efficacy of transdermal fentanyl in the treatment of postoperative pain: A double-blind comparison of fentanyl and placebo systems. Pain 40: 21-28.
- Lehmann KA, Einnolf C, Eberlein HJ, Nagel R (1990) Transdermal fentanyl for the treatment of pain after major urological operations. A randomized double-blind comparison with placebo using intravenous patient-controlled analgesia. Eur J Clin Pharmacol 41: 17-21.
- Van Bastelaere M, Rolly G, Abdullah NM (1995) Postoperative analgesia and plasma levels after transdermal fentanyl for orthopedic surgery: Double-blind comparison with placebo. J Clin Anesth 7: 706.
- Minville V, Lubrano V, Bounes V, Pianezza A, Rabinowitz A, et al. (2008) Postoperative analgesia after total hip arthroplasty: Patient-controlled analgesia versus transdermal fentanyl patch. J Clin Anesth. 20: 280-283.
- Abrisham JSM, Ghahramani R, Heiranizadeh N, Kermani-Alghoraishi M, Ayatollahi V, et al. (2014) Reduced morphine consumption and pain severity with transdermal fentanyl patches following total knee arthroplasty. Knee Surg Sports Traumatol Arthrosc 22: 1580-1584.
- Osipova NA, Petrova VV, Lastukhin AV, Kudriavtsev SB, Vashakmadze LA, et al. (2010) Prevention and treatment of postoperative pain syndrome in extensive thoracoabdominal oncological surgery. Anesteziol Reanimatol. 3: 29-33.
- Overdyk F, Dahan A, Roozekrans M, Van Der Schrier R, Aarts L, et al. (2014) Opioid-induced respiratory depression in the acute care setting: A compendium of case reports. Pain Manag. 4: 317-325.
- Apfel CC, Heidrich FM, Jukar-Rao S, Jalota L, Hornuss C, et al. (2012) Evidence-based analysis of risk factors for postoperative nausea and vomiting. Br J Anaesth 109: 742-753.
- Sandler AN, Baxter AD, Katz J, Samson B, Friedlander M, et al. (1994) A double-blind, placebo-controlled trial of transdermal fentanyl after abdominal hysterectomy. Analgesic, respiratory, and pharmacokinetic effects. Anesthesiology 81: 1169-1180.
- Sevarino FB, Paige D, Sinatra RS, Silverman DG (1997) Postoperative analgesia with parenteral opioids: Does continuous delivery utilizing a transdermal opioid preparation affect analgesic efficacy or patient safety? J Clin Anesth 9: 173-178.
- Miguel R, Kreitzer JM, Reinhart D, Sebel PS, Bowie J, et al. (1995) Postoperative pain control with a new transdermal fentanyl delivery system: A multicenter trial. Anesthesiology 83: 470-477.
- Lauretti GR, Mattos AL, Almeida R, Lima ICPR, Resende CS (2009) Efficacy of fentanyl transdermal delivery system for acute postoperative pain after posterior laminectomy. Poster Sessions / European Journal of Pain. 13: S55–S285.
- Barrera E, Fernandez–Galinski S, Ferrer MD, Escolano F, Puig M (2009) Postoperative analgesia induced by transdermal fentanyl in dorsal and lumbar spine arthrodesis: Poster Sessions / European Journal of Pain. 13: S255–S285.
- Tilbrook AJ, Clarke IJ (2006) Neuroendocrine mechanisms of innate states of attenuated responsiveness of the hypothalamopituitary adrenal axis to stress. Front Neuroendocrinol 27: 285-307.
- Kornick CA, Santiago-Palma J, Moryl N, Payne R, Obbens EAMT (2003) Benefit-risk assessment of transdermal fentanyl for the treatment of chronic pain. Drug Saf 26: 951-973.
Citation: Mohamad MF, Othman AH, Darwish AMM, Elzohry AAM (2018) Reducing Postoperative Opioid Consumption by Adding Transdermal Fentanyl Patches to Multimodal Analgesia after Breast Cancer Surgery. J Pain Relief 7:326. DOI: 10.4172/2167-0846.1000326
Copyright: © 2018 Mohamad MF, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Conferences
42nd Global Conference on Nursing Care & Patient Safety
Toronto, CanadaRecommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 3663
- [From(publication date): 0-2018 - Apr 04, 2025]
- Breakdown by view type
- HTML page views: 2823
- PDF downloads: 840