Recurrent Toxic Blooms of Alexandrium spp. in the East China Sea-Potential Role of Taiwan Warm Current in Bloom Initiation
Received: 01-Oct-2018 / Accepted Date: 16-Oct-2018 / Published Date: 22-Oct-2018
Abstract
Large-scale dinoflagellate blooms started to appear in the coastal waters adjacent to the Changjiang River estuary (CRE) in the East China Sea (ECS) at the beginning of the 21st century. The oceanographic and ecological mechanisms, as well as the impacts of these harmful algal blooms (HABs), received much attention over the last decade. During the studies in the coastal waters adjacent to the CRE from 2004 to 2007, recurrent blooms of Alexandrium spp. were observed for the first time. The major causative species was identified as Alexandrium catenella. Analysis of the samples collected during the blooms of Alexandrium spp. revealed the presence of paralytic shellfish toxins (PST) dominated by low-potency N-sulfocarbamoyl toxins C1 and C2. Toxin content in the Alexandrium cells ranged from 17.08 to 33.59 fmol. Cell-1. Blooms of Alexandrium spp. occurred generally in April and May, but the initiation time of the blooms varied from year to year. It’s suggested that initiation of the blooms have little connection with nutrients, as other algal blooms co-existed in this area were more significant in terms of scale and intensity. The initiation time of the blooms, however, seems to be more closely related to the seawater temperature at the bottom, which is affected by the intrusion of Taiwan Warm Current (TWC). Patches of the Alexandrium blooms were mainly found in the area of 29.0°-31.0°N, 122.0°-123.0°E, with water depth between 20 m and 50 m, but the distribution of the blooms varied from year to year. The distribution pattern, as well as the cell density of Alexandrium spp. during the blooms, was related to the initiation time of the blooms. It was proposed that intrusion of TWC in the sea area adjacent to the CRE may trigger the blooms of Alexandrium spp. and affect the distribution and intensity of the blooms. This hypothesis, however, requires more detailed studies on the blooms of Alexandrium spp. in this region.
Keywords: Algal blooms; Red tides; Neurotoxins; Paralytic shellfish poisoning; Alexandrium catenella
Regional Index Terms
China; East China Sea; Changjiang River estuary
Introduction
There is an apparent global increase and expansion of harmful algal blooms (HABs) over the last four decades [1-5]. Recurrent blooms of Alexandrium spp., for example, have been found in many coastal regions around the world, such as those in the Gulf of Maine, USA since 1972 [6,7], in the Bay of Fundy, eastern Canada since 1988 [8,9], along the north-west Mediterranean coast since 1998 [10], and in Hiroshima Bay, Japan [11], etc. Some species in genus Alexandrium can produce paralytic shellfish toxins (PST), which pose a potential threat to the health of human-beings following consumption of PSTcontaminated shellfish products.
Over 300 deaths were reported from intoxication caused by PST worldwide. Therefore, blooms of the toxic species in genus Alexandrium are a significant and increasing concern around the world.
In China, cells of Alexandrium spp. have been observed in coastal waters near the Nanhuangcheng Island in the Bohai Sea (BS) [11], Jiaozhou Bay in the Yellow Sea (YS), Xiamen coast in the East China Sea (ECS) [12] and Dapeng Bay of Shenzhen in the South China Sea (SCS) [13,14].
In an investigation of phycotoxins in the shellfish samples collected along the coast of China [15], PST were widely detected in shellfish samples collected from the BS in the north to the SCS in the south. Recent investigations revealed that coastal waters adjacent to the Changjiang River estuary (CRE) in the ECS is one of the major areas in China with PST contamination problems in shellfishes [16-18].
Both vegetative cells and cysts of Alexandrium spp. have been found in this region [19,20], but there was no report of blooms caused by Alexandrium spp. before the year 2000 [21].
Similarly, no information is available so far on the spatial-temporal variation pattern of Alexandrium cells in the ECS and the relationship between the bloom formation and environmental factors.
This paper explores information on the distribution pattern of Alexandrium blooms and PST profile in the coastal waters adjacent to the CRE in the ECS. In addition, the potential environmental factors that regulate outbreaks of the blooms are also discussed based on data of the 4-year cruises from 2004 to 2007.
Materials and Methods
Cruises
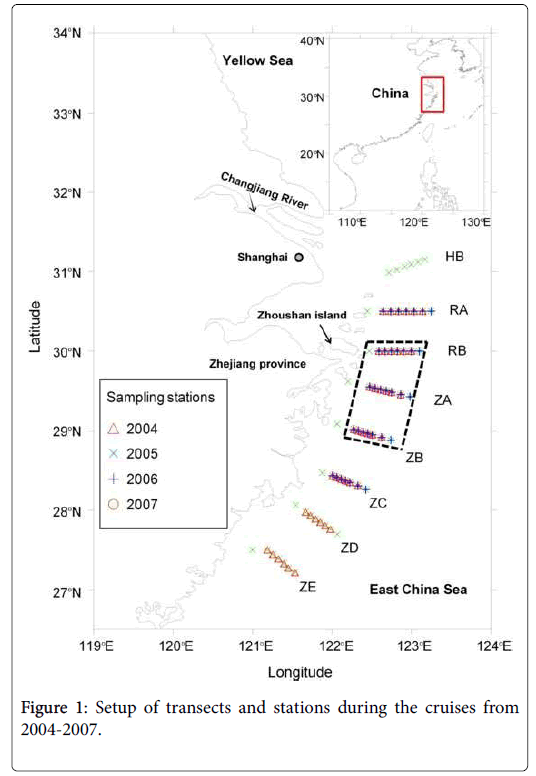
Four cruises were conducted in spring from 2004 to 2007. The study area was located in the coastal waters adjacent to the CRE in the ECS. Transects and stations of the cruises are shown in Figure 1. The dates of the cruises were April 2-May 13 for 2004, March 27-June 4 for 2005, March 30-May 30 for 2006, and April 4-May 21 for 2007, respectively. Data collected within the area surrounded by the dashed lines were used for analyses of relationships between the blooms of Alexandrium spp. and environmental factors.
