Recurrent TB Disease in Singapore: A Retrospective Case Control Study
Received: 26-Mar-2021 / Accepted Date: 09-Apr-2021 / Published Date: 16-Apr-2021 DOI: 10.4172/2332-0877.1000458
Abstract
Background: Previously treated Tuberculosis (TB) cases account for ~7%-8% of incident TB globally and in Singapore. Molecular fingerprinting has enabled the differentiation of these patients into relapsed or re-infection cases.
Methods: Patient demographics, disease characteristics and treatment information were obtained from the national TB notification registry and TB Control Unit. We performed a retrospective, case-control study to evaluate factors associated with recurrent TB disease in Singapore citizens and Permanent Residents with culture-positive TB from 2006 to 2013 and who developed a second episode of culture-positive TB up to 2016 using multivariable logistic regression analyses.
Results: 91 cases with culture-positive first and recurrent TB disease episodes were notified during the study period. Available DNA fingerprinting results for both episodes in 49 cases differentiated these into 28 relapsed and 21 re-infection cases. Recurrent TB was associated with age ≥ 60 years (adjusted odds ratio [aOR] 2.06, 95% confidence interval [CI] 1.14–3.73), male gender (aOR 2.29, 95% CI 1.23–4.43) and having concomitant pulmonary and extrapulmonary TB (aOR 2.76, 95% CI 1.43–5.36); and was less likely in persons of non-Malay ethnicity (aOR 0.50, 95% CI 0.26–0.95). Relapse was associated with having concomitant pulmonary and extrapulmonary TB (aOR 8.24, 95% CI 2.28–36.35), and sputum acid fast bacilli smear positivity (aOR 3.59, 95% CI 1.27–11.32).
Conclusion: Relapse and re-infection contributed to 57% and 43% respectively of recurrent TB in Singapore. Our study identified the hitherto unrecognized association of concomitant pulmonary and extrapulmonary TB disease with risk of relapse.
Keywords: Tuberculosis, Relapse, Exogeneous reinfection Extrapulmonary
Keywords
Tuberculosis; Relapse; Exogeneous re-infection; Extrapulmonary
Introduction
Persons with previously treated tuberculosis (TB) account for ~7% of incident TB cases globally [1]. Recurrence of TB disease after clinical cure / treatment completion of a previous episode may be due to endogenous re-activation of residual tuberculous bacilli from the original episode (referred to as relapse) or exogeneous re-infection [2]. Over the past two decades, molecular genotyping techniques has enabled the study of the diversity of Mycobacterium tuberculosis strains and has demonstrated that exogeneous re-infection plays a more important role in causing recurrent disease than previously thought [3]. It is likely that re-infection drives the TB epidemic in areas with high TB and HIV prevalence. Nonetheless, there does not appear to be a consistent correlation in the literature between the predominant cause of recurrent TB (i.e. relapse vs. re-infection) and geographical TB prevalence. Singapore is a densely populated island city-state with an intermediate TB incidence of 35-40 cases per 100,000 population and a very low HIV/AIDS notification rate of 7.9 per 100,000 population in 2018 (9). There are ~3,000 notified TB cases in the country annually, of whom ~50% are Singapore citizens or Permanent Residents (PRs) [4]. Among these, persons with a history of previously treated TB account for ~8% of notified TB episodes. We undertook a retrospective case-control study to investigate the characteristics of Singapore citizens and PRs with recurrent culture-positive TB (“recurrent TB”).
The availability of DNA fingerprinting results provided the opportunity to examine factors associated with recurrent disease due to relapse or exogeneous re-infection in our cohort [5].
Methods
TB disease is notifiable by law in Singapore. The national TB notification registry is also electronically linked to the two mycobacterial culture laboratories in Singapore, enabling complete capture of all positive.
Mycobacterium tuberculosis complex culture results. Singapore citizens and PRs aged 16 and above with first episode culturepositiveTB notified between 1 January 2006 and 31 December 2013 and who developed a second episode of culture-positive TB in the period up to 31 December 2016 were identified from the national TB notification registry.
