Recent Advances towards Understanding the Role of Opioid Receptor Phosphorylation
Received: 29-Dec-2015 / Accepted Date: 27-Jan-2016 / Published Date: 30-Jan-2016 DOI: 10.4172/2161-0681.1000264
Abstract
Opioid receptors, including mu (MOR for mu-opioid receptor), delta (DOR for delta-opioid receptor) and kappa (KOR for kappaopioid receptor), belong to the super family of G-protein coupled receptors (GPCRs). Those receptors, and particularly the MOR, are involved in pain control and are the targets of numerous drugs including morphine and its derivatives. The different opioid receptors are widely and differentially distributed throughout the human central nervous system and peripheral tissues [1]. Molecular studies revealed that MOR and DOR are highly expressed in brain regions with subtle differences; DOR are found at higher levels compared to MOR in cerebral cortex, putamen, nucleus accumbens, caudate nucleus, temporal lobe, and hippocampus. The cerebral distribution of DOR is in agreement with their involvement in motor as well as in cognitive functions. In contrast, higher levels of MOR are measured in cerebellum, the spinal cord and dorsal root ganglia suggesting that they may play an important role in the control of nociception. KOR are expressed in different brain regions although at moderate amounts and are implicated
Introduction
Opioid receptors, including mu (MOR for mu-opioid receptor), delta (DOR for delta-opioid receptor) and kappa (KOR for kappaopioid receptor), belong to the super family of G-protein coupled receptors (GPCRs). Those receptors, and particularly the MOR, are involved in pain control and are the targets of numerous drugs including morphine and its derivatives. The different opioid receptors are widely and differentially distributed throughout the human central nervous system and peripheral tissues [1]. Molecular studies revealed that MOR and DOR are highly expressed in brain regions with subtle differences; DOR are found at higher levels compared to MOR in cerebral cortex, putamen, nucleus accumbens, caudate nucleus, temporal lobe, and hippocampus. The cerebral distribution of DOR is in agreement with their involvement in motor as well as in cognitive functions. In contrast, higher levels of MOR are measured in cerebellum, the spinal cord and dorsal root ganglia suggesting that they may play an important role in the control of nociception. KOR are expressed in different brain regions although at moderate amounts and are implicated in pain perception, feeding and neuroendocrine functions.
It’s now well established that opioid receptors regulate numerous intracellular effectors through heterotrimeric Gi/o proteins [2]. Activation of potassium channels such as GIRK (G protein-coupled inwardly rectifying K+ channels) and inhibition of voltage-dependent Ca2+ channels result in hyperpolarization, decrease in neurotransmitters release and would participate to the anti-nociceptive effect of opioids. Inhibition of adenylyl cyclase, activation of phospholipase C/Ca2+ mobilization and mitogen-activated protein (MAP) kinases would rather be involved in receptor regulation and long-term opioid effects such as tolerance [3]. Upon sustained or chronic administration of opioids, a decrease in anti-nociceptive effects is generally observed and is defined as tolerance. In parallel at the cellular level, the decrease of signal transduction from the receptor is designed as desensitization. A lot of work has been conducted to decipher the molecular mechanisms of tolerance and desensitization. From the canonical model of GPCR regulation established by the Professor Robert J. Lefkowitz [4], who received the Nobel Prize in chemistry in 2012, receptor phosphorylation by kinases, including Gprotein receptor kinases (GRKs) was described as a critical step in desensitization. Receptor phosphorylation would enable recruitment of partners such as arrestins that promote G-protein uncoupling, receptor internalization but also signalling to other pathways (i.e. mitogen-activated protein kinases).
Opioid receptor phosphorylation
Since the first demonstration 20 years ago [5], different technical approaches with their own limitations have been developed to study opioid receptor phosphorylation: liquid chromatography-mass spectrometry techniques, antibodies directed against specific phospho- S/T, and site-directed mutagenesis. Since MOR is the main target of drugs, most of the studies have been conducted on this receptor type and to a lesser extent on DOR and KOR.
In vivo and in vitro proteomic studies from different laboratories revealed two major regions of the human and rodent MOR located at the carboxy-terminal tail whose phosphorylation state is modulated by agonist exposure [6-9]:
• Region 1 from amino acid 349 to 365 including the cluster 354TSST357.
• Region 2 from amino acid 366 to 382 including the motif 375STANT379.