Sample collection and preparation
Samples for identification and enumeration of Alexandrium cells: Water samples were collected from the surface (0-1 m), the bottom (1 m above the bottom) and the subsurface layer (the layer with the maximum Chl-a level as indicated by a Chl-a sensor on a YSI-6600, usually at the depth of 8-15 m) by a Niskin water sampler at the stations shown in Figure 1. Hydrographic data of the water column were collected with a SBE37-CTD (Seabird Inc., USA) at all stations.
If no maximum Chl-a layer was observed, a mid-water sample was collected at the depth of about 8-15 m depending on the water depth of the station. At some stations, supplementary samples were collected at the depth of 20 m or 30 m when necessary. For identification and enumeration of Alexandrium cells, 1 L seawater was collected and sieved onto a 20 μm mesh and backwashed with filtered seawater to a final volume of 20 ml. For stations with low levels of algal cells, 10 L of seawater was sieved. Small portions of the samples were used for live cell observation on board with a light microscope. Other samples were fixed with acid Lugol’s solution (3%-5%) for cell counting and for morphological observations in the laboratory with the light microscope (LM) and with scanning electron microscope (SEM).
Samples for analysis of PST: A volume of 100-1000 L of seawater at 5 selected stations containing high densities of Alexandrium cells was first concentrated to 100 ml with a 10 μm mesh, 5 ml were fixed with acid Lugol’s solution (3%-5%) to enumerate cells of Alexandrium spp. The remaining was filtered onto a GF/C glass-fiber filter and kept at -20°C for toxin analysis.
Sample treatment method followed. The filters were cut into pieces with scissors and put into 7 ml centrifuge tubes, 4 ml 0.05 M acetic acid solution was added into the tubes and the mixture was sonicated with a probe sonicator (200 W) for 5 min in an ice-bath until no intact cells were observed.
The mixture was then centrifuged at 10,000 rpm for 5 min, and the supernatant was collected. The residue was extracted again with 1 ml 0.05 M acetic acid solution, and the two supernatants were combined and filtered with a 0.22 μm membrane filter before analysis with HPLC.
A fraction of the extract was hydrolyzed to convert C toxins into the corresponding GTX toxins, using the following procedure: 68 μl of 1 M HCl was added to 300 μl sample solution, the mixture was kept at 90°C for 15 min, and 150 μl 1 M CH3COONa was added [22]. Both the hydrolyzed sample and the original sample were analyzed with HPLC.
A Waters high performance liquid chromatography system coupled with a fluorescence detector (HPLC-FLD) was used for the analysis of PSP samples. The toxins were separated with a C18 column (Phenomenex, 150 × 4.6 mm, 3 m). The method was used, with slight modifications. Two mobile phases (flow rate 0.8 ml min-1) were used in separation of GTX toxins and STX toxins, respectively.
For analysis of GTX toxins, the mobile phase was 1 mM sodium heptanesulfonic acid in 10 mM ammonium phosphate buffer (pH 7.1). For analysis of STX toxins, the mobile phase was 1.5 mM sodium heptanesulfonic acid in 30 mM ammonium phosphate buffer (pH 7.1), containing 5 percent acetonitrile. The oxidation solution and acid solution were prepared as described in [23]. The temperature of post column derivatization system was 80°C. The excitation and emission wave length used for detection of PSP toxins were 330 nm and 390 nm, respectively.
On board oceanographic data collection and nutrient analysis
Vertical profiles of temperature, salinity, and depth were obtained at every station with a SBE37-CTD (Seabird Inc., USA), while Chl-a and turbidity were measured using sensors installed on the YSI-6600 (YSI Inc., USA). 500 ml water samples were collected by a Niskin water sampler and then filtered through a GF/F filter (Whatman) for nutrient analysis on board by the spectrophotometric method described by [24] to obtain temporal and vertical distribution patterns of several important nutrients such as dissolved inorganic nitrogen (DIN), phosphate and silicate.
Results and Discussions
Causative species of the Alexandrium blooms
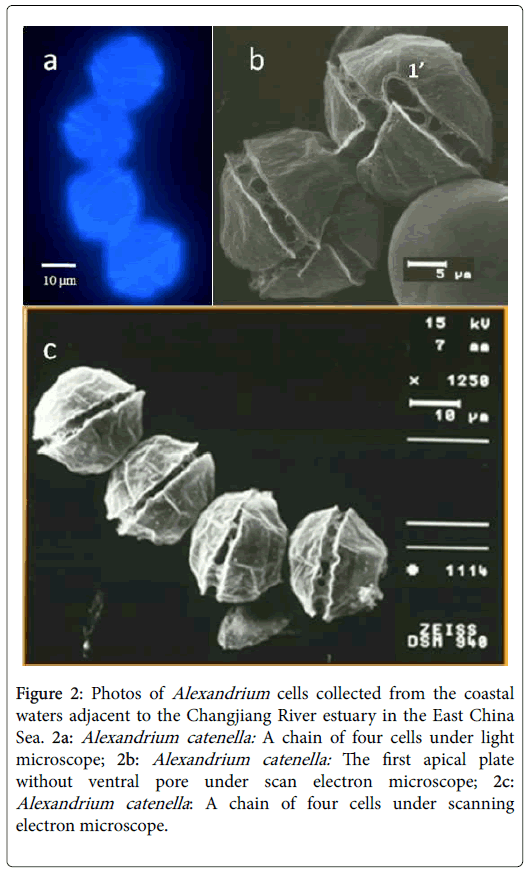
The live cells of Alexandrium spp. collected during the cruises were first observed under a light microscope on board. Based on the morphological features and chain-forming characteristics (chains with 4 or more cells), the major species of Alexandrium spp. was primarily identified as A. catenella (Figure 2). This result was further confirmed by the observation of fixed cells under scanning electron microscope, which showed that most of the Alexandrium cells had no ventral pore in plate 1’ (Figure 2). Cells of the A. tamarense , identified based on their morphological features (with ventral pore in plate 1), were also found occasionally in the phytoplankton samples.