For this study, “recurrent TB” was defined as a second episode of culture-positive TB disease occurring in persons who had completed treatment of a first episode of culture-positive TB.
Data analysed in this study pertained to the first disease episode and included age, sex, ethnic group, and presence of Diabetes Mellitus (DM) and HIV co-infection, sputum Acid Fast Bacilli (AFB) smear status, site(s) of disease (pulmonary TB [PTB] with or without concomitant extra-pulmonary disease), presence of cavitation on baseline Chest X-Ray (CXR), treatment regimen, mode of treatment delivery (whether Directly Observed Therapy [DOT] or Self- Administered Therapy [SAT]], AFB culture results at two months of treatment, and whether the duration of short-course TB therapy was extended beyond the conventional six months.
This information was obtained from clinical case records for patients who were treated at the TB Control Unit during the first episode of TB; otherwise, the information was extracted from the TB registry for patients treated in other institutions.
For the case-control study, cases were defined as patients with recurrent TB. Controls were culture-positive patients who were not notified with recurrent TB within the period of the study.
Two controls were randomly selected for each study case, matched by date of notification (+/- five days) [6-8].
For subgroup analyses, cases with available 24-loci mycobacterial interspersed repetitive unit variable number tandem repeat (MIRU-VNTR) and spacer oligonucleotide (spoligotyping) results for both TB episodes were classified into those with identical/ near-identical DNA fingerprinting results (‘relapse cases”) or different results (“re-infection cases”) for both episodes.
Statistical analysis
The Chi-square test or Fisher’s exact test, where appropriate, was used to compare baseline characteristics between individuals with and without recurrent TB.
No imputation was carried out for missing data.
We inserted an “unknown” category for variables with missing data. The main outcome was whether an individual had recurrent TB.
Crude and adjusted odds ratio were calculated using Firth’s logistic regression analyses.
Variables for the multivariable logistic regression model were selected through stepwise use of Akaike’s information criterion.
All p values reported were 2-sided and statistical significance was taken as p<0.05.
Statistical analyses were performed using SPSS version 24 (IB, USA) and R version 3.6.2 (R Foundation for Statistical Computing, Vienna, Austria) [9].
Results
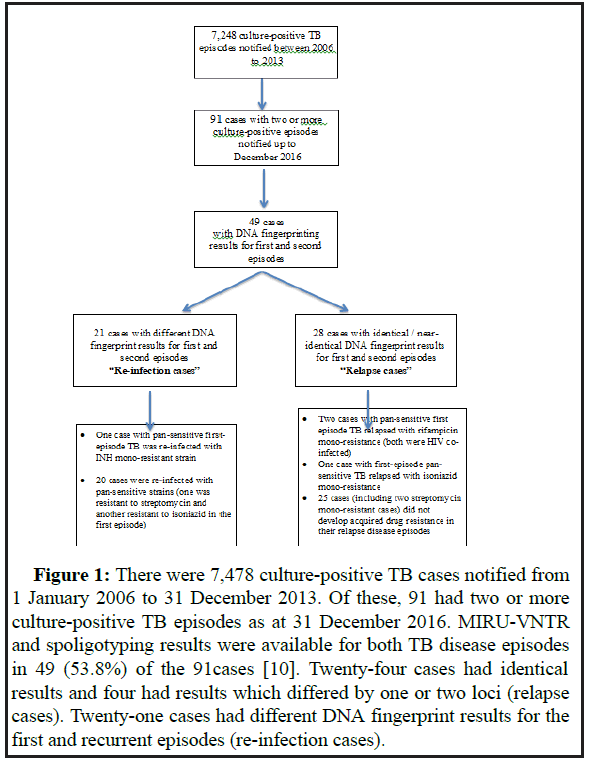
There were 7,478 culture-positive TB cases episodes notified between 1 January 2006 and 31 December 2013. Of these, 91 cases had two or more culture-positive TB episodes as at 31 December 2016 (Figure 1).