Those peptides exist in different forms: non-phosphorylated, monoor poly-phosphorylated. Among the 11 putative phosphorylation sites, 7 were shown to be phosphorylated. Two residues, S363 and T370, are mono-phosphorylated in the absence of agonist and not regulated by opioids. Upon agonist activation, increase in mono- and polyphosphorylated peptides from regions 1 and/or 2 was observed. For instance, a slight increase of the phospho-S375 was observed after morphine administration in MOR extracted from mouse brain [9]. Several studies compared to ability of different agonists to promote MOR phosphorylation and showed rather quantitative than qualitative differences [6,8,9]. Upon [D-Ala2-MePhe4-Gly5-ol]enkephalin (DAMGO) or etonitazene exposure, the level of the polyphosphorylated peptides, including the motif STANT, was increased to a higher extent than in the presence of morphine. Constantly, those studies also revealed that agonist mediated a hierarchical phosphorylation of the carboxy-terminal amino acids: phosphorylation of the S375 is first required to obtain a polyphosphorylated receptor [9]. This would suggest that MOR phosphorylation is a multi-step process.
The group of Stefan Schulz elegantly developed antibodies against specific phospho-S and T and confirmed the constitutive phosphorylation at S363 of the MOR but revealed marked differences in the ability of various opioid agonists to promote phosphorylation at T370, S375, T376 and T379 [10,11]. For instance, morphine increases phosphorylation at S375 but not at T370 in contrast to DAMGO. Phosphorylation occurs rapidly after agonist exposure: after 20 sec, DAMGO increases phospho-S375 followed by phospho-T370. The phosphorylation at T379 is detected after 1 min exposure while longer times are required to observe phospho-T376 [11]. This latter study also evidenced that agonist concentration influences the phosphorylation at those positions: low concentrations promote phosphorylation at S375 and T379 while high concentrations are required to observe phospho- T370 and -T376.
MOR phosphorylation at S375 involves different GRK members with contradictory data probably resulting from different experimental conditions (agonist concentration, agonist exposure) and models (cell lines). Both GRK2 and GRK3 were shown to phosphorylate S375 upon activation by DAMGO [12] while these data were not confirmed by others [7]. Regarding morphine, both in vitro and in vivo data indicate that GRK5 but also GRK3 mediate phosphorylation at S375 [12,13]. Other kinases such as PKC would also be involved in basal and heterologous MOR phosphorylation but at different amino acids (S363 and T370) [14].
Regarding KOR and DOR phosphorylation, data are scarcer. In a recent paper, using both phosphoproteomic and phospho-S and T antibodies, the group of Liu-Chen showed that U50488 increases mono- and poly-phosphorylation at different amino acids located at the carboxy-terminal tail of the KOR [15]. As demonstrated for the MOR, KOR undergoes a hierarchical phosphorylation with S369 and T363 as the primary sites followed by S356 and T357. Quantitative difference in the phosphorylation level both at the primary and secondary site of KOR were evidenced upon different agonist’s exposure.
When considering DOR, S363 was described as the primary phosphorylation site using site-directed mutagenesis [16]. This residue is phosphorylated by the GRK2 [17] and different levels of phospho- S363 are observed upon different agonists exposure [18].
Role of phosphorylation in receptor regulation
Data presented above indicate that opioid receptors can undergo a rapid and hierarchical phosphorylation upon agonist activation. From the model of GPCR regulation proposed by Lefkowitz, it is tempting to speculate that phosphorylation would be responsible for desensitization and opioid tolerance by promoting G-protein uncoupling and receptor internalization. To respond to this assumption, different experimental models with mutations of the phosphorylation sites were used.
The S375A MOR mutant displays no more desensitization both on the cAMP and the ERK1/2 pathways upon morphine exposure compared to wild type receptor indicating the critical role of this residue in receptor regulation [19]. However, when using the S375A MOR knock-in mouse the role of this amino acid in acute and chronic morphine tolerance was not confirmed [20]. The study of MOR phosphorylation and desensitization on the cAMP pathway or on the G-protein–activated inwardly rectifying potassium channel (GIRK) conductance also revealed that the two processes are not correlated [21,22]. In this latter paper, the authors showed a total inhibition of Met-enkephalin- but not morphine-induced MOR desensitization only when all the 11 putative phosphorylation sites of the carboxy-terminal tail of the MOR were mutated into A. A total blockade of desensitization is observed for morphine only upon PKC inhibition and when using the MOR mutant for the 11 S/T phosphorylation sites. This may suggest that morphine promotes phosphorylation in intracellular loops in a PKC-dependent manner which would further enable phosphorylation of the carboxy-terminal residues; this receptor poly-phosphorylation would be required for a complete desensitization upon morphine exposure. A similar conclusion was proposed for the KOR upon U50488 exposure [15]: both a strong (above a given threshold) and a poly-phosphorylation are required to obtain receptor internalization.