Figure 2: Photos of Alexandrium cells collected from the coastal waters adjacent to the Changjiang River estuary in the East China Sea. 2a: Alexandrium catenella: A chain of four cells under light microscope; 2b: Alexandrium catenella: The first apical plate without ventral pore under scan electron microscope; 2c: Alexandrium catenella : A chain of four cells under scanning electron microscope.
A. catenella, A. tamarense and A. fundyense were members of the A. tamarense species complex, which could be divided into different ribotypes termed as “Temperate Asian”, “North America”, and “Europe” etc., based on the sequences of small and large-subunit ribosomal RNA genes [25]. These ribotypes were later later renamed as Group I to V by [26]. Both morphotype A. catenella and A. tamarense were found in several of these ribotypes. In the previous studies, strains of A. catenella and A. tamarense established from the coastal waters adjacent to the CRE were all assigned to the Group IV of A. tamarense species complex, based on the sequences of large subunit ribosomal RNA gene and the ITS regions [27,28]. Recently, [29] revealed the presence of A. tamarense species complex (Group I) in the China Seas, but their distribution was confined in the BS and YS, and all the strains of A. tamarense and A. catenella established from the ECS and SCS up to now belonged to the Group IV of A. tamarense species complex. Therefore, the major causative species of the Alexandrium blooms in the sea area adjacent to the Changjiang River can be considered as A. tamarense species complex (Group IV). The morphological features of A. catenella and A. tamarense are quite similar, and the major difference is the absence (for A. catenella ) or presence (for A. tamarense ) of a ventral pore in the first apical plate. So the two species were not discriminated from each other during most of the investigations.
Toxin production and potential impacts of the Alexandrium blooms
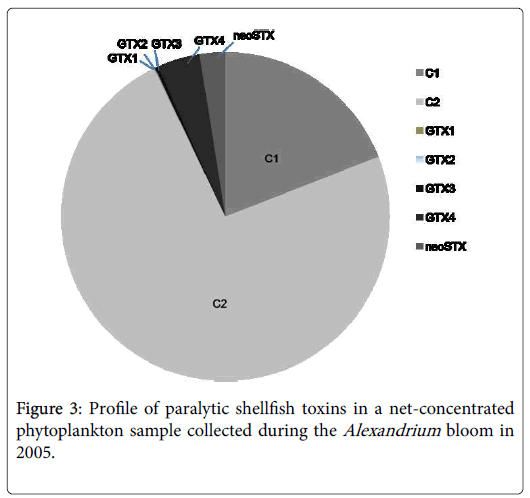
PST in the net-concentrated phytoplankton samples collected during the Alexandrium blooms were analyzed with the HPLC-FLD system. PST components, including C1, C2, GTX1, GTX2, GTX3, GTX4, and neoSTX, were detected. The dominant toxins were C1 and C2, which contributed more than 92% of the total PSP toxins (Figure 3). Toxin levels of the samples collected at the 5 stations in 2005 were summarized in Table 1, with a range of 17.08-33.59 fmol/cell and an average level at 24 fmol/cell. Compared to other toxic species in genus Alexandrium , such as A. fundyense in the Casco Bay (cellular toxin content 113-155 fmol cell-1) and A. catenella in the Argentine Sea (cellular toxin content 45.53-261.4 fmol cell-1) [30,31], A. catenella in the ECS produces much lower amounts of PST.
| C1 | C2 | GTX1 | GTX2 | GTX3 | GTX4 | neoSTX | Total | |
|---|---|---|---|---|---|---|---|---|
| 122.9190°E, 30.7510°N | 3.07 | 28.95 | 0.01 | 0.01 | 0.16 | 0.49 | 0.9 | 33.59 |
| 122.7831°E, 30.5618°N | 1.42 | 14.85 | 0.02 | 0.01 | 0.09 | 0.28 | 0.41 | 17.08 |
| 122.6110°E, 30.4992°N | 7.95 | 9.07 | 0.04 | 0.02 | 0.05 | 0.06 | 0.26 | 17.45 |
| 122.7470°E, 30.5123°N | 7.53 | 8.26 | 0.01 | 0 | 0.03 | 3.27 | 0.47 | 19.57 |
| 122.6023°E, 30.0017°N | 2.8 | 27 | 0 | 0 | 0.05 | 0.93 | 0.99 | 31.77 |
| Average | 4.55 | 17.63 | 0.02 | 0.01 | 0.08 | 1.01 | 0.61 | 23.89 |
| Standard deviation | 2.98 | 9.81 | 0.02 | 0.01 | 0.05 | 1.31 | 0.32 | 8.1 |
Table 1: Toxin content and composition of the net-concentrated phytoplankton samples collected during the Alexandrium bloom in 2005
Toxin profile is a relatively stable characteristic for a specific toxic species when grown under constant conditions. So far two different toxin profiles were found among the strains within the A. tamarense species complex (Group IV) isolated in China. Most of the strains from mainland China produce a large proportion of C1 and C2 toxins together with trace amount of other GTX toxins [32,33], while the strains from the coastal waters of Hong Kong produce a relatively high proportion of GTX toxins, particularly GTX1, 4, besides C toxins. Analysis of the strains of A. tamarense species complex (Group IV) established from the coastal waters adjacent to the CRE also found high proportion of C toxins (C1 and C2) similar to those of the field samples collected in the current study. This may give another clue that the bloom was caused by the A. tamarense species complex (Group IV). Toxin content calculated from the current study is in consistence with those strains of A. tamarense species complex (Group IV) established from the China Seas, with toxin level between 11.9 and 64.0 fmol cell-1.