Figure 1: There were 7,478 culture-positive TB cases notified from 1 January 2006 to 31 December 2013. Of these, 91 had two or more culture-positive TB episodes as at 31 December 2016. MIRU-VNTR and spoligotyping results were available for both TB disease episodes in 49 (53.8%) of the 91 cases [10]. Twenty-four cases had identical results and four had results which differed by one or two loci (relapse cases). Twenty-one cases had different DNA fingerprint results for the first and recurrent episodes (re-infection cases).
Fifty-six (61.5%) were treated at the TB Control Unit during the first disease episode. Forty-six (50.5%) developed recurrent disease within 24 months after completion of treatment of their first TB episode [11]. Among the 91 cases with recurrent TB, 84 (92.3%) had PTB in their first TB episode; among these, 23 (27.3%) had concomitant extra-pulmonary site disease. Seven (7.7%) had extra-pulmonary disease only (3 pleura, 3 lymphatic, 1 genito-urinary) [12].
Recurrent TB disease was significantly associated with age ≥ 60 years (adjusted odds ratio [aOR] 2.06, 95% confidence interval [CI] 1.14–3.73), male gender (aOR 2.29, 95% CI 1.23–4.43) and PTB with concomitant extra-pulmonary TB (aOR 2.76, 95% CI 1.43–5.36); and less likely to occur in persons of non-Malay ethnicity (aOR 0.50, 95% CI 0.26–0.95) (Table 1).
| Cases | Controls | Univariable model | Multivariabl e model | p-value | |
|---|---|---|---|---|---|
| (N=91) | (N=182) | OR (95% CI) | p-value | aOR (95% CI) | |
| n (%) | n (%) | ||||
| 61 (67.0) | 145 (79.7) | 1.00 (reference) | 1.00 (reference) | ||
| 30 (33.0) | 37 (20.3) | 1.92 (1.09–3.38) | 0.024 | 2.06 (1.14–3.73) | 0.017 |
| 17 (18.7) | 61 (33.5) | 1.00 (reference) | 1.00 (reference) | ||
| 74 (81.3) | 121 (66.5) | 2.15 (1.20–4.03) | 0.01 | 2.29 (1.23–4.43) | 0.008 |
| 23 (25.3) | 33 (18.1) | 1.00 (reference) | 1.00 (reference) | ||
| 68 (74.7) | 149 (81.9) | 0.65 (0.36–1.20) | 0.167 | 0.50 (0.26–0.95) | 0.035 |
| 59 (64.8) | 131 (72.0) | 1.00 (reference) | |||
| 32 (35.2) | 51 (28.0) | 1.39 (0.81–2.38) | 0.225 | ||
| 84 (92.3) | 171 (94.0) | 1.00 (reference) | 1 | ||
| 7 (7.7) | 11 (6.0) | 1.32 (0.49–3.40) | 0.569 | ||
| 67 (73.6) | 160 (87.9) | 1.00 (reference) | 1.00 (reference) | ||
| 24 (26.4) | 22 (12.1) | 2.59 (1.37–4.93) | 0.004 | 2.76 (1.43–5.36) | 0.002 |
| 50 (54.9) | 120 (65.9) | 1.00 (reference) | |||
| 41 (45.1) | 62 (34.1) | 1.58 (0.95–2.65) | 0.078 | ||
| 31 (34.1) | 82 (45.1) | 1.00 (reference) | |||
| 60 (65.9) | 98 (53.8) | 1.61 (0.96–2.73) | 0.07 | ||
| 0 (0.0) | 2 (1.1) | 0.52 (0.004–6.67) |
0.66 | ||
| 4 (4.4) | 7 (3.8) | 1.00 (reference) | |||
| 52 (57.1) | 120 (65.9) | 0.73 (0.22–2.66) | 0.61 | ||
| 35 (38.5) | 55 (30.2) | 1.07 (0.31–4.01) | 0.919 | ||
| 36 (39.6) | 64 (35.2) | 1.00 (reference) | |||
| 55 (60.4) | 118 (64.8) | 0.83 (0.49–1.39) | 0.473 | ||
| 71 (78.0) | 140 (76.9) | 1.00(reference) | |||
| 11 (12.1) | 26 (14.3) | 0.85 (0.39–1.77) | 0.674 | ||
| 9 (9.9) | 16 (8.8) | 1.13 (0.47–2.60) | 0.776 |
Table 1: Odds ratios of candidate predictors for recurrent tuberculosis.
