Real-World Evidence for the Widespread Effects of Fixed-Site High- Frequency Transcutaneous Electrical Nerve Stimulation in Chronic Pain
Received: 03-Sep-2018 / Accepted Date: 20-Sep-2018 / Published Date: 27-Sep-2018 DOI: 10.4172/2167-0846.1000329
Keywords: Chronic pain; Widespread; Transcutaneous electrical nerve stimulation; Wearable; Pain relief devices
Introduction
The prevalence of chronic pain among adults in the U.S. is 30% [1], at an annual economic cost of $600 billion [2]. Many people with chronic pain also have debilitating comorbidities including low quality sleep, anxiety, depression and poor overall health [3]. Prescription opioids are frequently used for chronic pain despite concerns about adverse events, addiction and long-term efficacy [4,5]. Alternatives such as non-steroidal anti-inflammatory drugs and antiepileptics also have side effects [6,7] and abuse potential [8]. For these reasons, there is a need for non-pharmacological treatments for chronic pain [9].
Transcutaneous electrical nerve stimulation (TENS) is a noninvasive treatment for chronic pain that has no major side effects. Conventional TENS is delivered through surface electrodes at a frequency and intensity that produces a strong, nonpainful sensation. The resulting stimulation of large diameter, deep tissue afferents [10], produces pain relief by decreasing central excitability and increasing central inhibition [11,12]. TENS provides pain relief or improves function in multiple types of chronic pain [11,12-19].
In traditional practice, TENS electrodes are applied over or adjacent to the targeted pain. Remote placement of electrodes are recommended if localized placement is undesirable due to skin irritation or allodynia, or impractical as in the case of phantom limb pain [20]. Stimulation proximal to the pain, distal to the pain, contralateral to the pain, within the same dermatomes as the pain and extra-segmentally have all been shown to be effective [21-30]. These remote analgesic effects provide a mechanism of action for fixed-site high-frequency TENS (FS-TENS) [31]. In this approach, the stimulator is designed for a predetermined location rather than for co-localization with pain. The advantage of FSTENS relative to traditional TENS is that a single target site enables wearable device designs that promote frequent use and may include embedded sensors to adaptively control stimulation and to measure objective outcomes such as patient activity and sleep. FS-TENS on the calf has been shown to be effective in chronic lower extremity and low back pain [31,32]. Encouraging preliminary results have also been obtained in chemotherapy induced neuropathic pain [33] and restless leg syndrome [34], FS-TENS on the upper arm has been found to be effective in reducing migraine pain [29].
The widespread effects of TENS are defined as pain relief beyond the anatomical site of stimulation [24]. Most studies of the relationship between stimulation sites and the location of TENS effects have been conducted in animal models [23,35] and with experimental pain in healthy humans [22,36]. The few studies that examined this question in chronic pain generally had short duration and small sample size [21,24,37]. The objective of this study was to confirm that FS-TENS produces widespread effects in a real-world chronic pain population.
This was examined by determining if distally placed (i.e., on calf) FS-TENS is effective for proximal chronic pain. The analyses were performed on self-administered FS-TENS for chronic pain. Two experimental predictions were derived from the widespread response of FS-TENS. The first was that participants with distal and proximal chronic pain would have comparable treatment outcomes. The second was that the strength of the dose-response association between FSTENS and treatment outcomes would be similar in participants with distal and proximal pain.
