Research Article Open Access
Recruitment in a Pediatric Clinical Research Trial Targeting Underserved Populations: Efforts and Challenges
| Kathy A. Ireland1, Aaron J. Manders1*, Barbara E. Corkey2 and Carine M. Lenders1 | |
| 1Boston Medical Center, USA | |
| 2Boston University School of Medicine, USA | |
| Corresponding Author : | Aaron J. Manders, MS, RD, LDN Boston Medical Center, 88 E. Newton St. Vose 507 Boston, MA 02118, USA Tel: 6174143582 E-mail: aaron.manders@bmc.org |
| Received May 06, 2015; Accepted May 26, 2015; Published June 05, 2015 | |
| Citation: Ireland KA, Manders AJ, Corkey BE, Lenders CM (2015) Recruitment in a Pediatric Clinical Research Trial Targeting Underserved Populations: Efforts and Challenges. J Obes Weight Loss Ther 5:262. doi:10.4172/2165-7904.1000262 | |
| Copyright: © 2015 Ireland KA, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | |
Visit for more related articles at Journal of Obesity & Weight Loss Therapy
Abstract
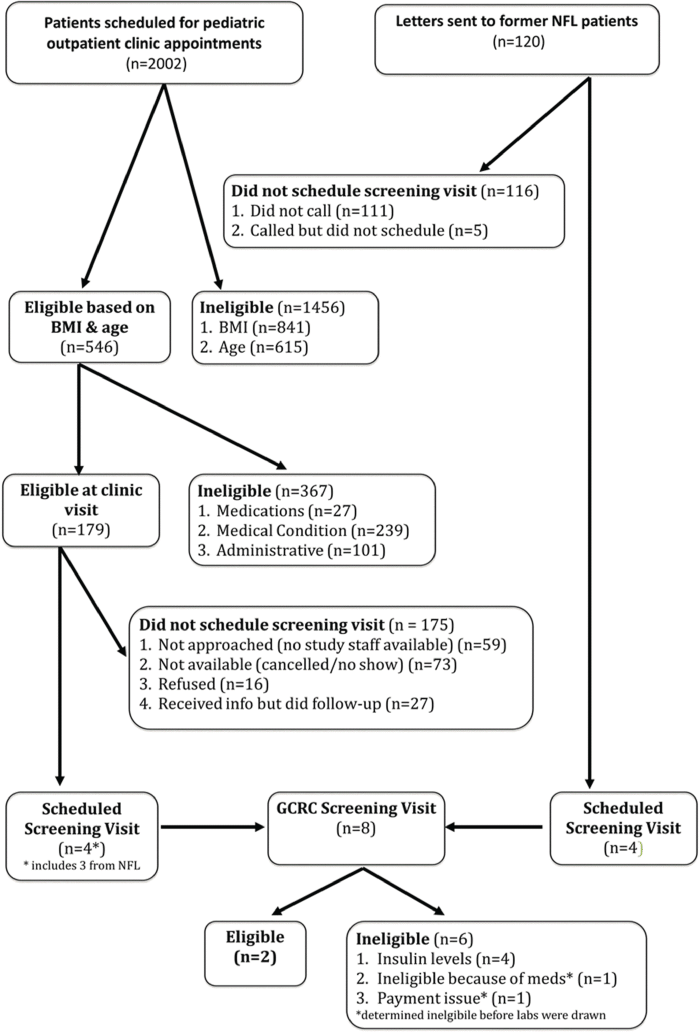
Introduction: To describe recruitment difficulties in a pediatric clinical trial targeting underserved pediatric obese populations. Methods: We planned a 6-month randomized, double-blind, placebo-controlled clinical trial of glutamine vs. placebo, to reduce HOMA_IR and weight gain in obese adolescents. Participation required 5 visits at a research center at 8:00 AM. Cash incentives were provided at visits. After recruitment difficulties, study design was modified and recruitment efforts were intensified over a 14-week period. Subjects were recruited from Boston Medical Center’s (BMC) pediatric outpatient clinics including the pediatric obesity program (NFL) which was staffed by members of the research team. Results: 2002 adolescents were evaluated: 546 met BMI and age criteria. After further exclusions, 179 were eligible for a screening visit but only 4 attended. Additionally, 120 recruitment letters were sent to NFL patients, resulting in 4 attending a screening visit. Seven of the 8 adolescents attending a screening visit were from NFL, and 2 were randomized but subsequently lost to follow-up. Discussion: Recruitment of pediatric patients from low-income and minority populations at BMC to a clinical trial is difficult. Challenges included strict inclusion/exclusion criteria and rigid appointment schedules. Existing patientclinician relationships may increase recruitment. Future trials should use more flexible study designs.