MIRU-VNTR and spoligotyping results were available for both TB disease episodes in 49 (53.8 %) of the 91 cases with recurrent TB. There was no significant difference in age, gender, ethnicity, presence of DM or HIV, sputum AFB smear, presence of cavity, disease site (PTB with or without concomitant extra-pulmonary disease), sputum AFB culture status at two months of treatment, treatment delivery mode and duration of treatment between the cases with and without available DNA fingerprinting results for both TB episodes. Of the 49 cases with available genotyping, 24 had identical results and four had results which differed by one or two loci for the first and recurrent disease episodes - these 28 cases were classified as “relapse cases”. Twenty-one cases with different MIRU-VNTR results for the first and recurrent episodes were classified as “re-infection” cases.There was no significant difference in age, gender, ethnicity, and proportion infected with the Beijing strain between the relapse and re-infection groups. The median time to disease recurrence was significantly shorter in those who relapsed (22 months, range 2-68) compared with those who were re-infected (49 months, range 4-110) p=0.003) (Table 2).
| Variable | Relapse cases | Re-infection cases | p-value^ |
|---|---|---|---|
| (N=28) | (N=21) | ||
| Age group, n (%) | 1 | ||
| <60 years | 20 (71.4%) | 15 (71.4%) | |
| = 60 years | 8 (28.6%) | 6 (28.6%) | |
| Gender, n (%) | 1 | ||
| Female | 7 (25%) | 5 (23.8%) | |
| Male | 21 (75%) | 16 (76.2%) | |
| Ethnic group, n (%) | 0.359 | ||
| Malay | 11(39.3%) | 5 (23.8%) | |
| Non-Malay | 17 (60.7%) | 16 (76.2%) | |
| Median time to disease recurrence | |||
| (months) | 22 | 49 | 0.003 |
| Infecting strain, n (%) | 1 | ||
| Beijing | 18 (64.3%) | 13 (61.9%) | |
| Non-Beijing | 10 (35.7%) | 8 (38.1%) |
^P values comparing relapse and reinfection cases are from Fisher’s exact test for categorical variables and Mann-Whitney U test for the continuous variable.
Table 2: Comparison between characteristics of relapse and re-infection cases.
Relapse cases were more likely to have PTB and concomitant extrapulmonary disease (aOR 8.24, 95% CI 2.28–36.35), and to be sputum AFB smear positive (aOR 3.59, 95% CI 1.27–11.32) Table 3.
| Cases | Controls | Univariable model | p-value | Multivariable emodel | p-value |
|---|---|---|---|---|---|
| (N=28) | (N=56) | OR (95% CI) | aOR (95% CI) | ||
| n (%) | n (%) | ||||
| 20 (71.4) | 45 (80.4) | 1.00 (reference) | |||
| 8 (28.6) | 11 (19.6) | 1.64 (0.57–4.59) | 0.349 | ||
| 7 (25.0) | 16 (28.6) | 1.00 (reference) | |||
| 21 (75.0) | 40 (71.4) | 1.17 (0.43–3.34) | 0.762 | ||
| 11 (39.3) | 14 (25.0) | 1.00 (reference) | 1.00 (reference) | ||
| 17 (60.7) | 42 (75.0) | 0.52 (0.20–1.36) | 0.179 | 0.38 (0.12–1.17) | 0.092 |
| 22 (78.6) | 40 (71.4) | 1.00 (reference) | 1.00 (reference) | ||