Methods
Study design
This retrospective cohort study was conducted on individuals selfadministering FS-TENS for chronic pain who contributed to an online database. The analyses were performed on an anonymized image of the database taken on March 5, 2018. Data were uploaded to the database via a mobile application linked to the participant’s FS-TENS device. The database includes data on device usage, demographics, chronic pain characteristics, daily pain ratings, and objective measurements of sleep and activity. The data is either automatically collected by the device (usage and objective measurements) or entered by the participant using the mobile application (demographics, pain characteristics, and pain ratings). This study evaluated device usage, demographics, a subset of chronic pain characteristics, and pain ratings. Device usage data included daily records of stimulation intensity, duration of stimulation, and whether the device was used overnight. Demographic data included the participants’ age, sex, height, and weight. Chronic pain characteristics included pain duration, painful health conditions, anatomic pain distribution, frequency of pain, pain pattern, and weather sensitivity. The choices for duration were “≤3 months”, “<1 year”, “1–3 years”, “4-10 years”, “>10 years”, and “not sure”. Painful health conditions were one or more among 15 medical conditions associated with chronic pain. Pain distribution was one or more among 10 body sites. Pain location was not qualified as unilateral or bilateral because of the potential for contralateral secondary hyperalgesia and allodynia [38]. Pain frequency was selected from among “every day or most days”, “several times a week”, “several times a month”, or “not sure”. Pain pattern captured the participant’s response to the question “When is your pain at its worst?” and was selected from among “morning”, “at night / while sleeping”, “when active”, “when resting”, “all the time” or “not sure”. Those participants selecting “all the time” were defined as having constant pain. Weather sensitivity denoted the participant’s selection of “yes”, “no” or “not sure” to the question “Does weather affect your chronic pain?” Additional data about triggering weather conditions (e.g., rain, cold) were collected but not analyzed in this study.
Pain intensity and interference were rated on an 11-point numerical rating scale derived from the Brief Pain Inventory-Short Form [39]. Four pain domains were assessed: average pain intensity over the past 24-hours (pain intensity), pain interference with sleep over the past 24-hours (sleep interference), pain interference with activity over the past 24-hours (activity interference) and pain interference with mood over the past 24-hours (mood interference). As a real-world study of self-administered TENS, there was no control exerted over when and how often participants rated their pain. As a result, the conditions under which pain was assessed, such as during rest or movement, were unknown.
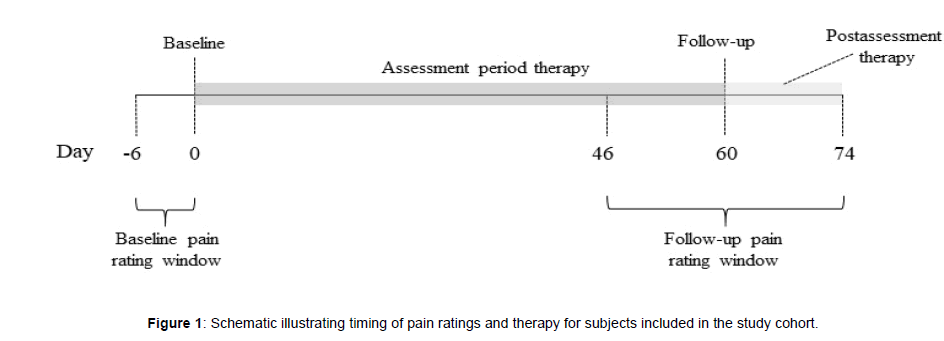
All participants were eligible for inclusion in the study, but only those that provided a complete set of demographic data, pain characteristics, and rated their pain intensity and interference with sleep, activity and mood at baseline and at follow-up were included. Participants with unspecified pain duration, pain duration of less than 3 months, an unspecified pain frequency, or a pain frequency less than several times a week were excluded because they were unlikely to have chronic pain. Figure 1 depicts the data collection timeline for participants included in the study. A valid baseline pain rating occurred on the first day (day 0) of device use or within the prior 6 days. A valid follow-up pain rating occurred between days 46 and 74 (i.e., ± 2 weeks of day 60). If more than one pain rating was available within the baseline or follow-up window, the rating closest to day 0 or day 60, respectively, was used. If more than one pain rating was available on a given day, the earliest was used.
All study participants consented to the use of their de-identified data for clinical research by establishing a database account. This study was institutional review board exempt because the investigators used a database without personal identifying information (see Code of Federal Regulations, Title 45, Department of Health and Human Services, Part 46, Protection of Human Subjects, Section 101(b)(4)).
