Conference Proceeding Open Access
Redistribution of Elements in Microbial Biomass in the Process of Silver and Gold Nanoparticles Synthesis Studied by Neutron Activation Analysis
| Marina Vladimirovna Frontasyeva1*, Inga Zinicovscaia1,2, Sergey Sergeevich Pavlov1, Andrei Yurevich Dmitriev1, Tamaz Kalabegishvili3,4, Ivane Murusidze4 and Elena Kirkesali1,3 | |
| 1Joint Institute for Nuclear Research, Dubna, Russia | |
| 2The Institute of Chemistry of the ASM, Chisinau, Moldova | |
| 3I. Javakhishvili State University, E. Andronikashvili Institute of Physics, Georgia | |
| 4Ilia State University, Georgia | |
| Corresponding Author : | M.V. Frontasyeva Joint Institute for Nuclear Research 6 Joliot-Curie Str, 1419890, Dubna, Russia Tel: +7(49621)65064 Fax: +4(49621) 65085 E-mail: marina@nf.jinr.ru |
| Received July 20, 2013; Accepted October 29, 2013; Published November 05, 2013 | |
| Citation: Frontasyeva MV, Zinicovscaia I, Pavlov SS, Dmitriev AY, Kalabegishvili T, et al. (2013) Redistribution of Elements in Microbial Biomass in the Process of Silver and Gold Nanoparticles Synthesis Studied by Neutron Activation Analysis. J Bioremed Biodeg S18:001. doi:10.4172/2155-6199.S18-001 | |
| Copyright: © 2013 Frontasyeva MV, et al. This is an open-a ccess article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | |
Related article at Pubmed Pubmed  Scholar Google Scholar Google |
|
Visit for more related articles at Journal of Bioremediation & Biodegradation
Abstract
Reactor neutron activation analysis was applied to characterize the elemental content of a microbial biomass in the process of synthesis of silver and gold nanoparticles from silver nitrate and chloroauric acid solutions. It was established that this process is accompanied by redistribution of some essential elements such as Ca, Cl, Fe, K, Mg, Mn, Na, P, U, Zn in microbial biomass both in case of different concentration loadings of silver and gold in aqueous solutions of silver nitrate and chloroauric acid, respectively, and in case of different incubation times. The role of cell surface adsorption in the formation of nanoparticles is discussed
| Keywords |
| Neutron; Analysis; Nanoparticles; Synthesis; Microorganisms |
| Introduction |
| Neutron activation analysis (NAA) is a powerful analytical technique widely used for determination of major, minor and trace element content in a great variety of biological objects including microorganisms [1-11]. In our earlier investigations the conventional instrumental NAA (INAA) as well as activation with epithermal neutrons was efficiently used for analytical purposes in the microbial biotechnology of pharmaceutical substances and sorbents [11-14]. NAA was also used to trace the process of silver and gold nanoparticle formation by microorganisms used as “nanofactories” for intracellular and extracellular synthesis of nanoparticles. Microorganisms grab target ions from the environment and then turn the metal ions into the elemental metal through enzymes generated by the cell activities [15,16]. Synthesis of silver and gold nanoparticles based on Spirulina platensis and actinomycetes biomass was studied in our investigations at different loadings of AgNO3 and HAuCl4 and different incubation time [17]. NAA and other analitical and optical methods weren used for characterisation of obtained nanomaterials [18-20]. Besides total silver and gold concentrations, NAA allowed determination of matrix and trace elements in the microbial biomass. In the present paper the behaviour of some selected elements such as Ca, P, Mg, Na, K, U, in the varying condition of nanoparticles formation is described. |
| Materials and Methods |
| Materials |