| 6 (21.4) | 16 (28.6) | 0.71 (0.24–1.95) | 0.513 | 0.32 (0.08–1.07) | 0.065 |
| 25 (89.3) | 52 (92.9) | 1.00 (reference) | |||
| 3 (10.7) | 4 (7.1) | 1.60 (0.34–7.09) | 0.536 | ||
| 18 (64.3) | 51 (91.1) | 1.00 (reference) | 1.00 (reference) | ||
| 10 (35.7) | 5 (8.9) | 5.31 (1.72–18.14) | 0.004 | 8.24 (2.28–36.35) | 0.001 |
| 15 (53.6) | 41 (73.2) | 1.00 (reference) |
|||
| 13 (46.4) | 15 (26.8) | 2.33 (0.92– 6.01) |
0.075 | ||
| 9 (32.1) | 31 (55.4) | 1.00 (reference) |
1.00 (reference) | ||
| 19 (67.9) | 24 (42.9) | 2.64 (1.05– 6.97) |
0.038 | 3.59 (1.27–11.32) | 0.016 |
| 0 (0.0) | 1 (1.8) | 1.11 (0.01–22.57) | 0.953 | 0.76 (0.00–17.78) | 0.872 |
| 2 (7.1) | 1 (1.8) | 1.00 (reference) | |||
| 16 (57.1) | 37 (66.1) | 0.26 (0.02–2.14) | 0.206 | ||
| 10 (35.7) | 18 (32.1) | 0.34 (0.03–2.91) | 0.319 | ||
| 11 (39.3) | 20 (35.7) | 1.00 (reference) | |||
| 17 (60.7) | 36 (64.3) | 0.85 (0.34– 2.17) |
0.738 | ||
| 23 (82.1) | 42 (75.0) | 1.00 (reference) | |||
| 2 (7.1) | 6 (10.7) | 0.70 (0.12–2.99) | 0.638 | ||
| 3 (10.7) | 8 (14.3) | 0.74 (0.17–2.69) | 0.663 |
Adjusted for ethnic group, diabetes, TB site and smear result.
Abbreviations: AFB: Acid Fast Bacilli; aOR: adjusted Odds Ratio; CI: Confidence Interval; CXR: Chest X-Ray; DOT: Directly Observed Therapy; OR: Odds Ratio; PTB:Pulmonary Tuberculosis; SAT: Self-Administered Therapy.
Table 3: Odds ratios of candidate predictors for relapsed cases.
Relapse was not associated with age, gender, ethnicity, DM, HIV co-infection, cavitation on baseline CXR, sputum AFB culture status at two months of treatment, mode of treatment delivery and duration of TB treatment.
Re-infection cases
There was no statistically significant difference between re-infection cases and controls in terms of age, gender, ethnicity, presence of DM or HIV, concomitant PTB and extrapulmonary disease, bacteriological burden (smear and cavitary status), sputum AFB culture status at two months of treatment, mode of treatment delivery and duration of TB treatment (Table 4).
| Cases | Controls | Univariable model | p-value | Multivariabl e model | p-value |
|---|---|---|---|---|---|
| (N=21) | (N=42) | OR (95% CI) | A OR (95% CI) | ||
| n (%) | n (%) | ||||
| 15 (71.4) | 35 (83.3) | 1 (reference) | 1 (reference) | ||
| 6 (28.6) | 7 (16.7) | 1.98 (0.58–6.72) | 0.269 | 2.90 (0.78–11.16) | 0.111 |
| 5 (23.8) | 19 (45.2) | 1(reference) | 1 (reference) | ||
| 16 (76.2) | 23 (54.8) | 2.49 (0.83–8.34) | 0.105 | 2.52 (0.81–8.86) | 0.111 |
| 5 (23.8) | 7 (16.7) | 1(reference) | |||
| 16 (76.2) | 35 (83.3) | 0.63 (0.18–2.30) | 0.477 | ||
| 11 (52.4) | 30 (71.4) | 1(reference) | 1(reference) | ||
| 10 (47.6) | 12 (28.6) | 2.23 (0.77–6.56) | 0.14 | 2.47 (0.82–7.88) | 0.11 |
| 18 (85.7) | 37 (88.1) | 1 (reference) | |||
| 3 (14.3) | 5 (11.9) | 1.29 (0.27–5.42) | 0.733 | ||
| 16 (76.2) | 38 (90.5) | 1(reference) | 1(reference) | ||