TENS
All contributors to the database used the same over-the-counter TENS device (Quell®, NeuroMetrix Inc., Waltham, MA, USA). Instructional materials were those normally included with commercial purchase. The user guide describes device placement, explains the importance of stimulation at a “strong but comfortable” level, and suggests daily use with at least 3 hours of stimulation. The device design and use have been previously described in detail [31,32] and therefore will be briefly described here. The device is placed on the upper calf and is comprised of a one-channel electrical stimulator, a stretchable band to secure the stimulator to the leg, and an electrode array [31]. When placed on the upper calf, the electrode array wraps around the leg and therefore should overlap areas innervated by sensory dermatomes S2 through L4. The electrode array is comprised of 4 hydrogel pads, each approximately 30 cm2, configured as two electrodes. The peak output voltage and current are 100 V and 100 mA, respectively. The stimulation waveform is biphasic with a pulse duration of 280 μsec. The inter-pulse intervals are random such that the mean simulation frequency is 80 Hz and uniformly distributed between 60 and 100 Hz. Prior to first use, the device is algorithmically calibrated to a “strong but comfortable” level [31]. Subsequent stimulation is automatically controlled, although the user may manually decrease or increase the intensity. Each therapy session is 60 minutes, with sessions automatically starting every other hour while the device is worn [40,41].
An important variable in the present study was FS-TENS dose. There is no accepted definition for dose in a TENS application. Stimulation intensity has been shown to influence efficacy in experimental human pain [36,40,41] and acute pain [42-44]. Animal studies show that TENS anti-hyperalgesia requires sufficient intensity to activate deep tissue afferents [10]. No studies have systematically evaluated the effect of stimulation intensity on TENS efficacy in chronic pain, although several investigators commented on a possible relationship [17,21,37,45]. Another parameter that may influence TENS dose is the frequency of TENS use [16]. Regular use of TENS appears to have a cumulative effect on outcomes that may outlast the end of therapy [46-48]. The reason for this behavior is unknown but may involve a reversal in central sensitization or an increase in activity which leads to lower pain [16].
In the present study, FS-TENS dose was characterized by stimulation intensity (measured in milliamps) and utilization. The latter parameter represents the percentage of days, with a minimum of 30 minutes [49], of stimulation, during the assessment period. It is a measure of how regularly FS-TENS was used. A small value indicates infrequent use. A large value indicates regular use. A prior study of FS-TENS in chronic pain suggested a dose-response association between utilization and pain outcomes [32].
Stratification into distal and proximal pain
Study participants were stratified into distal and proximal pain groups. The distal pain group was comprised of participants whose pain distribution included, but was not limited to, foot or leg pain. Foot pain was included despite its location distal to the FS-TENS application site because of potential stimulation of sensory afferents innervating the foot and ankle. The proximal pain group was comprised of participants who did not identify either foot or leg pain and reported at least one pain site among hips, low back, trunk, upper extremities, neck and head.
Outcome measures
The outcome measures were changes in pain intensity, sleep interference, activity interference and mood interference from baseline to the 60-day (± 14 days) follow-up assessment. The outcomes measures were based on the differences of two 24-hour recall pain ratings (one at baseline, one at follow-up), which may be as reliable for detecting treatment effects as differences of multiple ratings [50]. The minimal clinically important difference (MCID) was defined as 1 point [51].