Some previous studies reported the contamination of shellfish by PST in the coastal waters adjacent to the CRE. Compared to other coastal regions affected by Alexandrium blooms in China, however, the status of PST contamination in the CRE region is not that serious, generally being 0-64 μg STX equiv./100 g fresh shellfish soft tissue, and the maximum toxin content in shellfish recorded was 64 μg STX equiv./100 g in Scapharca subcrenata collected from Daishan Island of Zhoushan in 2006. In the northern Yellow Sea, PST content detected in shellfish could be as high as 640 μg STX equiv./100 g in scallops Patinopecten yessuensis. In the SCS, PST content in scallops Chalmys nobilis could reach 100 μg STX equiv./100 g. Both were above 80 μg STX equiv./100 g, the acting limit for shellfish safety in China and many other countries. Toxin content and composition of the Alexandrium tamarense species complex (Group IV) in this region may partially account for the relatively lower PST levels in shellfish, as the strains of A. tamarense species complex (Group IV) from this region produce not only much lower level of toxins (~ 24 fmol cell-1), but also the low-potency components of PST (C1, C2) (Table 1 and Figure 3).
Dynamics of the Alexandrium blooms
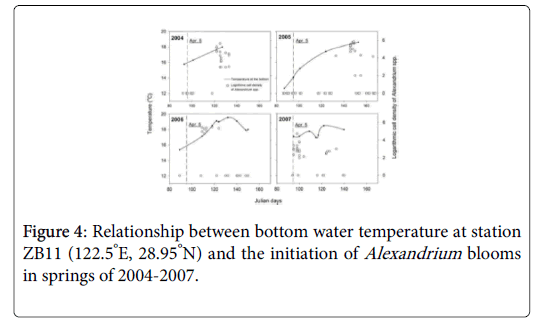
Alexandrium blooms occurred every spring from 2004 to 2007, and duration of the blooms was usually between 1-2 weeks in the sea area adjacent to the CRE (Figure 4). The blooms appeared from May 2 to 10 in 2004 (Julian days 122-130), May 26-30 in 2005 (Julian days 146-150), April 19-May 4 in 2006 (Julian days 109-124) and April 5-10 in 2007 (Julian days 95-100), respectively.
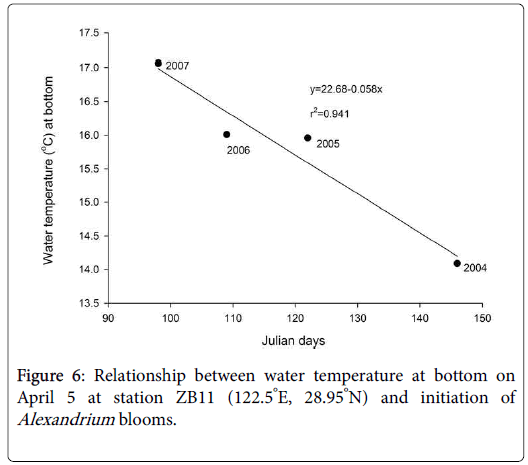
The vertical dashed lines indicated the date of April 5, on which the bottom temperature was adopted to analyze its relationship with the initiation of Alexandrium blooms in Figure 6.
A. tamanrense spp. was not the only bloom-causative species in the coastal waters adjacent to CRE in spring 2004 to 2007. The major algal blooms in this area were caused by the diatom Skeletonema costatum in early spring (around Julian day 90-100) followed by dinoflagellates like Prorocentrum donghaiense and Karenia mikimotoi (around Julian day 130-150). Generally, two peaks of chlorophyll a would be observed during spring (Figure 5, in the year 2005). The first peak represented the diatom bloom in early spring, and the other peak was a bloom of dinoflagellates.
Interestingly, blooms of Alexandrium spp . did not occur synchronously with those of otherdinoflagellates, such as P. donghaiense and K. mikimotoi. Alexandrium spp. bloomed early with the diatoms in 2007, late during the blooms of other dinoflagellate species in 2004 and 2005, and between the diatom bloom and the dinoflagellate bloom in 2006 (Figure 5).
