Editorial Open Access
Recent Advances in Application of Tissue Engineering to Cancer Biology
Sumit Lal*Harvard Medical School, Harvard Univeristy, USA
- Corresponding Author:
- Sumit Lal
Harvard Medical School, Harvard Univeristy, USA
Tel: 857-247-6408
E-mail: lal@hms.harvard.edu
Received date December 12, 2013; Accepted date December 13, 2013; Published date December 26, 2013
Citation: Lal S (2013) Recent Advances in Application of Tissue Engineering to Cancer Biology. J Biomim Biomater Tissue Eng 18:e102. doi:10.4172/1662-100X.1000e102
Copyright: © 2013 KLal S. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Biomimetics Biomaterials and Tissue Engineering
Tissue engineering is an interdisciplinary field that applies the principles of engineering (materials science and biomedical engineering) and the life sciences (biochemistry, genetics, cell and molecular biology) to develop biological substitutes of human body parts to improve, replace or restore their biological functions [1]. Tissue engineering started as a research field dedicated to regeneration of skin, bone, and cartilage. More recently tissue engineering is being investigated for reconstruction of more complex, vascularised tissues, such as the liver or pancreas and development of novel 3D models of solid tumours [2].
Cancer biologists use monolayers of tumour cells for pre clinical drug testing. These 2D models of tumour cells lack in vivo tumour behaviour therefore are not ideal models for pre-clinical drug testing [3,4]. As a result of current 2D model inadequacies only 5% of cancer drug candidates enter clinical trials to receive approvals from U.S. Food and Drug Administration [5]. This represents a huge burden, both financially and clinically, as limited resources are devoted to compounds that will never demonstrate clinical benefit [6]. On the contrary 3D models of solid tumours closely resemble in vivo tumour microenvironment and metabolic characteristics [7]. 3D models of solid tumour consist of numerous cells grown in close contact in the presence of extra cellular matrix [8]. It is known that multicellular spheroids (a kind of 3D tumour model) show greater drug resistance than monolayer of tumour cells and closely resemble the drug resistance offered by solid tumours in vivo [9]. The reason for this is tumour cells when cultured in 3D in the presence of ECM change shape, lose polarity and form disorganized proliferative masses or aggregates similar to those seen in tumour progression in vivo [10,11]. Thus 3D tumour models are of increased biological significance and clinical relevance [12].
One of the major differences between 2D monolayers and 3D tumour models is the presence of extracellular matrix. Extra cellular matrix binds to cell surface adhesion molecules such as integrin and plays a vital role in development of tumours [12]. Naturally occurring as well as synthetic extra cellular matrix has been used to generate 3D tumour models. Among naturally occurring extra cellular matrix, matrigel, which consists of mainly type IV collagen and laminin has been extensively used [13]. Apart from matrigel, type I collagen gels has also been consistently used to develop 3D cancer models. It is suggested that cancer metastasis requires cancer cells to interact with a stromal environment that is often dominated by cross-linked networks of collagen. Therefore 3D gels of native type I or IV collagen are used recreate an in vivo like environment for migrating cancer cells [14-17]. Another important factor in extra cellular matrix gels is their microstructure. Gel microstructure is often hard to control as it depends on a number of factors including crosslinking agent, temperature and pH. Due to these and other limitations of naturally derived gels synthetic hydrogel-like biomaterials and scaffolds have been developed. These synthetic gels and scaffolds mimic key features of extra cellular matrix. Synthetic hydrogel offer may salient features including consistent matrix morphology, degradation rates and mechanical properties [15].
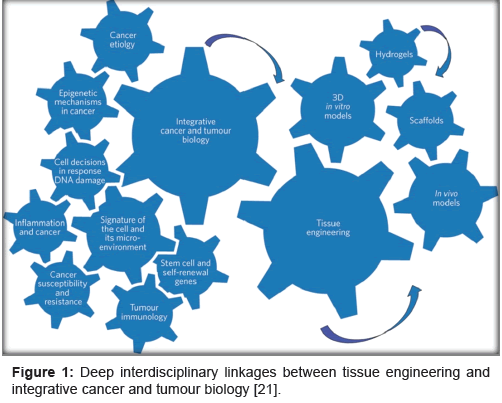
Over time different kinds of synthetic hydrogels and scaffolds have been developed from a wide variety of polymers. Out of which, macromers or copolymers of polyethylene glycol are the most common. The reason for this is the fact that polyethylene glycol readily forms a 3D polymeric network upon physical or chemical cross-linking. These 3D polymeric network can even be made biodegradable by insertion of functional united that can be cleaved enzymatically [18]. Combination of Arg-Gly-Asp (RGD) and aligante has also been used to make extra cellular matrix gel. One of the unique features of this gel is its controllable mechanical properties. Due to this many recent studies have used this gel system [19]. A more recent alternative to use of hydrogel and scaffold is 3D cell culture system. These systems consist of carcinoma cell seeded scaffolds. The angiogenic characteristics of these systems are found to be similar to 3D in vitro model and in vivo tumor xenograft experiments, but different from those observed in routine 2D cell culture system suggesting that these 3D cell culture systems are promising 3D models of in vivo tumour xenografts [20]. All the above suggests that deep interdisciplinary linkages between tissue engineering and integrative cancer and tumour biology (Figure 1) [21].