Recently, using similar mutants of the carboxy-terminal tail of the MOR, Birdsong and colleagues suggested that phosphorylation of S and T residues would have distinct functions: phosphorylation of both TSST and STANT motifs (i.e., poly-phosphorylation) are necessary to promote desensitization on the outward potassium currents while phosphorylation of the TSST region would act as a allosteric modulator for ligand binding [23]. MOR desensitization partially requires GRK2 and 3 but maybe a non-dependent phosphorylation mechanism as inhibition of various kinases has no effect. Furthermore, reduction of S375 phosphorylation totally disrupts interaction between receptor and arrestins, severely impairs receptor internalization but partially reduced desensitization of the outward potassium currents [24]. This would suggest that desensitization and internalization would share common but also different regions of the carboxy-terminal tail of the MOR and the relationship between those processes are highly complex.
Conclusion
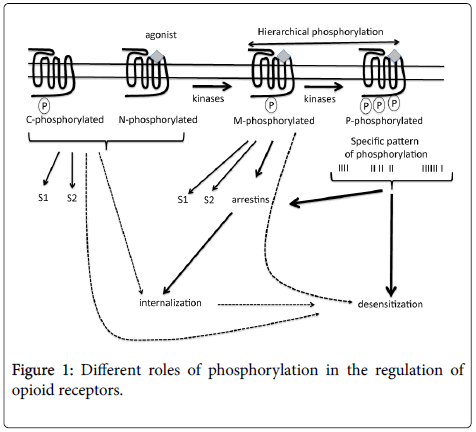
In the absence of agonist, opioid receptors exist as nonphosphorylated (N-phosphorylated) and constitutively phosphorylated (C-phosphorylated) at residues phosphorylated notably by PKC but not by agonists (Figure 1). Actually, several questions remain concerning the proportion of those constitutively phosphorylated receptor, their cellular localization (at cell surface or in intracellular compartments) and their roles. But given the involvement of PKC in morphine-mediated MOR desensitization [25], one can speculate that those constitutively phosphorylated amino acids could modulate further receptor phosphorylation and could participate to mechanisms implicated in tolerance.
C-phosphorylated: constitutively phosphorylated receptor; Nphosphorylated: non-phosphorylated receptor; M-phosphorylated: mono-phosphorylated receptor; P-phosphorylated: polyphosphorylated receptor; S1: signalling pathway 1; S2: signalling pathway 2; the importance of mechanism is related to the thickness of the arrows.
Several lines of evidence support the notion that opioid receptors undergo a GRK-mediated hierarchical phosphorylation; it means that kinases proceed by a multistep sequences to generate polyphosphorylated receptors (P-phosphorylated). This suggests that either the carboxy-terminal tail of the receptor contains an inhibitory motif preventing poly-phosphorylation or internalization as hypothesized by Whistler et al. [26] or due to steric hindrance, only the primary phosphorylation site could be phosphorylated. This first receptor phosphorylation is a very rapid process (less than 1 min) that would enable further phosphorylation. Several factors were identified to influence phosphorylation: the type of agonist (partial/total/biased agonism) and its concentration, the time of exposure, the expression level of kinases and phosphatases. So, it’s not surprising to note some discrepancies form literature data concerning this point.
There is now accumulating evidence which indicates that phosphorylation is not mandatory to promote desensitization and internalization but would increase such processes [27,28]. However, to obtain a profound and rapid desensitization and internalization, a poly-phosphorylation of receptor is required. As binding of a given agonist to receptors promotes selective conformations which enable phosphorylation at specific residues, a specific phosphorylation signature is observed for each opioid agonist. This is like a barcode which favours further interactions with selective partners and determines their conformations (i.e., arrestins) resulting in specific responses [29]. The minor role of phosphorylation in desensitization could explain the lack of a direct correlation between desensitization and internalization that were extensively studied. Whereas the role of receptor phosphorylation in tolerance is highly complex (see for review [30]), it is now clear that antinociceptive tolerance is associated with the activation of abnormal compensatory signalling pathways from opioid receptors (i.e., adenylyl cyclase superactivation, N-methyl-Daspartate receptors). For instance, it is possible to reverse morphineinduced tolerance when associating methadone with chronic morphine exposure to block such compensatory signalling pathways [31].