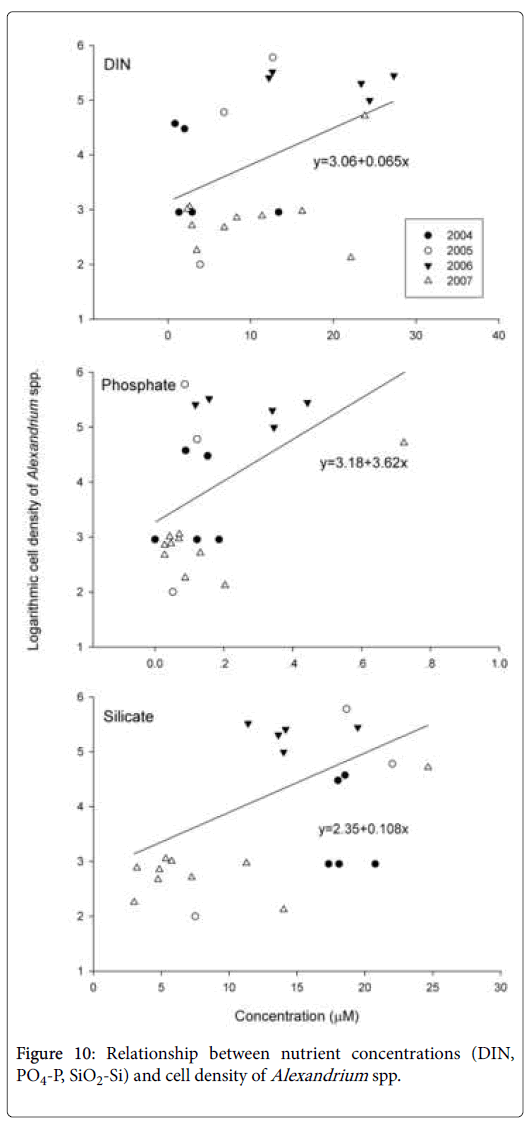
Nutrients should have little effects on the initiation of the Alexandrium blooms, as other algal blooms co-existed in this area, such as those of S. costatum and P. donghaiense , were more significant in terms of scale and intensity. Cell densities of S. costatum and P. donghaiense during the blooms could reach 107 cells/L. The Alexandrium cells, however, did not contribute substantially to the total algal biomass due to the relatively low cell density during the blooms (normally less than 105 cells l-1). The initiation date of the blooms of Alexandrium spp. seems to be more closely related to the seawater temperature at the bottom. Figure 4 illustrates the relationship between water temperature at the bottom in earlier April (~Julian day 95) and the cell density of Alexandrium spp. in station ZB11 (122.5°E, 28.95°N), a representative station for the Alexandrium blooms. The investigation results showed that Alexandrium spp. bloomed earlier if the bottom water temperature increased earlier and vice versa. For example, Alexandrium bloomed in early April in 2007 when the bottom temperature was the highest in early April. In 2005, bloom of Alexandrium didn’t occur until late May, as the temperature was the lowest observed in early April. This bloom occurred some 50 days later than that in 2007. A strong negative correlation (R2=-0.941) was observed between the outbreak date (Julian days) of Alexandrium blooms and the water temperature at the bottom in early April (~ Julian day of 95) as shown in Figure 6.
Previous studies on the Alexandrium blooms in the Gulf of Maine suggested that cysts were the important seed source and key to the initiation of the blooms [34,35] also found the same phenomenon along the Catalan coast in the Mediterranean Sea. In our study area in the ECS, Alexandrium cysts have been widely found in the surface sediment [36], particularly in the region southeast to the Zhoushan Island [37]. The distribution pattern of Alexandrium cysts matched well with the distribution pattern of the Alexandrium blooms documented in the current study. These cysts could serve as a seed source for the recurrent Alexandrium blooms in this area found that the density of Alexandrium cysts in surface sediment of ECS had a clear seasonal variation pattern, with the highest values in summer and autumn, intermediate in winter, and lowest in spring. This may reflect cyst germination (for the bloom) in spring and encystment in summer after the blooms of Alexandrium [38] studied the germination rate of cysts isolated from surface sediments in the ECS and the survival rate of the germinated cells. Cysts of Alexandrium spp. could germinate between 9°C and 21°C, but germination was the fastest when temperature was above 15°C. Therefore, warmer temperature at the sea bottom in spring would lead to large and synchronized cell germination at the time of bloom initiation.
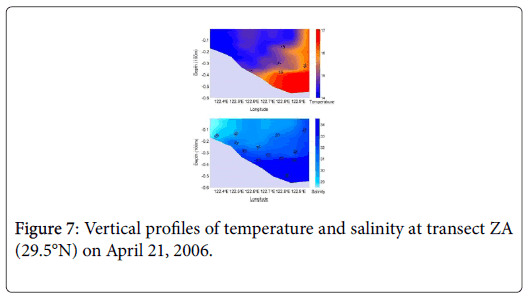
In the coastal waters adjacent to the CRE, intrusion of the Taiwan Warm Current (TWC) at the bottom layer from early spring to late summer is a unique physical oceanographic process in this region. Many studies observed a clear invasion of warm water at the bottom of the bloom area in early spring, a typical upwelling phenomenon driven by TWC. Our investigation also confirmed that the warm water intrusion (Figure 7) into the bottom in the study area was evident in spring but the degree of temperature increase was different from year to year [39]. Therefore, we deduced that outbreak of the Alexandrium blooms in this region might be a process driven by the intrusion of TWC and its associated temperature increase at the bottom, which promoted the germination of the cysts in the surface sediment. Besides, the intrusion of TWC (seawater with much higher salinity compared to the Changjiang diluted water) at the bottom also contributes to the formation of stable stratification, which is beneficial for the development Alexandrium blooms.
Distribution of the Alexandrium blooms
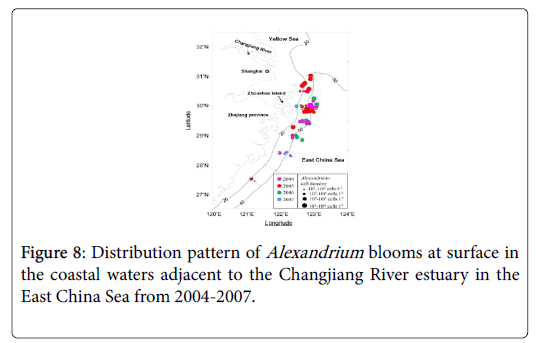
During the four-year investigations, patches of dense Alexandrium cells were mainly found in the area of 29.0-31.0°N, 122.0-123.0°E where the water depth was between 20 m and 50 m (Figure 8). Density of the Alexandrium cells in the patches was generally higher than 105 cells/L. The area of one patch sometimes covered an area of about 400 km2.