Part of the reason for this is that existing methods for imaging and analysing cell function and protein distribution are not compatible with 3D tumour models [22]. Therefore new methods need to be developed that take into consideration differences between 2D and 3D tumour models. Despite of all these challenges it is clear that 3D tumour models are superior to 2D models. 3D tumour models are not only ideal systems to model tumour cell behaviour and drug response but also novel candidates for elucidation of fundamental understanding of biological processes. Additionally, 3D tumour models are also ideal for studying the influence of biomechanical forces on tumour formation and homeostasis.
Conclusively, current 3D tumour model systems have limitations in mimicking the tumour behaviour in vivo but the innovation of new hydrogels, scaffolds and co-culture systems will help cancer biologist’s leverage on the novelties of 3D tumour model systems for pre clinical drug testing and studying pathways responsible for the growth and metastasis of cancer.
References
- Cowan CM, Soo C, Ting K, Wu B (2005) Evolving concepts in bone tissue engineering. Curr Top Dev Biol 66:239-285.
- Place ES, Evans ND, Stevens MM (2009) Complexity in biomaterials for tissue engineering. Nat Mater 8: 457-470.
- Bissell MJ, Radisky D (2001) Putting tumours in context. Nat Rev Cancer 1: 46-54.
- Jacks T, Weinberg RA (2002) Taking the study of cancer cell survival to a new dimension. Cell 111: 923-935.
- Kola I, Landis J (2004) Can the pharmaceutical industry reduce attrition rates? Nat Rev Drug Discov 3: 711-720.
- Sawyers C (2004) Targeted cancer therapy. Nature 432: 294-301.
- Ingber DE (2008) Can cancer be reversed by engineering the tumor microenvironment? Semin Cancer Biol 18: 356-364.
- Kenny HA, Krausz T, Yamada SD, Lengyel E (2007) Use of a novel 3D culture model to elucidate the role of mesothelial cells, fibroblasts and extra-cellular matrices on adhesion and invasion of ovarian cancer cells to the omentum. Int J Cancer 121: 1463-1472.
- Miller BE, Miller FR, Heppner GH (1985) Factors affecting growth and drug sensitivity of mouse mammary tumor lines in collagen gel cultures. Cancer Res 45: 4200-4205.
- Kenny PA, Lee GY, Myers CA, Neve RM, Semeiks JR, et al. (2007) The morphologies of breast cancer cell lines in three-dimensional assays correlate with their profiles of gene expression. Mol Oncol 1: 84-96.
- Partanen JI. Nieminen AI, M√?¬§kel√?¬§ TP, Klefstrom J (2007) Suppression of oncogenic properties of c-Myc by LKB1-controlled epithelial organization. Proc Natl Acad Sci USA 104: 14694-14699.
- Buck CA, Horwitz AF (1987) Cell surface receptors for extracellular matrix molecules. Annu Rev Cell Biol 3: 179-205.
- Chan BP, Leong KW (2008) Scaffolding in tissue engineering: general approaches and tissue-specific considerations. Eur Spine J 17: 467-479.
- Moss NM, Liu Y, Johnson JJ, Debiase P, Jones J, et al. (2009) Epidermal growth factor receptor-mediated membrane type 1 matrix metalloproteinase endocytosis regulates the transition between invasive versus expansive growth of ovarian carcinoma cells in three-dimensional collagen. Mol Cancer Res 7: 809-820.
- Sabeh F, Shimizu-Hirota R, Weiss SJ (2009) Protease-dependent versus -independent cancer cell invasion programs: three-dimensional amoeboid movement revisited. J Cell Biol 185: 11-19.
- Hotary KB, Allen ED, Brooks PC, Datta NS, Long MW, et al. (2003) Membrane type I matrix metalloproteinase usurps tumor growth control imposed by the three-dimensional extracellular matrix. Cell 114: 33-45.
- Cavallo-Medved D, Rudy D, Blum G, Bogyo M, Caglic D, et al. (2009) Live-cell imaging demonstrates extracellular matrix degradation in association with active cathepsin B in caveolae of endothelial cells during tube formation. Exp Cell Res 315: 1234-1246.
- Papavasiliou G, Songprawat P, Luna VP, Hammes E, Morris M, et al. (2008) Three-dimensional patterning of poly(ethylene glycol) hydrogels through surface-initiated photopolymerization. Tissue Eng Part C: Methods 14: 129-140.
- Fischbach C, Kong HJ, Hsiong SX, Evangelista MB, Yuen W, et al. (2009) Cancer cell angiogenic capability is regulated by 3D culture and integrin engagement. Proc Natl Acad Sci USA 106: 399-404.
- Fischbach C,Chen R, Matsumoto T, Schmelzle T, Brugge JS, et al. (2007) Engineering tumors with 3D scaffolds. Nat Meth 4: 855-860.
- Hutmacher DW (2010) Biomaterials offer cancer research the third dimension. Nat Mat 9: 90-93.
- Sameni M, Cavallo-Medved D, Dosescu J, Jedeszko C, Moin K et al. (2009) Imaging and quantifying the dynamics of tumor-associated proteolysis. Clin Exp Metastasis 26: 299-309.
Relevant Topics
Recommended Journals
Article Tools
Article Usage
- Total views: 14027
- [From(publication date):
January-2014 - Jul 19, 2025] - Breakdown by view type
- HTML page views : 9330
- PDF downloads : 4697