| 5 (23.8) | 4 (9.5) | 2.85 (0.72–11.92) | 0.134 | 3.90 (0.89–18.61) | 0.071 |
| 15 (71.4) | 26 (61.9) | 1(reference) | |||
| 6 (28.6) | 16 (38.1) | 0.67 (0.21–1.98) | 0.478 | ||
| 9 (42.9) | 16 (38.1) | 1 (reference) | |||
| 12 (57.1) | 25 (59.5) | 0.85 (0.30–2.46) | 0.763 | ||
| 0 (0.0) | 1 (2.4) | 0.58 (0.00–12.02) | 0.736 | ||
| 1 (4.8) | 1 (2.4) | 1 (reference) | |||
| 12 (57.1) | 25 (59.5) | 0.49 (0.04–6.50) | 0.557 | ||
| 8 (38.1) | 16 (38.1) | 0.52 (0.04–7.08) | 0.592 | ||
| 7 (33.3) | 19 (45.2) | 1 (reference) | |||
| 14 (66.7) | 23 (54.8) | 1.60 (0.56–4.84) | 0.381 | ||
| 16 (76.2) | 32 (76.2) | 1 (reference) | |||
| 4 (19.0) | 6 (14.3) | 1.36 (0.34–5.18) | 0.652 | ||
| 1 (4.8) | 4 (9.5) | 0.66 (0.06–3.94) | 0.663 |
Abbreviations: AFB: Acid Fast Bacilli; aOR: adjusted Odds Ratio; CI: Confidence Interval; CXR: Chest X-Ray; DOT: Directly Observed Therapy; OR: Odds Ratio; PTB: Pulmonary Tuberculosis; SAT: Self-Administered Therapy.
Table 4: Odds ratios of candidate predictors for re-infection cases.
Cases with PTB and concomitant extrapulmonaryTB disease in the first episode.There were 23 cases of recurrent TB disease who had PTB and concomitant extrapulmonary TB disease in their first disease episode. A wide variety of extrapulmonary sites were involved, the most common being the pleura (56.5%). The majority (70%) had recurrent disease confined to the lungs. Fifteen cases had available DNA fingerprinting results for both episodes; of these, 10 were relapse cases (three were HIV co-infected) and five were re-infection cases (one was HIV co-infected) (Table 5).
| Relapsed cases (N=10) | Re-infection cases (N=5) | Cases without DNA fingerprinting results for both disease episodes (N=8) | ||
|---|---|---|---|---|
| Sites of extrapulmonary disease in first TB episode |
Pleura (3) | Pleura (3) | Pleura (4) | |
| Larynx (2) | Pleura+lymphatic (2) | Genitourinary (1) | ||
| Pleura+lymphatic (1) | Gastrointestinal (1) | |||
| Skeletal (1) | Skeletal (2) | |||
| CNS (1) | ||||
| Gastrointestinal (1) | ||||
| Genitourinary +lymphatic (1) | ||||
| HIV co-infected | 3 | 1 | Nil | |
| (one developed RIF mono- resistance in relapsed episode) | (infected with INH resistant strain in first episode, re- infected with pan- susceptible strain) | |||
| Skeletal (1) | Pleura+Lymphatic (1) | |||
| CNS (1) | ||||
| Genitourinary +Lymphatic (1) |
||||
| Sites of disease in recurrent TB episode | Pulmonary only (6) | Pulmonary (5) only |
Pulmonary (5) only | |
| Pulmonary +GastrointestinaI (1) |
Pulmonary+eye (1) | |||
| Pulmonary+lymphatic (1) | Pulmonary+GastrointestinaI (1) | |||
| Pulmonary +lymphatic +CNS (1) | Skeletal (1) | |||
| CNS only (1) | ||||
CNS: Central Nervous System
Table 5: Extrapulmonary sites of disease in 23 recurrent TB cases with PTB and concomitant extrapulmonary TB in their first TB episode.