| New bacterial strains of actinomycetes such as Streptomyces glaucus 71MD and Streptomyces sp. 211A (isolated from the rhizosphere of soybeans grown in Georgia), actinomycetes belonging to arthrobacter genera - Arthrobacter globiformis 151B and Arthrobacter oxydans 61B (isolated from the basalt rocks collected from the Kazreti region of Georgia) and blue-green algae Spirulina platensis (strain IPPAS B-256) were used to study synthesis of silver and gold nanoparticles. The actinomycetes and Spirulina platensis strains were grown as described elsewhere [18-20]. The bacterial cells were harvested after 5-6 days of cultivation and then were washed twice in distilled water. In the first series of experiments the dose dependency of the silver and gold nanoparticles formation was study. The wet microbial biomass was resuspended in 250 ml Erlenmeyer flasks with 100 ml aqueous AgNO3 or HAuCl4 solutions at different concentrations (10-2 –10-4 M) and incubated at the room temperature for 5 days being shaken continuously. In the second series of experiments the temporal dependency of silver and gold nanoparticles formation was studied. The harvested mycelial mass was resuspended in 250 ml Erlenmeyer flasks in 100 ml of 10-3 M aqueous AgNO3 (silver nitrate) solution and in HAuCl4 (chloroauric acid) solution, respectively. The resulted mixtures were put again into the shaker at the room temperature and pH 5-8 for different periods of time (1–12 days). For NAA the bacterial cells in each case were harvested by centrifugation at 12000g for 20 min. The wet biomass was placed in an adsorption-condensation lyophilizer [21] and dried to a constant weight and packed in polyethylene bags and aluminum cups for short and long-term irradiations, respectively. |
| Methods |
| The elemental concentrations of bacterial samples were determined using NAA at the IBR├ó┬?┬?2 reactor of the Frank Laboratory of Neutron Physics (FLNP), JINR, Dubna, Russia. The description of the irradiation channels and the pneumatic transport system REGATA of the IBR├ó┬?┬?2 are given by Frontasyeva MV et al. [22]. The temperature in the irradiation channels of the IBR-2 reactor does not exceed 60–70 °C, which allows irradiation of biological samples. |
| The IBR-2 pulsed fast reactor with a Cd-coated channel provides the activation with thermal, epithermal and fast neutrons. Thermal NAA takes advantage of the high intensity of neutrons available from the thermalization of fission neutrons and the large thermal neutron cross sections for most isotopes. Epithermal neutron activation analysis (ENAA) is a useful extension of INAA in that it enhances the activation of a number of trace elements relative to the major matrix elements. ENAA has certain advantages over conventional instrumental activation analysis for many trace elements in terms of improvement in precision and lowering of detection limits, reduction of high matrix activity. |
| The concentrations of elements based on short-lived radionuclides were determined by irradiation for 60 s under a thermal neutron fluency rate of approximately 1.6×1013 n cm-2 s-1. After decay for 3 and 15 min the samples were measured for 3 and 15 min, respectively. To determine long-lived isotopes a cadmium-screened irradiation channel under a resonance neutron fluency rate of approximately 3.31×1012 n cm-2 s-1 was used. The samples were irradiated for 5 days, repacked and then measured twice after decay for 4 and 20 days. The counting time varied from 30 min to 1.5 hours. |
| The spectra of induced γ-activity were processed using Genie 2000 and concentrations were calculated using software developed at the FLNP JINR [23]. |
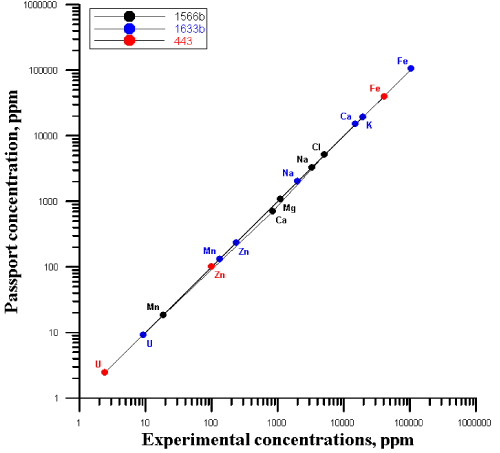
| The quality was assured by the use of the certified reference materials: Trace Elements in Marine Sediment (IAEA 433), Oysters Tissue (1566b), Coal Fly Ash (1633b), liquid standards of Au and Ag. The correlation between the certified (recommended) values of concentrations and the experimentally obtained ones is presented in Figure 1. |
| All standard and the test samples were irradiated together with flux monitor which was prepared by evaporating certified Au solution as 1000 μg/mL in 2% (v/v) HNO3 (Inorganic ventures manufacturer). A solution of 10 ml was pipetted by the digital Socorex Acura electro with an accuracy of ± 2.5%. |