Statistical analyses
Device usage, demographics, and pain characteristics were quantified by the mean and standard deviation (SD) if numerical variables (e.g., age), and by proportions if categorical variables (e.g., sex, weather sensitivity). The statistical significance of group comparisons was evaluated by the two-group t-test for numerical variables and Pearson’s chi-squared test for categorical variables. Correction for multiple tests were not performed because the group comparisons were primarily descriptive rather than for evaluation of hypotheses. Although pain measures defined over a numerical rating scale are technically ordinal variables, they were treated as numerical variables for analyses. The FS-TENS dose-response association was characterized by the linear relationship between stimulation intensity or utilization and the outcome measures (e.g., between utilization and pain intensity) determined from multivariable regression. The term “association” is used to emphasize that the causality can only be inferred but not proven from observational data. Statistical significance (p<0.05) was required to confirm a dose-response association. The potential moderating effect of distal pain on the FS-TENS dose-response association was examined by moderated multivariable regression. A moderator (i.e., distal pain in the present study) is a variable that influences the direction and/or strength of an association between an independent variable (i.e., dose in the present study) and the dependent variable (i.e., pain outcomes in the present study). Four models were created with the baseline to followup change in average pain, sleep interference, activity interference and mood interference as the dependent variables. Each model had the same 15 independent variables and 1 first-order interaction term. The independent variables were age, sex (0 male, 1 female), BMI, baseline pain intensity, baseline sleep, activity and mood interference, number of painful health conditions, pain duration >3 years (0, ≤3 years; 1, >3 years), daily pain (0, pain several times per week; 1, pain everyday), constant pain (0, no; 1, all day pain), and weather sensitivity (0, pain not weather sensitive; 1, pain weather sensitive), distal pain (0, no distal pain; 1, distal pain), FS-TENS utilization and FS-TENS stimulation intensity. The number of pain sites was not included in the models because it was highly correlated with the distal pain indicator. An interaction term between the distal pain indicator variable and utilization represented the potential moderating effects of distal pain on the dose-response association. Utilization was chosen for the moderation analysis because it had the strongest univariate association to pain outcomes among all the FS-TENS dose parameters. Furthermore, utilization was highly correlated to most other dose parameters and therefore served as a proxy for FS-TENS dose.
The robustness of the regression models was characterized by the unadjusted R2 and the F-statistic. Both conventional and standardized coefficients were reported. Variables with non-zero coefficients (twotailed p<0.05) were considered statistically significant. A negative coefficient indicated that the variable was associated with a reduction in the pain measure (i.e., dependent variable) from baseline to followup, and therefore corresponded to an improvement in pain. A positive coefficient denoted that the variable was associated with an increase in the pain measure, thereby attenuating effectiveness. The marginal effects of distal pain on utilization were visualized by plotting the relationship with all other variables at their mean value. All analyses were performed with Stata Version 15.1 (StataCorp, College Station, TX).
Results
Study population
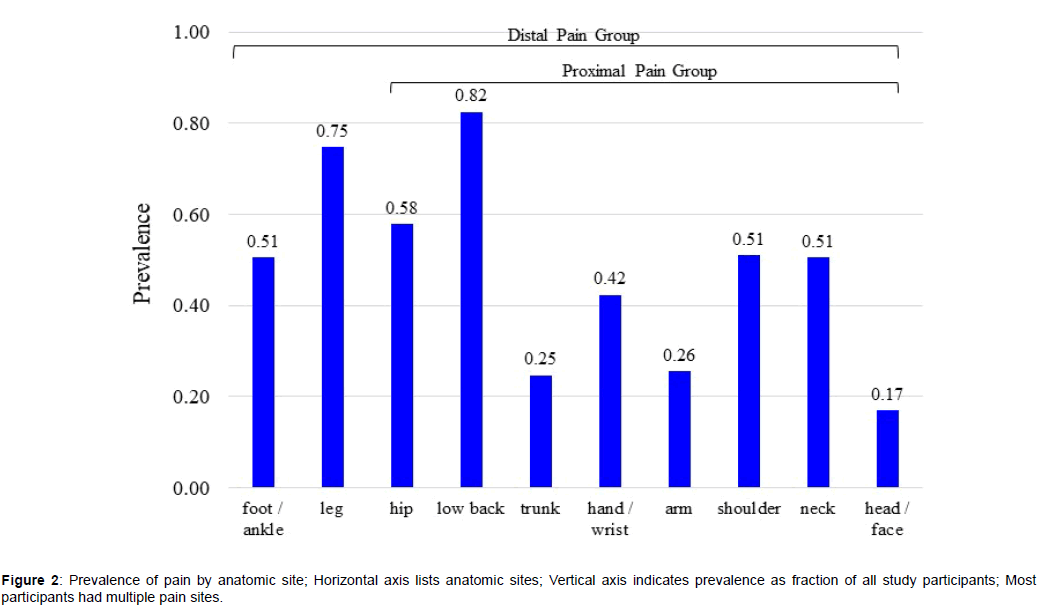
A total of 1676 FS-TENS users met the inclusion and exclusion criteria. Study participants had a mean of 4.9 (SD 2.5) pain sites. A limited pain distribution (1-3 sites) was reported by 33.8% of participants and an extensive pain distribution (6-10 sites) was reported by 36.2% [52]. Figure 2 shows the prevalence of pain sites. There were 296 (17.7%) participants with proximal pain and 1380 (82.3%) participants with distal pain.