Methods: We planned a 6-month randomized, double-blind, placebo-controlled clinical trial of glutamine vs. placebo, to reduce HOMA_IR and weight gain in obese adolescents. Participation required 5 visits at a research center at 8:00 AM. Cash incentives were provided at visits. After recruitment difficulties, study design was modified and recruitment efforts were intensified over a 14-week period. Subjects were recruited from Boston Medical Center’s (BMC) pediatric outpatient clinics including the pediatric obesity program (NFL) which was staffed by members of the research team.
Results: 2002 adolescents were evaluated: 546 met BMI and age criteria. After further exclusions, 179 were eligible for a screening visit but only 4 attended. Additionally, 120 recruitment letters were sent to NFL patients, resulting in 4 attending a screening visit. Seven of the 8 adolescents attending a screening visit were from NFL, and 2 were randomized but subsequently lost to follow-up.
Discussion: Recruitment of pediatric patients from low-income and minority populations at BMC to a clinical trial is difficult. Challenges included strict inclusion/exclusion criteria and rigid appointment schedules. Existing patient-clinician relationships may increase recruitment. Future trials should use more flexible study designs.
Given the lack of clinical trials in pediatric and minority populations and the public health call to reduce risk of T2DM, we sought to conduct a 6-month randomized, double-blind, placebo-controlled clinical trial of pharmacological doses of glutamine versus placebo in obese Hispanic adolescents with a family history of T2DM and who receive care at an urban outpatient medical center. The purpose of the study was to determine the effect of pharmacological doses of glutamine on weight gain and HOMA-IR (insulin resistance) among Hispanic adolescents. Participation in the study required morning visits to a research center to obtain fasting bloodwork. Ultimately, the study was redesigned due to inability to recruit eligible participants. The objective of this article is to describe the barriers to participation observed in this trial of underserved pediatric minorities and provide recommendations for future clinical trials in such a population.
Participation in the study required five visits to the Clinical Transitional Science Institute (CTSI, formerly, General Clinical Research Center) over a 6-month period: a baseline visit to determine eligibility (week-4), a run-in visit to determine if the subject was able to comply with study protocol (week-2), and randomization (week 0), mid-point (week 12), and final (week 24) visits. All visits were conducted at 8:00 AM so that fasting blood work could be obtained (Table 1).
However, given recruitment difficulties, the study design was altered multiple times over the following years. Initially, pediatric patients enrolled in the study were Hispanic individuals with a family history of T2DM. The inclusion criteria were then expanded to include individuals from all races/ethnicities with or without a family history of T2DM (Table 2). Despite this change, there was little improvement in recruitment. As a result, we decided to reevaluate and improve our recruitment strategies.
Recruitment efforts were intensified over a period of 14 weeks during the summer of 2010. A team of research assistants, a dietitian, student volunteers, a physician nutrition specialist, and a consultant with expertise in recruitment for studies targeting similar populations worked together to maximize recruitment of eligible patients in the outpatient clinics. Each week, the research team received a list of all patients scheduled for visits at pediatric outpatient primary care and specialty clinics at BMC the following week. The list included patients scheduled for visits in a variety of outpatient clinics including the pediatric obesity clinic, Nutrition and Fitness for Life (NFL). Investigators of this study were also clinicians at the NFL clinic.
Using the electronic medical record (EMR), the research team conducted chart reviews to determine the potential eligibility of patients with appointments in the various clinics based on criteria including age, medical history, and medications (Table 2). A member of the research team was assigned to meet eligible patients at their appointment and inform them of the study. Eligible patients were given a pamphlet describing the study and provided with the opportunity to further discuss the study. Individuals interested in enrolling in the study were then scheduled for an appointment for a baseline/screening visit at the CTSI. The family received a reminder call the day before the visit with directions to the CTSI and instructions to arrive fasting. Parent consent and participant consent or assent was obtained based on age.