| The calculation of the activities of the isotopes in the standards, samples and flux monitors was performed by means of program Genie 2000 (CANBERRA) using program of the interactive analysis of peaks S506 of the same company. After calculating the activities, the program for calculating the concentrations was used [23]. |
| Results and Discussion |
| Silver and gold nanoparticles produced by Spirulina platensis as well as Actinomycetes can find wide applications in medicine. This requires the precise analysis of the elemental content of their biomass. NAA was chosen as the most appropriate multi-element technique. In Table 1 the chemical composition of Streptomyces glaucus 71MD grown in glucose-medium after interaction with silver nitrate is presented. A total of 10 major, minor and trace element were identified. |
| The concentrations of heavy metals in biomass are in the order of μg/g and do not exceed tolerance limits for living organisms. This observation is also supported in W.H.O (1996) report and by Mertz [24,25]. |
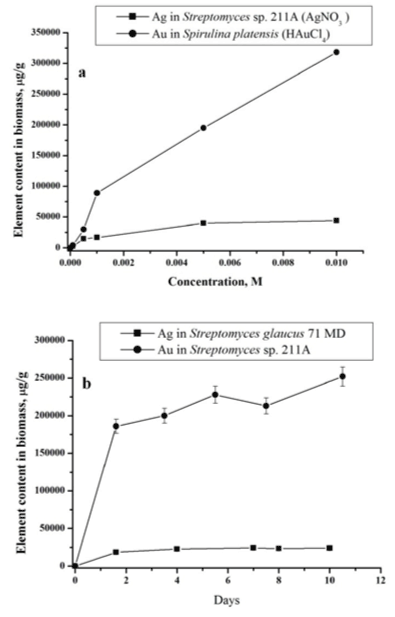
| The process of gold and silver uptake by microorganisms was studied in dependence of AgNO3 and HAuCl4 concentrations in the nutrient medium. The examples of data obtained by NAA for actinomycete Streptomyces sp. 211A and microalgae Spirulina platensis at different concentration loadings of AgNO3 and HAuCl4 are presented in Figure 2a. As follows from the obtained data in all studied cases with increase of silver nitrate and chloroauric acid concentrations in solution, silver and gold total content in biomass increases. |
| In Figure 2b the total concentrations of silver in Streptomyces glaucus 71 MD and gold Streptomyces sp. 211A biomass for different incubation time are presented. Obtained data show that uptake of silver and gold includes two phases: a rapid one and a slower one. In the first ‘rapid’ stage (1 day), the metal ions are adsorbed onto the surface of microorganism. The nanoparticles production takes place extracellularly, as was shown in our previous study [19,20]. |
| Bacteria of genus Streptomyces are gram-positive bacteria. The cell wall of gram-positive bacteria contains a second bilayer of waxy lipids in the form of mycolic acids in addition to the thicker peptidoglycan layer, polysaccharides, acids, and proteins. Functional groups within these biomolecules provide the amino, carboxylic, sulfydryl, phosphate, amino and thiol groups that can bind metal ions. The role of surface adsorption in nanoparticles formation was also demonstrated by our results obtained for studied samples using equilibrium dialysis and explained with Freundlich model [18]. |
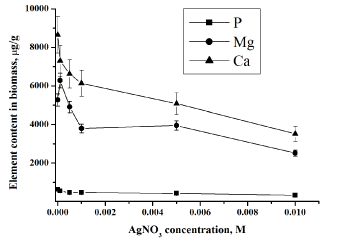
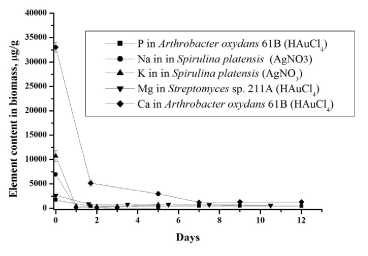
| In the second ‘slow’ stage (more then 1 day), the metal ions are transported across the cell membrane into the cytoplasm, followed by enzymatic reduction of metals ions to the metallic form. The nanoparticles production takes place intracellularly [19,20]. The total concentrations of metals increase slowly. Similar results were obtained for all studied bacteria using NAA as well as AAS [19,20]. In bacteria treated with silver and gold salts some similarity in the behaviour of the essential and trace elements is observed (Figures 3 and 4). |
| Silver cations exhibit broad antimicrobial action even at low concentrations. The biological role of gold is not well-defined. The ions of metals can bind to biological molecules containing thiol, amino, carboxylate, imidazole, or phosphate groups, inhibiting activities that are vital to the bacteria’s regulatory processes and cause bacterial inactivation [26]. |
| Data obtained for phosphorus in the studied bacterial samples support this fact (Figure 3). All living organisms need phosphorus to function, along with other elements such as hydrogen, oxygen, carbon, nitrogen and sulphur. The phosphate ion, PO43−, plays several essential roles in cells: it maintains the structure of DNA and RNA, combines with lipids to make cell membranes and transports energy within the cell through the molecule adenosine triphosphate (ATP). |