Demographic and pain characteristics
Table 1 compares demographics and pain characteristics between the two groups. The distal pain group was heavier, had more pain sites and painful health conditions, longer pain duration, a higher prevalence of constant pain and weather sensitivity, and higher baseline pain intensity and pain interreference. The distribution of painful health conditions is shown in Table 2. The distal pain group had a higher prevalence of arthritis, fibromyalgia, diabetes, restless leg syndrome, previous arm/hand injury, previous leg/foot injury and headaches/migraine. There were no differences between groups for spine conditions.
| Characteristic | Proximal | Distal | p |
|---|---|---|---|
| Pain | Pain | ||
| (N=296) | (N=1380) | ||
| Female (%) | 56.5 | 59.5 | 0.399 |
| Age, years | 55.2 (14.0) | 55.5 (13.4) | 0.676 |
| BMI, kg/m2 | 28.3 (5.7) | 31.0 (7.2) | 0 |
| No. pain sites | 2.9 (1.7) | 5.3 (2.5) | 0 |
| No. painful health conditions | 3.0 (1.9) | 4.0 (2.4) | 0 |
| Pain >3 years (%) | 69.9 | 75.8 | 0.035 |
| Constant pain (%) | 40.9 | 52.5 | 0 |
| Daily pain (%) | 95.6 | 96.8 | 0.3 |
| Weather sensitive (%) | 51.4 | 65.4 | 0 |
| Baseline pain, 0-10 NRS | |||
| Pain intensity | 6.1 (1.9) | 6.3 (2.0) | 0.019 |
| Sleep interference | 4.9 (2.9) | 5.5 (2.9) | 0.002 |
| Activity interference | 6.2 (2.5) | 6.7 (2.4) | 0 |
| Mood interference | 6.0 (2.7) | 6.4 (2.6) | 0.04 |
| Statistical significance of group differences determined by two-group t-test for continuous variables and Pearson’s chi-squared test for categorical variables. | |||
| No. pain sites, see Figure 2 for list. | |||
| No. painful health conditions, see Table 2 for list. | |||
Table 1: Demographics and self-reported baseline pain characteristics.
| Condition | Proximal | Distal | p |
|---|---|---|---|
| Pain | Pain | ||
| (N=296) | (N=1380) | ||
| Musculoskeletal | |||
| Arthritis | 49 | 65.5 | <0.001 |
| Fibromyalgia | 18.2 | 27.7 | <0.001 |
| Spinal | |||
| Herniated disc | 28 | 31.9 | 0.195 |
| Spinal stenosis | 29.4 | 29.3 | 0.968 |
| Neuropathic | |||
| Diabetes | 8.5 | 14.9 | 0.003 |
| Complex regional pain syndrome | 13.5 | 16.7 | 0.171 |
| Shingles / post herpetic neuralgia | 4.1 | 5.2 | 0.405 |
| Restless leg syndrome | 9.1 | 22.5 | <0.001 |
| Multiple sclerosis | 1.4 | 2.2 | 0.364 |
| Previous injury | |||
| Back | 43.2 | 43.8 | 0.851 |
| Neck | 26 | 24 | 0.461 |
| Arm / hand | 16.6 | 21.8 | 0.044 |
| Leg / foot | 5.1 | 23.8 | <0.001 |
| Cancer | 3.7 | 5.9 | 0.14 |
| Headaches / migraine | 21.3 | 26.8 | 0.049 |
| Other | 25.3 | 33.6 | 0.006 |
| Statistical significance of group differences determined by Pearson’s chi-squared test. | |||
Table 2: Prevalence of self-reported painful health conditions.