Additionally, letters informing potential subjects of the study were sent to patients ages 12-19 who attended the NFL clinic at BMC. Along with the letter, a study brochure and flyer were sent. Patients were encouraged to call the research team if interested in participating in the study. Interested and eligible patients who called were scheduled for a baseline/screening visit in the CTSI. All individuals also received incentives during the study: 20 dollars at signing the consent form, 30 dollars at the baseline visit, and 50 dollars at each of their following three study visits. This study was approved by the Institutional Review Board (IRB) at Boston University.
Forty percent (n=73) of these patients (n=179) either cancelled or did not keep their appointment, which is consistent with the appointment cancellation/no-show rate of outpatient pediatric appointments at BMC. Another 32% (n=59) were not approached because an investigator was not available at the time of the appointment. This left only 26% (n=47) patients available to be approached by a member of the study team. Of the patients approached, 9.3% (n=4) agreed to participate in the study. Three of the 4 (75%) who agreed to participate were patients of the NFL clinic. Recruitment via letters sent to former NFL patients (n=120) yielded 4 (3.3%) additional interested patients. In total, 8 subjects attended a screening visit at the CTSI. Eighty-eight percent (n=7/8) of them had an existing or previous relationship with NFL.
At the baseline visit at the CTSI, 2 subjects were determined to be ineligible; one because of the start of a new medication and one because of inability to provide documentation to receive payment. Of the 6 subjects who had blood-work done, 33% (n=2) had a fasting insulin ≥ 104.2 pmol/L, which was higher than the expected rate of 20%. One subject was lost to follow-up prior to the first visit while the other subject was lost to follow-up before the final visit, resulting in a study completion rate of 0%.
With these findings, the study team decided that recruitment efforts had failed and a new course of action was needed to conduct the trial. As a result, the research team decided to recruit patients directly from the NFL clinic. However after 8 months, only one additional subject enrolled in the study. Anecdotally, potential subjects expressed an unwillingness and/or inability to attend 8:00 AM study visits on school days and the desire to continue lifestyle counseling in the NFL clinic while enrolled in the study.
In April 2012 the study was stopped due to poor recruitment with the intention of changing the design of the study again. Following these changes, we received approval from the IRB to conduct the study at the NFL clinic, where patients received lifestyle counseling. At this time, the study was no longer a double-blinded, placebo-controlled, randomized clinical trial and was now open labeled. Patients were either randomized to take glutamine supplements for 12-14 weeks while receiving NFL lifestyle counseling or not to take glutamine supplements while receiving NFL lifestyle counseling. Notable changes in the study design included: 1) no minimum insulin level required, 2) recruitment from NFL clinic only, 3) shorter study duration and study visits taking place at the NFL clinic at the time of a regularly scheduled patient appointment, and 4) non-fasting blood work was drawn (Table 3). A more accessible study with more flexible visits was restarted in April 2013. Since revising our study protocol, we have screened more than 300 potentially eligible participants in the NFL clinic over a 16-month period, of which 24 provided consent, 12 were enrolled, and 10 have completed the study (two were lost to follow up).
Our study observations confirm findings from other studies [14-17]. As observed in our study, many factors may create an environment that makes recruitment more challenging, including work demands, ability to travel, and limited access to child care [14,16]. One barrier identified in our study was the requirement to arrive at 8:00 AM for a fasting blood draw. This may be especially difficult for single parents working multiple jobs and struggling to provide basic needs. Similarly, this time may be hard on adolescents who tend enjoy sleeping later, especially during summer months. Therefore, scheduling appointments after school and working hours may improve recruitment rates and retention of patients.
The initial focus of our study on Hispanic populations may have helped obtain a more homogeneous sample but may also have negatively affected our ability to recruit patients. Poor parental understanding [18] and language barriers have been found to be a discouraging factor for Hispanic participation [14]. More liberal eligibility criteria may help encourage recruitment but may also limit the strength of the conclusions. Unfortunately, both pediatric and minority populations are underrepresented in research, which is a cause of concern for discrimination [8,11,14,15,18]. Potential factors that may have contributed to underrepresentation of children in obesity trials include few funding opportunities and the requirement of more rigorous oversight [8,11]. Another factor is parental consent. Some authors report that parents from lower socioeconomic and education background are more likely to consent to a study [19,20] but their understanding of risk is lower [21,22] and their trust in research may thus be compromised [23-26]. Others [14,15] suggest that minorities are no less willing to participate in research. Therefore, efforts should be made to understand underrepresentation of pediatric patients and minorities.