The distribution pattern of blooms varied from year to year. The bloom occurred mainly in the sea area southeast to the Zhoushan Island when Alexandrium bloomed early in 2007, east to the Island when Alexandrium bloomed in mid-season in 2004 and 2006, and northeast to the Island when Alexandrium bloomed late in 2005 (Figure 8). It seems that the distribution pattern is closely related to the initiation time of the blooms. When the bloom started earlier, the distribution of the blooms was more southwards. And the blooms appeared more northwards when the bloom started later. Besides, cell density of Alexandrium spp. also varied from year to year (Figure 8), with a potential linkage to the initiation of the blooms. Cell density recorded was relatively higher when Alexandrium blooms occurred later, such as those in 2004, 2005 and 2006 (105-106 cells/L), and cell density was much lower when Alexandrium bloomed earlier in 2007. The maximum cell density recorded in 2007 was less than 104 cells/L.
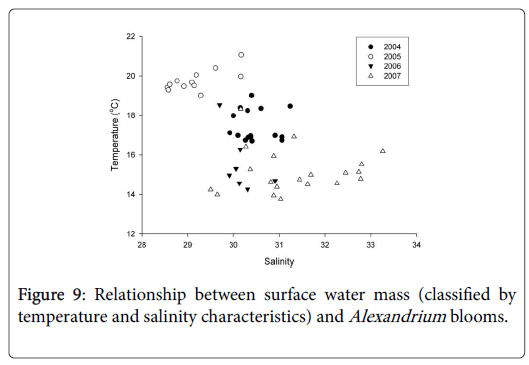
Water temperature and salinity in the bloom area of Alexandrium spp. were analyzed to reveal the relationship between cell density of Alexandrium spp. and their distribution. It was suggested that the blooms occurred in different water masses in the four years, depending upon the timing of the blooms (Figure 9). If Alexandrium spp. bloomed earlier, for example, in 2007, the cells of Alexandrium spp. were mainly distributed in the water mass from the open sea, with relatively lower temperature (~13°C-15°C) and higher salinity (~30-33). If Alexandrium spp. bloomed later, like that in 2005, the cells were mainly distributed in the coastal waters with relatively higher temperature (~19°C-21°C) and lower salinity (~28.5-30). This might explain the obscured difference in cell density between the blooms occurred early in 2007 versus that occurred later in 2005.
Coastal water rich in nutrients would support a much higher cell density of Alexandrium spp. than the water mass from the open sea (Figure 10). Besides, coastal water is rich in growth stimulants like humic substances, which will also promote the growth of Alexandrium spp. [40]. Meanwhile, the relatively high temperature at the surface would also favor the growth of Alexandrium spp. if they bloomed later. Therefore, the distribution pattern and cell density of Alexandrium blooms was related to the initiation time of the blooms.
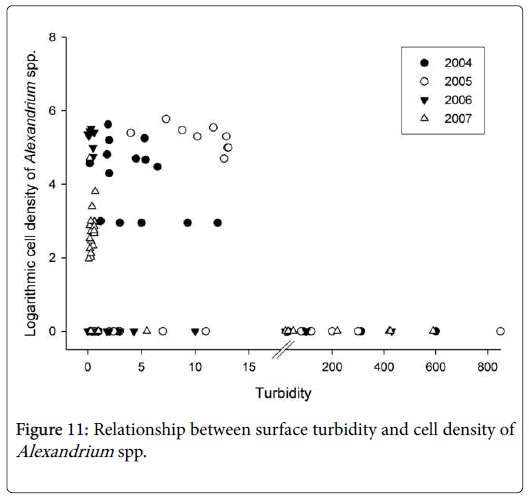
Another finding regarding the distribution of Alexandrium blooms is that Alexandrium spp. bloomed only in seawater where turbidity was less than 13 NTU (Figure 11). The CRE and Zhoushan archipelago are surrounded by turbid seawater in winter and spring as a result of huge amounts of sediment carried by the Changjiang River and resuspended by strong winds in those seasons. The turbid water could partially explain why Alexandrium blooms showed such a distribution pattern as seen in Figure 8. Due to the high turbidity, the blooms were always insulated from the inshore area west to the Zhoushan archipelago, where the main shellfish mariculture operations are located. This may protect the mariculture zone from being affected by the blooms. Recently, however, there is a decreasing trend of sediments carried by the Changjiang River following the construction of the Three Gorge Dam [41]. The Alexandrium blooms could therefore extend closer to the shellfish farms if the sediment carried by the Changjiang River continues to decrease. Someday this trend may result in conditions that no longer prevent the Alexandrium blooms from reaching the inshore area [42-47]. The persistence of recurrent Alexandrium blooms may lead to more serious problem of PST contamination in shellfish in the future.
Conclusion
In the current study, we found recurrent Alexandrium blooms from 2004-2007 in the coastal waters adjacent to CRE. The dominant species was identified as Alexandrium catenella . During the blooms, PST dominated by the low-potency N-sulfocarbamoyl toxins C1 and C2 were detected. Patches of dense Alexandrium cells were mainly distributed in the surface waters near Zhoushan Island with water depth between 20 m and 50 m. The distribution pattern and intensity of the blooms were closely related to the initiation of the blooms, which had a significant relationship with water temperature at the bottom. It was proposed that intrusion of TWC and its associated temperature increase at the bottom may trigger the initiation of the Alexandrium blooms in the ECS, and subsequently affect the distribution and intensity of the blooms. The persistence of the Alexandrium blooms, with the long-term change of the turbidity in the coastal waters adjacent to CRE, may lead to increased risk of PSP poisoning in this region.