FS-TENS dose
Table 3 compares FS-TENS dose in the two groups. There were no statistically significant differences. Utilization in the proximal pain group was 71.9% (SD 27.0%) and 73.3% (SD 26.7%) for the distal pain group. The median utilization was 80.3% in the proximal pain group and 82.0% in the distal pain group (p=0.335, two-sample Wilcoxon ranksum test). Among all participants, utilization was highly correlated to night utilization (r=0.56, p<0.001), hours/day (r=0.53, p<0.001) and hours/week (r=0.78, p<0.001) but not to stimulation intensity (r=-0.02, p=0.364).
| Parameter | All Participants | Proximal Pain | Distal | p |
|---|---|---|---|---|
| (N=1676) | (N=296) | Pain | ||
| (N=1380) | ||||
| Utilization (%) | 72.9 (26.8) | 71.9 (27.0) | 73.2 (26.7) | 0.386 |
| Night utilization (%) | 33.1 (31.7) | 33.2 (32.8) | 33.0 (31.4) | 0.952 |
| Hours/day | 6.6 (2.8) | 6.5 (2.8) | 6.6 (2.8) | 0.419 |
| Hours/week | 36.4 (23.3) | 35.1 (23.0) | 36.7 (23.3) | 0.284 |
| Stimulation intensity (mA) | 26 (14) | 25 (12) | 26 (14) | 0.239 |
| p, two-group t-test. | ||||
Table 3: FS-TENS dose characteristics.
Pain outcomes
The baseline to follow-up assessment period was 58.6 (SD 5.9) days in the proximal pain group and 58.9 (SD 5.6) days in the distal pain group. Table 4 shows the changes in the four pain measures for all participants and for those with high utilization (>90%). There were no statistically significant differences between the two groups for any pain measure.
| All Participants | Participants with Utilization >90% | |||||
|---|---|---|---|---|---|---|
| Pain Measure | Proximal Pain | Distal | p | Proximal Pain | Distal | p |
| (N=296) | Pain | (N=110) | Pain | |||
| (N=1380) | (N=548) | |||||
| Pain intensity | -0.38 (2.4) | -0.44 (2.5) | 0.723 | -0.98 (2.3) | -0.91 (2.3) | 0.787 |
| Sleep interference | -0.37 (3.0) | -0.40 (3.0) | 0.858 | -1.0 (2.8) | -0.85 (3.0) | 0.616 |
| Activity interference | -0.85 (2.8) | -1.1 (2.8) | 0.259 | -1.7 (2.9) | -1.5 (2.7) | 0.363 |
| Mood interference | -0.92 (3.0) | -1.1 (2.8) | 0.311 | -1.7 (3.1) | -1.6 (2.9) | 0.841 |
| Negative values indicate improvement in pain measure. | ||||||
| p, two-group t-test. | ||||||
Table 4: Change in pain outcomes from baseline to follow-up.
Moderated multivariable regression
Table 5 shows the results of multivariable regression modeling. BMI and sex were dropped from the models because they were not statistically significant for any pain outcome and reduced the sample size due to missing data (BMI, 384 participants; sex, 281 participants). The overall models were statistically significant at the p<0.001 level and explained 25-31% of the variance in pain outcomes. Age, baseline pain measures, number of painful health conditions, constant pain, weather sensitivity and utilization were statistically significant predictors of most pain outcomes. The strongest predictor of improvement was the baseline pain measure corresponding to the pain outcome (e.g., baseline pain intensity for change in pain intensity outcome). An elevated baseline mood interference was associated with worsening of pain intensity, sleep interference and activity interference. All four pain outcomes exhibited a dose-response association with utilization. Distal pain moderated (i.e., statistically significant interaction term) the association between utilization and the sleep and activity outcomes. The positive sign of the interaction term indicates that distal pain weakened the association between utilization and sleep / activity interference. Figure 3 graphically shows the marginal effects of distal pain on these relationships.