Acquisition of drug resistance
None of the 91 recurrent TB patients had multidrug-resistant TB (i.e. strains resistant to at least rifampicin and isoniazid) in their first or recurrent disease episodes. Six cases had new drug-resistant recurrent episodes, of whom four had available MIRU and spoligotyping results for both disease episodes (Figure 1). All six cases had pan-sensitive TB in their first episode: two HIV co-infected patients relapsed with rifampicin mono-resistant TB, one patient relapsed with isoniazid-resistant TB, one patient was re-infected with an isoniazid-resistant strain,while the remaining two patients with isoniazid mono-resistant recurrent TB disease (one of whom was HIV co-infected) did not have available DNA fingerprinting results for both episodes. Three cases had streptomycin mono-resistant TB for both first and recurrent episodes–two were relapse cases, while the thi rd case had no available DNA fingerprinting result.All three patients who relapsed with acquired drug resistance did not receive DOT and were not treated under the national TB programme for their first TB episode.
Discussion
In our case-control study, age ≥ 60 years, male gender, Malay ethnicity and PTB with concomitant extrapulmonary TB in the first disease episode were found to be significantly associated with recurrent TB disease. This information is useful to raise awareness as to the local epidemiological risk groups for TB disease recurrence. Consistent with the TB literature, recurrent disease due to relapse occurred significantly earlier than that from exogeneous re-infection.
The odds of disease relapse were significantly higher in persons with PTB and concomitant extra-pulmonary disease, and with sputum AFB smear positivity in the first episode. Our study did not identify any factors associated with exogeneous re-infection. A key objective of TB treatment is to eradicate populations of persisting bacilli to achieve durable cure (i.e. to prevent relapse). The risk of relapse arises when there is suboptimal bacteriologic response to treatment of the first episode – this may be due to high bacteriological burden, or treatment factors such as inappropriate regimens, poor adherence or drug pharmacokinetic/pharmacodynamic factors affecting therapeutic drug levels in individual patients. Indicators of disease burden such as cavitation/extensive disease on chest radiograph, and slower response to treatment as indicated by lack of sputum culture conversion at two months of treatment, presence of cavity on end-of-treatment chest radiograph, and lack of weight gain during the intensive phase of treatment have been shown to be associated with risk of relapse. Our finding that baseline sputum AFB smear positivity was significantly associated with relapse is not unexpected as this is an indicator of high initial disease burden. To our knowledge, concomitant PTB and extrapulmonary TB has thus far not been reported in the literature as a risk factor for relapse. As persons with multi-site TB disease presumably have higher bacterial burden, this association is biologically plausible. Interestingly, 70% of these patients relapsed with pulmonary TB only. Current international guidelines recommend extending the continuation phase of the standard six-month short-course therapy by three months (i.e. a total of nine months of treatment) in persons with cavitation on their baseline CXR who have positive cultures at two months of treatment (15). Other factors to consider in the decision to prolong treatment in patients with either baseline CXR cavitation or positive culture at two months (but not both) are being > 10% below ideal body weight, being an active smoker, having diabetes mellitus, HIV co-infection or any other immunosuppressing condition, or having extensive disease on chest radiograph. Pertaining to extrapulmonary disease, these guidelines recommend extension of treatment duration only for CNS and skeletal TB. That our study found no association of relapse risk with cavitation on baseline CXR or sputum AFB culture at 2 months of treatment may have been influenced by the practice in TB Control Unit of routinely extending the treatment continuation phase for persons with these factors. Our finding that persons with concomitant PTB and extrapulmonary disease involving a variety of extrapulmonary sites are at risk of relapse may suggest the need for extending treatment in these patients, regardless of the site of extrapulmonary disease.