In conclusion, a lot of work has been conducted on the role of opioid phosphorylation and desensitization/tolerance but obviously, their relationships are not as simple as initially expected from the model of GPCR regulation. Most of studies focused on the carboxyterminal tail of opioid receptors but other intracellular regions could also participate in those regulations. Despite the recent determination of crystal structure of opioid receptors, we have no idea about conformational changes of the carboxy-terminal tail upon agonist binding. Furthermore, this highly flexible region could undergo various modifications (phosphorylation, palmitoylation, ubiquitination) which modulate further interactions between opioid receptors and partners. Future challenges will consist in determining the signatures of the receptor that are responsible for the activation of the signalling pathways associated with tolerance. Thus, we could expect to obtain more potent analgesic drugs.
References
- Peng J, Sarkar S, Chang SL (2012) Drug and Alcohol Dependence. Drug Alcohol Depend 124:223-228.
- Law PY, Wong YH, Loh HH (2000) Molecular mechanisms and regulation of opioid receptor signaling. Annu Rev PharmacolToxicol 40:389-430.
- Christie MJ (2008) Cellular neuroadaptations to chronic opioids: tolerance, withdrawal and addiction. Br J Pharmacol 154:384-396.
- Shenoy SK, Lefkowitz RJ (2011) ß-Arrestin-mediated receptor trafficking and signal transduction. Trends PharmacolSci 32:521-533.
- Pei G, Kieffer BL, Lefkowitz RJ, Freedman NJ (1995) Agonist-dependent phosphorylation of the mouse delta-opioid receptor: involvement of G protein-coupled receptor kinases but not protein kinase C. MolPharmacol 48:173-177.
- Lau EK, Trester-Zedlitz M, Trinidad JC, Kotowski SJ, Krutchinsky AN, et al. (2011) Quantitative encoding of the effect of a partial agonist on individual opioid receptors by multisite phosphorylation and threshold detection. Sci Signal 4:ra52.
- Moulédous L, Froment C, Dauvillier S, Burlet-Schiltz O, Zajac JM, et al. (2012) GRK2 protein-mediated transphosphorylation contributes to loss of function of µ-opioid receptors induced by neuropeptide FF (NPFF2) receptors. J BiolChem 287:12736-12749.
- Chen YJ, Oldfield S, Butcher AJ, Tobin AB, Saxena K, et al. (2013) Identification of phosphorylation sites in the COOH-terminal tail of the µ-opioid receptor. J Neurochem 124:189-199.
- Moulédous L, Froment C, Burlet-Schiltz O, Schulz S, Mollereau C (2015)Phosphoproteomic analysis of the mouse brain mu-opioid (MOP) receptor. FEBS Lett 589:2401-2408.
- Doll C, Konietzko J, Pöll F, Koch T, Höllt V, Schulz S (2011) Agonist-selective patterns of µ-opioid receptor phosphorylation revealed by phosphosite-specific antibodies. Br J Pharmacol 164:298-307.
- Just S, Illing S, Trester-Zedlitz M, Lau EK, Kotowski SJ, et al. (2013) Differentiation of opioid drug effects by hierarchical multi-site phosphorylation. MolPharmacol 83:633-639.
- Doll C, Pöll F, Peuker K, Loktev A, Glück L, et al.(2012) Deciphering µ-opioid receptor phosphorylation and dephosphorylation in HEK293 cells. Br J Pharmacol 167:1259-1270.
- Glück L, Loktev A, Moulédous L, Mollereau C, Law PY, Schulz S: Loss of Morphine Reward and Dependence in Mice Lacking G Protein-Coupled Receptor Kinase 5. Biol Psychiatry 76: 767-774.
- Illing S, Mann A, Schulz S (2014) Heterologous regulation of agonist-independent µ-opioid receptor phosphorylation by protein kinase C. Br J Pharmacol 171:1330-1340.
- Chen C, Chiu Y-T, Wu W, Huang P, Mann A, et al. (2015) Determination of sites of U50,488H-promoted phosphorylation of the mouse kappa opioid receptor (KOPR): Disconnect between KOPR phosphorylation and internalization. Biochem J.