| Average Pain | Sleep Interference | Activity Interference | Mood Interference | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variable | B | SE B | β | B | SE B | β | B | SE B | β | B | SE B | β |
| Average pain | -0.762 | 0.039 | -0.600† | 0.098 | 0.046 | 0.063* | 0.125 | 0.043 | 0.086* | 0.089 | 0.046 | 0.06 |
| Sleep interference | 0.071 | 0.024 | 0.084* | -0.675 | 0.029 | -0.637† | 0.069 | 0.027 | 0.070* | 0.077 | 0.028 | 0.076* |
| Activity interference | 0.058 | 0.034 | 0.057 | -0.038 | 0.041 | -0.03 | -0.8 | 0.039 | -0.694† | -0.035 | 0.041 | -0.029 |
| Mood interference | 0.128 | 0.03 | 0.137† | 0.154 | 0.037 | 0.135† | 0.164 | 0.034 | 0.155† | -0.57 | 0.036 | -0.524† |
| Utilization | -0.022 | 0.008 | -0.234† | -0.027 | 0.01 | -0.238† | -0.032 | 0.009 | -0.309† | -0.026 | 0.01 | -0.240† |
| Stimulation intensity | -0.005 | 0.004 | -0.027 | -0.005 | 0.005 | -0.023 | -0.006 | 0.005 | -0.03 | -0.006 | 0.005 | -0.029 |
| Distal pain | -0.436 | 0.416 | -0.066 | -0.77 | 0.501 | -0.095 | -1.196 | 0.466 | -0.160* | -0.641 | 0.495 | -0.084 |
| Distal pain x Utilization | 0.006 | 0.009 | 0.093 | 0.014 | 0.011 | 0.171* | 0.017 | 0.01 | 0.220* | 0.007 | 0.011 | 0.089 |
| No. painful health conditions | 0.06 | 0.025 | 0.057* | 0.075 | 0.031 | 0.057* | 0.074 | 0.029 | 0.062* | 0.063 | 0.03 | 0.051* |
| Pain for >3 years | 0.055 | 0.13 | 0.01 | -0.043 | 0.157 | -0.006 | 0 | 0.146 | 0 | 0.012 | 0.155 | 0.002 |
| Daily pain | 0.189 | 0.302 | 0.014 | 0.322 | 0.364 | 0.019 | 0.215 | 0.339 | 0.014 | 0.096 | 0.36 | 0.006 |
| Constant pain | 0.273 | 0.114 | 0.055* | 0.449 | 0.137 | 0.074† | 0.231 | 0.128 | 0.041 | 0.43 | 0.136 | 0.075* |
| Weather sensitive | 0.38 | 0.118 | 0.074† | 0.425 | 0.143 | 0.068† | 0.297 | 0.133 | 0.051* | 0.177 | 0.141 | 0.03 |
| Age | -0.017 | 0.004 | -0.091† | -0.019 | 0.005 | -0.086† | -0.018 | 0.005 | -0.085† | -0.024 | 0.005 | -0.113† |
| R2 | 0.28 | 0.31 | 0.296 | 0.252 | ||||||||
| F(14, 1516) | 42.2† | 48.5† | 45.6† | 36.4† | ||||||||
| Column labels are as follows: B, coefficient; SE B, standard error of coefficient; β, standardized coefficient. | ||||||||||||
| Statistically significant predictors indicated by shaded background (probability that coefficient is different than zero: * p<0.05, † p<0.001). | ||||||||||||
Table 5: Predictors of pain outcomes from moderated multivariable regression.
Figure 3: Relationship between FS-TENS utilization and baseline to follow-up change in sleep and activity interference; Horizontal access indicates utilization; Vertical axis indicates change in sleep or activity interference on 0-11 point NRS; Blue, subjects with proximal pain; Green, subjects with distal pain; Error bars represent standard error; Participants with proximal pain (blue) have a steeper slope and therefore a stronger dose-response association for these two pain outcomes.