Some authors [27] have shown higher recruitment rates in clinics where investigators already have an existing relationship with the potential research subjects, indicating that patients may be more willing to enroll in a study when they know an investigator personally, which is consistent with our findings. However, this dual relationship also raises concerns about coercion. Patients may confuse experimental care with standard of care. In that case, the investigators should work diligently to clearly explain both types of treatment [27] and to provide material that is written at an appropriate reading level [18]. Warner et al. [28] suggested that while challenging, conducting research in low-income and predominately minority populations is feasible but requires additional strategies. These authors used several strategies for aiding enrollment and retention including night and weekend appointments, ample study staff, and call centers to give patient appointment reminders. In our study, we were unable to approach 59 potentially eligible participants due to limited research staff resources. Conducting research in populations from minority and low income background requires creativity, time, and resources. Unfortunately, these requirements are scarce and funding for pediatric research remains limited [11]. Like Warner et al. [28] we believe that more funding is needed to improve recruitment in clinical trials in institutions targeting underserved pediatric populations.
In summary, our study recruitment challenges confirm previous findings of difficulties enrolling pediatric, minority, and underserved populations. However, we found that the location of the experiment and the flexibility of schedules were key to improving recruitment. More studies are needed to maximize participation of underserved populations to randomized clinical trials.
We are grateful for the suggestions provided by Anne Merewood and her team regarding recruitment of study subjects at Boston Medical Center Pediatric. In addition, we would like to thank the outpatient clinicians for their patience and support and are indebted to our patients and families for participating in this study. All authors have read and approved the final version this paper.
References
- Ogden CL, Carroll MD, Kit BK, Flegal KM (2012) Prevalence of obesity and trends in body mass index among US children and adolescents, 1999-2010. JAMA 307: 483-490.
- Flegal KM, Carroll MD, Kit BK, Ogden CL (2012) Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999-2010. JAMA 307: 491-497.
- Finkelstein EA, Trogdon JG, Cohen JW, Dietz W (2009) Annual medical spending attributable to obesity: payer-and service-specific estimates. Health Aff (Millwood) 28: w822-831.
- Cawley J, Meyerhoefer C (2012) The medical care costs of obesity: an instrumental variables approach. J Health Econ 31: 219-230.
- Berrington de Gonzalez A, Hartge P, Cerhan JR, Flint AJ, Hannan L, et al. (2010) Body-mass index and mortality among 1.46 million white adults. N Engl J Med 363: 2211-2219.
- Kitahara CM, Flint AJ, Berrington de Gonzalez A, Bernstein L, Brotzman M, et al. (2014) Association between class III obesity (BMI of 40-59 kg/m2) and mortality: a pooled analysis of 20 prospective studies. PLoS Med 11: e1001673.
- National Institutes of Health (1998) Policy and Guidelines on the Inclusion of Children as Participants in Research Involving Human Subjects.
- Tishler CL, Reiss NS (2011) Pediatric drug-trial recruitment: enticement without coercion. Pediatrics 127: 949-954.
- Hussain-Gambles M, Leese B, Atkin K, Brown J, Mason S, et al. (2004) Involving South Asian patients in clinical trials. Health Technology Assessment 8.
- Rochon PA, Mashari A, Cohen A, Misra A, Laxer D, et al. (2004) The inclusion of minority groups in clinical trials: problems of under representation and under reporting of data. Account Res 11: 215-223.
- Hay WW Jr, Gitterman DP, Williams DA, Dover GJ, Sectish TC, et al. (2010) Child health research funding and policy: imperatives and investments for a healthier world. Pediatrics 125: 1259-1265.
- Apovian C, Bigornia S, Cullum-Dugan D (2009) Milk-Based Nutritional Supplements in Conjunction With Lifestyle Intervention in Overweight Adolescents. Infant, child & adolescent nutrition 1: 37-44.