Limitations
After the four cruises which were conducted in spring from 2004 to 2007, there were no cruises related to the toxic blooms of Alexandrium spp. in the East China Sea. Hence, the recent data cannot be provided, and then it’s impossible to explain the influence on the current situations.
Acknowledgements
This work was supported by the National Basic Research Priority Program (2010CB428700), the National Natural Science Foundation of China (41076071, 41176100, 41121064), and the National Basic Research Priority Program (2001CB409700).
References
- Anderson DM (1989) Toxic algal blooms and red tides: A global perspective. Elsevier 2: 11-16.
- Hallegraeff GM (1993) A review of harmful algal blooms and their apparent global increase. Phycologia 32: 79-99.
- Heisler J, Glibert PM, Burkholder JM, Anderson DM, Cochlan W, et al. (2008) Eutrophication and harmful algal blooms: A scientific consensus. Harmful Algae 8: 3-13.
- Li S, Xu HP, Guo XL (2012) Satellite remote sensing of harmful algal blooms (HABs) and a potential synthesized framework. Sensors 12: 7778-7803.
- Hans Paerl (2018) Mitigating toxic planktonic cyanobacterial blooms in aquatic Ecosystems facing increasing anthropogenic and climatic pressures. Toxins 10: 76.
- Mulligan HF (1973) Probable causes for the 1972 red tide in the Cape Ann region of the Gulf of Maine. J Fisheries Res Board Canada 30: 1363-1366.
- Townsend DW, Pettigrew NR, Thomas AC (2001) Offshore blooms of the red tide dinoflagellate, Alexandrium sp., in the Gulf of Maine. Cont Shelf Res 21: 347-369.
- Martin JL, Page F, Strain M, LeGresley MM (2005) Alexandrium fundyense vertical distribution patterns during 1982, 2001 and 2002 in the offshore Bay of Fundy. J Ocean Univ China 52: 2569-2592.
- Martin JL, Hanke AR, LeGresley MM (2009) Long term phytoplankton monitoring, including harmful algal blooms, in the Bay of Fundy, eastern Canada. J Sea Res 61: 76-83.
- Vila M, Garces E, Maso M, Camp J (2001) Is the distribution of the toxic dinoflagellate Alexandrium catenella expanding along the NW Mediterranean coast? Mar Ecol Prog Ser 23: 497-514.
- Itakura S, Yamaguchi M, Yoshida M, Fukuyo Y (2002) The seasonal occurrence of Alexandrium tamarense (Dinophyceae) vegetative cells in Hiroshima Bay, Japan. Fisheries Science 68: 77-86.
- Song XK, Ma JX, Liu YH, Liu LJ, Ma YQ, et al. (2009) Evolution and formation causes of Alexandrium tamarense red tide in the sea area of the Nanhuangcheng island. Trans Oceanol Limnol 4: 93-98.
- Lin YS (1996) Toxic dinoflagellate Alexandrium tamarense blooms in shrimp pond of Xiamen. J Ocean Univ China 15: 16-18.
- Qi YZ, Qian F (1994) Taxonomic studies on red tide causative dinoflagellates in Dapeng bay, South China Sea. Oceanol Limnol Sin 25: 206-210.
- Zhou M, Li J, Luckas B, Yu R, Yan T, et al. (1999) A recent shellfish toxin investigation in China. Mar Pollut Bull 39: 331-334.
- Jiang TJ, Chen JF, Zou YL, Liu JS, Yang WD (2003) Paralytic shellfish toxins in shellfish from the coast of high frequent harmful algae blooms occurrence areas in East China Sea and South China Sea. Chinese J Appl Ecol 14: 1156-1160.
- Wang JH, Qin YT, Liu CC, Sun YW, Cheng XS, et al. (2007) A background investigation of toxic algae and bio-toxin in the Yangtze estuary. Trans Oceanol Limnol 1: 52-61.
- Hu HY, Tang JL, Huang B, Mao HY, Wang JY, et al. (2008) The shellfish-bearing PSP in areas of high red tide occurrence of the Zhoushan fishing ground. Oceanol Limnol Sin 39: 475-481.
- Qi YZ, Hong Y, Zheng L, Kulis DM, Anderson DM (1996) Dinoflagellate cysts from recent marine sediments of the South and East China Seas. Asian Mar Biol 13: 87-103.
- Wang ZH, Matsuoka K, Qi YZ, Lu SH (2003) Distribution of cysts of toxic Alexandrium spp. and Gymnodinium catenatum along the Chinese coastal waters. Oceanol Limnol Sin 34: 422-430.
- Wang JH, Qin YT, Liu CC, Sun YW, Cheng XS, et al. (2006) A preliminary investigation of potentially toxic algae and bio-toxin in the Changing Estuary. Mar Environ Sci 25: 15-19.
- Yu RC, Hummert C, Luckas B, Qian PY, Li J, et al. (1998) A modified HPLC method for analysis of PSP toxins in algae and shellfish from China. Chromatographia 48: 671-676.
- Oshima Y, Sugino K, Yasumoto T (1989) Latest advances in HPLC analysis of paralytic shellfish toxins. Mycotoxins and phycotoxins 88: 319-326.
- Zhang Z, Yang SL, Li P (2011) Quantifying the influence of water impoundment phases I and II of the three gorges reservoir on downstream suspended sediment flux. Acta Geographica Sinica 65: 623-631.
- Scholin CA, Hallegraef GM, Anderson DM (1995) Molecular evolution of the Alexandrium tamarense ‘species complex’ (Dinophyceae): Dispersal in the North American and West Pacific regions. Phycologia 34: 472-485.