DNA fingerprinting has shed light on the relative contribution of relapse (57%) versus re-infection (43%) to the burden of recurrent TB in Singapore. The risk of exogeneous re-infection is influenced by the prevalence and transmission of TB in the community and host immunological factors. That exogeneous re-infection accounted for almost half of the recurrent TB cases may not be surprising as Singapore is a densely populated, intermediate TB incidence (albeit low HIV-incidence) country. The rate of relapsed culture-positive TB in Singapore was reassuringly low during the study period. We believe that this is testimony to the effectiveness of the Singapore TB Elimination Programme (STEP) which has, since 1998, utilized inperson DOT for the majority of the country’s TB. The STEP’s Treatment Surveillance Module which actively tracks all TB cases in Singapore until their final outcome, has also served to ensure high treatment completion rates nationally.It is noteworthy that all three patients who relapsed with acquired drug-resistant disease were not treated under the national TB programme and did not receive DOT during their first TB episode. Two were HIV co-infected at the time of their first TB episode. This observation is consistent with the established fact that HIV co-infected TB patients are at high risk for acquisition of drug (particularly rifampicin) resistance and underscores the vital role of DOT in these persons to achieve best treatment outcomes. A strength of our study was the complete capture of all culture positive cases in Singapore. A study limitation was the lack of MIRU-VNTR and spoligotyping results for both disease episodes in 46% of the cohort, resulting in a small sample size for analysis. However, we believe that these cases were representative of the cohort as there was no difference in characteristics between those with and without available DNA fingerprinting results for both disease episodes. The unavailability of Whole Genome Sequencing (WGS) for our cases was another study limitation, as MIRU-VNTR and spoligotyping methods are not discriminatory enough to conclusively exclude re-infection in those with identical DNA fingerprints for both episodes particularly in our setting with high proportion of infection due to the Beijing family spoligotype. Socio-economic and lifestyle factors, which may be important determinants of risk for exogenous re-infection, were also not captured and analyzed in this study.
Conclusion
Our study provides insights into recurrent TB disease in Singapore and its associated risk factors. Our finding that patients with concomitant PTB and extra-pulmonary TB are significantly more likely to relapse has thus far not been reported in the literature. We have identified a risk group for whom measures to mitigate this outcome may be considered.
References
- McIvor A, Koornhof H, Kana BD (2017) Relapse, re-infection and mixed infections in tuberculosis disease. Pathog Dis.
- Lambert ML, Hasker E, Van Deun A (2003) Recurrence in tuberculosis: Relapse or reinfection?. The Lancet Infectious Diseases 3: 282-287.
- Verver S, Warren RM, Beyers NM (2005) Rate of reinfection tuberculosis after successful treatment is higher than rate of new tuberculosis. Am J RespirCrit Care Med 171: 1430-5.
- Mirsaeidi M, Sadikot RT (2018) Patients at high risk of tuberculosis recurrence. Int J Mycobacteriol 7: 1-6.
- Panjabi R, Comstock GW, Golub JE (2007) Recurrent tuberculosis and its risk factors: adequately treated patients are still at high risk. Int J Tuberc Lung Dis 11828-37.
- Hermans SM, Zinyakatira N, Caldwell J, Cobelens FGJ, Boulle A, et al. High rates of recurrent tuberculosis disease: a population-level cohort study. Clinical Infectious Diseases.
- Aber VR, Nunn AJ (1978) Factors affecting relapse following short- course chemotherapy. Bull int Union Tuberc 53: 276-80.
- Benator D, Bhattacharya M, Bozeman L (2002) Rifapentine and isoniazid once a week versus rifampicin and isoniazid twice a week for treatment of drug-susceptible pulmonary tuberculosis in HIV-negative patients: a randomised clinical trial. Lancet 360: 528-34.
- Jo KW, Yoo JW, Hong Y (2014) Risk factors for 1-year relapse of pulmonary tuberculosis treated with a 6-month daily regimen. Respir Med 108: 654-9
- Khan A, Sterling TR, Reeves R (2006) Lack of weight gain and relapse risk in a large tuberculosis treatment trial. Am J RespirCrit Care Med 174: 344-48.
- Hamilton CD, Stout JE, Goodman PC (2008) The value of end-of- treatment chest radiograph in predicting pulmonary tuberculosis relapse. Int J Tuberc Lung Dis 12: 1059-64.
- Chee CB, Emmanuel SC, Wang YT (1997) A brave STEP forward – the Singapore Tuberculosis Elimination Programme. Singapore Med J 38: 359-60.
Citation: Chee CBE (2021) Recurrent TB Disease in Singapore: A Retrospective Case Control Study. J Infect Dis Ther 9: 458. DOI: 10.4172/2332-0877.1000458
Copyright: © 2021 Chee CBE. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 1858
- [From(publication date): 0-2021 - Apr 26, 2025]
- Breakdown by view type
- HTML page views: 1149
- PDF downloads: 709