- Lee JM, Okumura MJ, Davis MM, Herman WH, Gurney JG (2006) Prevalence and determinants of insulin resistance among U.S. adolescents: a population-based study. Diabetes Care 29: 2427-2432.
- Wendler D, Kington R, Madans J, Van Wye G, Christ-Schmidt H, et al. (2006) Are racial and ethnic minorities less willing to participate in health research? PLoS Med 3: e19.
- Kressin NR, Meterko M, Wilson NJ (2000) Racial disparities in participation in biomedical research. J Natl Med Assoc 92: 62-69.
- Calderón JL, Baker RS, Fabrega H, Conde JG, Hays RD, et al. (2006) An ethno-medical perspective on research participation: a qualitative pilot study. MedGenMed 8: 23.
- Heller C, Balls-Berry JE, Nery JD, Erwin PJ, Littleton D, et al. (2014) Strategies addressing barriers to clinical trial enrollment of underrepresented populations: a systematic review. ContempClin Trials 39: 169-182.
- Tait AR, Voepel-Lewis T, Malviya S (2003) Participation of children in clinical research: factors that influence a parent's decision to consent. Anesthesiology 99: 819-825.
- Harth SC, Thong YH (1990) Sociodemographic and motivational characteristics of parents who volunteer their children for clinical research: a controlled study. BMJ 300: 1372-1375.
- Harth SC, Johnstone RR, Thong YH (1992) The psychological profile of parents who volunteer their children for clinical research: a controlled study. J Med Ethics 18: 86-93.
- Young-Hyman D, Herman LJ, Scott DL, Schlundt DG (2000) Care giver perception of children's obesity-related health risk: a study of African American families. Obes Res 8: 241-248.
- Tait AR, Voepel-Lewis T, Malviya S (2003) Do they understand? (part I): parental consent for children participating in clinical anesthesia and surgery research. Anesthesiology 98: 603-608.
- Reverby SM (2001) More than fact and fiction. Cultural memory and the Tuskegee Syphilis Study. Hastings Cent Rep 31: 22-28.
- Gamble VN (1993) A legacy of distrust: African Americans and medical research. Am J Prev Med 9: 35-38.
- Thomas SB, Quinn SC (1991) The Tuskegee Syphilis Study, 1932 to 1972: implications for HIV education and AIDS risk education programs in the black community. Am J Public Health 81: 1498-1505.
- Corbie-Smith G, Thomas SB, Williams MV, Moody-Ayers S (1999) Attitudes and Beliefs of African Americans Toward Participation in Medical Research. Journal of General Internal Medicine 14: 537-546.
- Macneill V, Nwokoro C, Griffiths C, Grigg J, Seale C (2013) Recruiting ethnic minority participants to a clinical trial: a qualitative study. BMJ Open 3.
- Warner ET, Glasgow RE, Emmons KM (2013) Recruitment and retention of participants in a pragmatic randomized intervention trial at three community health clinics: Results and lessons learned. BMC Public Health 13192.
Tables and Figures at a glance
| Table 1 | Table 2 | Table 3 |
Figures at a glance
 |
| Figure 1 |
Relevant Topics
- Android Obesity
- Anti Obesity Medication
- Bariatric Surgery
- Best Ways to Lose Weight
- Body Mass Index (BMI)
- Child Obesity Statistics
- Comorbidities of Obesity
- Diabetes and Obesity
- Diabetic Diet
- Diet
- Etiology of Obesity
- Exogenous Obesity
- Fat Burning Foods
- Gastric By-pass Surgery
- Genetics of Obesity
- Global Obesity Statistics
- Gynoid Obesity
- Junk Food and Childhood Obesity
- Obesity
- Obesity and Cancer
- Obesity and Nutrition
- Obesity and Sleep Apnea
- Obesity Complications
- Obesity in Pregnancy
- Obesity in United States
- Visceral Obesity
- Weight Loss
- Weight Loss Clinics
- Weight Loss Supplements
- Weight Management Programs
Recommended Journals
Article Tools
Article Usage
- Total views: 14305
- [From(publication date):
June-2015 - Apr 03, 2025] - Breakdown by view type
- HTML page views : 9793
- PDF downloads : 4512