- Kouhen OM, Wang G, Solberg J, Erickson LJ, Law PY, et al. (2000) Hierarchical phosphorylation of delta-opioid receptor regulates agonist-induced receptor desensitization and internalization. J BiolChem 275:36659-36664.
- Marie N, Aguila B, Hasbi A, Davis A, Jauzac P, et al. (2008) Different kinases desensitize the human delta-opioid receptor (hDOP-R) in the neuroblastoma cell line SK-N-BE upon peptidic and alkaloid agonists. Cell Signal 20:1209-1220.
- Aguila B, Coulbault L, Boulouard M, Léveillé F, Davis A, et al. (2007) In vitro and in vivo pharmacological profile of UFP-512, a novel selective delta-opioid receptor agonist; correlations between desensitization and tolerance. Br J Pharmacol 152:1312-1324.
- Schulz S, Mayer D, Pfeiffer M, Stumm R, Koch T, et al. (2004) Morphine induces terminal micro-opioid receptor desensitization by sustained phosphorylation of serine-375. EMBO J 23:3282-3289.
- Grecksch G, Just S, Pierstorff C, Imhof AK, Glück L, et al. (2011) Analgesic tolerance to high-efficacy agonists but not to morphine is diminished in phosphorylation-deficient S375A µ-opioid receptor knock-in mice. J Neurosci 31:13890-13896.
- Nowoczyn M, Marie N, Coulbault L, Hervault M, Davis A, et al. (2013)Remifentanil produces cross-desensitization and tolerance with morphine on the mu-opioid receptor. Neuropharmacology 73:368-379
- Yousuf A, Miess E, Sianati S, Du YP, Schulz S, et al. (2015) Role of Phosphorylation Sites in Desensitization of µ-Opioid Receptor. MolPharmacol 88:825-835.
- Birdsong WT, Arttamangkul S, Bunzow JR, Williams JT (2015) Agonist Binding and Desensitization of the µ-Opioid Receptor Is Modulated by Phosphorylation of the C-Terminal Tail Domain. MolPharmacol 88:816-824.
- Lowe JD, Sanderson HS, Cooke AE, Ostovar M, Tsisanova E, et al. (2015) Role of G Protein-Coupled Receptor Kinases 2 and 3 in µ-Opioid Receptor Desensitization and Internalization. MolPharmacol 88:347-356.
- Bailey CP, Oldfield S, Llorente J, Caunt CJ, Teschemacher AG, et al (2009) Involvement of PKC alpha and G-protein-coupled receptor kinase 2 in agonist-selective desensitization of mu-opioid receptors in mature brain neurons. Br J Pharmacol 158:157-164.
- Whistler JL, Tsao P, Zastrow von M (2001) A phosphorylation-regulated brake mechanism controls the initial endocytosis of opioid receptors but is not required for post-endocytic sorting to lysosomes. J BiolChem 276:34331-34338.
- Qiu Y, Law PY, Loh HH (2003) Mu-opioid receptor desensitization: role of receptor phosphorylation, internalization, and representation. J BiolChem 278:36733-36739.
- Qiu Y, Loh HH, Law PY (2007) Phosphorylation of the delta-opioid receptor regulates its beta-arrestins selectivity and subsequent receptor internalization and adenylyl cyclase desensitization. J BiolChem 282:22315-22323.
- Nobles KN, Xiao K, Ahn S, Shukla AK, Lam CM, et al. (2011) Distinct phosphorylation sites on the ß(2)-adrenergic receptor establish a barcode that encodes differential functions of ß-arrestin. Sci Signal 4:ra51.
- Allouche S, Noble F, Marie N (2014) Opioid receptor desensitization: mechanisms and its link to tolerance. Front Pharmacol 5:280.
- Posa L, Accarie A, Noble F, Marie N (2015) Methadone Reverses Analgesic Tolerance Induced by Morphine Pretreatment. Int J Neuropsychopharmacol.
Citation: Daccache G, Allouche S (2016) Recent Advances towards Understanding the Role of Opioid Receptor Phosphorylation. J Clin Exp Pathol 6:264. DOI: 10.4172/2161-0681.1000264
Copyright: © 2016, Daccache G, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 11613
- [From(publication date): 2-2016 - Jul 02, 2025]
- Breakdown by view type
- HTML page views: 10694
- PDF downloads: 919