- Lilly E L, Halanych KM, Anderson DM (2007) Species boundaries and global biogeography of the Alexandrium tamarense complex (Dinophyceae). J Phycol 43: 329-1338.
- Tang XH, Yu RC, Yan T, Wang YF, Zhou MJ (2006) Sequence analysis and molecular phylogenetics of ribosomal RNA gene of Alexandrium (Dinophyceae) strains in China. Oceanol Limnol Sin 37: 529-535.
- Wang DZ, Lin L, Gu HF, Chan LL, Hong HS (2008) Comparative studies on morphology, ITS sequence and protein profile of Alexandrium tamarense and A. catenella isolated from the China Sea. Harmful Algae 7: 106-113.
- Gu HF, Zeng N, Liu TT, Yang WD, Müller A, et al. (2013) Morphology, toxicity, and phylogeny of Alexandrium (Dinophyceae) species along the coast of China. Harmful Algae 27: 68-81.
- Montoya NG, Fulco K, Carignan MO, Carreto JI (2010) Toxin variability in cultured and natural populations of Alexandrium tamarense from southern South America-Evidences of diversity and environmental regulation. Toxicon 56: 1408-1418.
- Poulton NJ, Keafer BA, Anderson DM (2005) Toxin variability in natural populations of Alexandrium fundyense in Casco Bay, Maine – Evidence of nitrogen limitation. Deep Sea Res Part 2 Top Stud Oceanogr 52: 2501-2521.
- Anderson DM, Kulis, Qi Y Z, Zheng L, Lü S, et al. (1996) Paralytic shellfish poisoning in Southern China. Toxicon 14: 579-590.
- Wang Y, Teng L (2006) Distribution of dinoflagellate cysts from Changiiang River Estuary in the spring of 2004. Ecol Sci 25: 131-134
- Anderson DM, Burkholder M, Cochlan W, Glibert P, Gobler C, et al. (2008) Harmful algal blooms and eutrophication: Examining linkages from selected coastal regions of the United States. Harmful Algae 8: 39-53.
- Bravo I, Vila M, Maso M, Figueroa R, Ramilo I (2008) Alexandrium catenella and Alexandrium minutum blooms in the Mediterranean Sea: Toward the identification of ecological niches. Harmful Algae 7: 515-522.
- Fu Y, Wang ZH, Kang W, Lü SH, Gu YG, et al. (2009) Vertical distribution of dinoflagellate resting cysts in recent sediments from the Changjiang River Estuary and adjacent waters. Journal of Jinan University (Natural Science) 30: 106-111.
- Wang Z, Qi Y, Lu S, Wang Y, Matsuoka K (2004) Seasonal distribution of dinoflagellate resting cysts in surface sediments from Changjiang River Estuary. Phycol Res 52: 387-395.
- Gu HF, Fang Q, Li RX, Lan DZ, Zhu MY (2003) Preliminary study on dinoflagellate cysts in changing river estuary. Oceanologia et Limnologia Sinica 35: 413-423
- Zhu DD, Lu DD, Wang YF, Su JL (2009) The low temperature characteristics in Zhejiang coastal region in the early spring of 2005 and its influence on harmful algae bloom occurrence of Prorocentrum donghaiense. Acta Oceanologica Sinica 31: 31-39.
- Gagnon R, Levasseur M, Weise AM, Fauchot J, Campbell GC, et al. (2005) Growth stimulation of Alexandrium tamarense (Dinophyceae) by humic substances from the Manicouagan River (Eastern Canada). J Phycol 41: 489-497.
- Bao WJ, Cao S, Lin H (2010) Analysis the change of mud-sand before and after of water impoundment of the three gorges reservoir in Datong station. Sci Rep 31: 21-23.
- Jungsu P, Jared C, Younggyu S, Keug-Tae K, Woo Lee (2017) Recent advances in ultrasonic treatment: Challenges and field applications for controlling harmful algal blooms (HABs). Ultrason Sonochem 38: 326-334.
- Keith D, Anderson DM, Marcos M, Beatriz R, Joe S, et al. (2016) Forecasting the risk of harmful algal blooms. Harmful Algae 53: 1-7.
- Kevin GS, Gregory JD, Gary JK (2003) Harmful algal blooms: Causes, impacts and detection. J Ind Microbiol Biotechnol 30: 383-406.
- Wang DZ, Zhang SG, Gu HF, Chan LL, Hong HS (2006) Paralytic shellfish toxin profiles and toxin variability of the genus Alexandrium (Dinophyceae) isolated from the Southeast China Sea. Toxicon 48: 138-151.
- Yuan-zheng X, Shuang-shuang W, Qiu-yan X, Dong-mei L, Bei-wei Z (2010) Pollution survey of paralytic shellfish poison (PSP) from aquaculture zones of Changhai’s sea area in Dalian. Food Mach 26: 54-56.
- Chuan-song Z, Xiu-Lin W, De-Di Z, Jian-Tao W (2007) The nutrients at prophase of large scale harmful algal blooms in the east China Sea in spring. Periodical Ocean U China 37: 1002-1006.
Citation: Wang YF, Yu RC, Lu DD, Lu SH, Zhub DD, et al. (2018) Recurrent Toxic Blooms of Alexandrium spp. in the East China Sea-Potential Role of Taiwan Warm Current in Bloom Initiation. J Ecol Toxicol 2: 115.
Copyright: © 2018 Wang YF, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Usage
- Total views: 2978
- [From(publication date): 0-2018 - Apr 05, 2025]
- Breakdown by view type
- HTML page views: 2146
- PDF downloads: 832