| Cell membranes, which are vital to the maintenance of intra and extracellular ion concentrations, appear to be important ion permeability barriers in microorganisms. The two ions which are most conspicuously regulated are sodium and potassium. The cell membrane is 100 times more permeable to potassium than sodium. The concentration of sodium is highest in extracellular fluid while potassium is highest in intracellular fluid [27]. Decrease of sodium concentration (Figure 4) can be explained by its replacement with silver ions [26,27] supporting the fact that one of mechanism of metal-microorganisms interaction is ion exchange mechanisms. In case of potassium its decrease can be explained in two ways by (i) disturbance of permeability of cytoplasmic membrane and (ii) replacement by silver. |
| Silver ions also displace other essential metal ions, such as Ca2+ and Mg2+ (Figures 4) [26]. Magnesium is typically the most abundant divalent cation inside prokaryotic cells [28]. Many enzymes require magnesium for their normal catalytic activity; it is required for maintaining pH balance, and for iron transport and metabolism. Ca2+ is much less abundant in bacteria than Mg2+, being presented in only low micromolar concentrations. Calcium plays an important role in stabilizing the structure of cell walls. It has been considered a cation, largely involved in coordinating to some extracellular enzymes and in specialized functions like sporulation [28]. Release of calcium and magnesium from bacterial cells in case of gold ions confirm penetration of AuCl4− inside the bacterial cell and destruction of DNA and RNA structures. The decrease of the metal content in the microbial biomass indicates that the silver nitrate and chloroauri├?┬ü acid solutions disturb the permeability of the microbial cell membranes. |
| Conclusions |
| Neutron activation analysis has been proved to be a powerful method to study microbial synthesis gold and silver nanoparticles. The investigation of the production dynamics of Au and Ag nanoparticles shows that it is a two-stage process - a rapid adsorption of metals on the surface of microorganisms is followed by their slow enzymatic reduction in the bacterial cells. The behavior of essential elements in the process of nanoparticle formation indicates the toxic impact of silver nitrate and chloroauric acid on the studied microorganisms. The symbiotic behaviour of such essential elements as Ca, K, Mg, Na, P, has been established. In these experiments, the concentrations of heavy metals in microbial biomass have not exceeded permissible levels, therefore the biomass with produced gold and silver nanoparticles can be used for medical purposes. |
| Acknowledgement |
| The authors acknowledge the Georgian scientists for experimental materials. The work was supported by the Ukrainian Science and Technology Centre (STCU) Grants ├ó┬?┬? 4744 and JINR Grant ├ó┬?┬? 13-402-03. |
References
|
Tables and Figures at a glance
| Table 1 |
Figures at a glance
 |
 |
 |
 |
| Figure 1 | Figure 2 | Figure 3 | Figure 4 |
Relevant Topics
- Anaerobic Biodegradation
- Biodegradable Balloons
- Biodegradable Confetti
- Biodegradable Diapers
- Biodegradable Plastics
- Biodegradable Sunscreen
- Biodegradation
- Bioremediation Bacteria
- Bioremediation Oil Spills
- Bioremediation Plants
- Bioremediation Products
- Ex Situ Bioremediation
- Heavy Metal Bioremediation
- In Situ Bioremediation
- Mycoremediation
- Non Biodegradable
- Phytoremediation
- Sewage Water Treatment
- Soil Bioremediation
- Types of Upwelling
- Waste Degredation
- Xenobiotics
Recommended Journals
Article Tools
Article Usage
- Total views: 13884
- [From(publication date):
specialissue-2014 - Apr 05, 2025] - Breakdown by view type
- HTML page views : 9392
- PDF downloads : 4492