Discussion
The objective of this study was to confirm the widespread effects of FS-TENS in a real-world, chronic pain cohort. The study had two key findings. First, pain outcomes were similar in participants with distal and proximal pain. Second, the dose-response association in the proximal pain group was as strong as in the distal pain group. Taken together, these findings suggest that FS-TENS has widespread effects that improve outcomes in participants with pain proximal to the stimulation site.
Participants in the distal pain group and in the proximal pain group had similar demographic characteristics, differing only in BMI. The distal pain group was heavier by 2.7 kg/m2, which is consistent with the association between obesity and lower extremity pain [53]. There were clinically significant differences in pain characteristics between the two groups. The distal pain group reported more pain sites than their proximal pain counterparts. Pain sites are correlated [52] and therefore differences in the total number of pain sites in the two groups were expected. A greater number of pain sites is also associated with worse health [54], lower quality of life,55 and increased severity of chronic pain [54,55]. The distal pain group had more painful health conditions, longer pain duration and higher baseline pain intensity and pain interference with function. These participants were also more likely to report constant pain and weather sensitivity than those in the proximal pain group.
There were associations between distal pain and specific health conditions. The elevated risks of self-reported arthritis, diabetes, previous leg/foot injury and restless leg syndrome in participants with distal pain were consistent with the established link between these conditions and lower extremity pain. The increased risk of fibromyalgia among participants with distal pain may relate to the greater number of pain sites in this group. For example, 43% of the distal pain group had 6 or more pain sites compared to only 7% in the proximal pain group. Number of pain sites is a key component of fibromyalgia diagnostic criteria [56]. The reasons for the increased rate of previous arm/ hand injury and headaches/migraine in participants with distal pain is unclear. It is possible that the association to head pain is mediated through comorbid conditions such as fibromyalgia [57].
FS-TENS usage was the same in the two groups. This result is surprising because the distal pain group had more severe chronic pain. Device usage in this study was higher than in most TENS studies [49], but comparable to previous reports for successful long-term users of TENS [45,58]. In the study by Johnson and colleagues,45 subjects selfreported 39.7 (SD 19.8) hours/week of TENS use compared to 36.4 (SD 23.3) hours/week in the present study (Table 3). In the study by Fishbain, et al. [58], subjects self-reported a mean utilization of approximately 50% in the 2-months prior to assessment and 6.1 (SD 5.2) hours/day of TENS use. These values match the 73% utilization and 6.6 (SD 2.8) hours/day in the current study (Table 3).
The distal pain and proximal pain groups both demonstrated clinically meaningful improvement in pain outcomes from baseline to follow-up (Table 4). The mean reduction in activity and mood interference was at or near the MCID of 1 point for all participants in both groups. The mean reduction in pain intensity and sleep interference was at or near the MCID for participants with high utilization. Although the proximal pain group did not have pain at the site of FSTENS application, there were no differences in pain outcomes between this group and the distal pain group. This result is consistent with prior animal [12,59,60], and human studies [12,22-24,29], showing a broad anatomic effect of TENS and support that FS-TENS has widespread effects. An alternative explanation is that the FS-TENS acted entirely through non-specific (e.g., placebo) mechanisms in all participants irrespective of stimulation site. This possibility cannot be dismissed, however it is well accepted that TENS has specific analgesic effects, particularly when applied to the site of pain [20,44]. Another possibility is that FS-TENS acted through specific mechanisms in participants with distal pain but through a placebo effect in participants with proximal pain. While this mode of action cannot be excluded, it is not a parsimonious explanation for the results. Several recent studies have identified predictors of placebo response in chronic pain therapeutic trials [61,62]. An elevated baseline pain intensity consistently predicted a stronger placebo response. In the present study, the proximal pain group had lower pain intensity, decreased pain interference, and fewer pain sites than the distal pain group. Participant expectations of treatment efficacy are also associated with placebo effects [62-64]. It is doubtful that participants with proximal pain were more likely to anticipate positive results than those with distal pain given the upper calf placement of the FS-TENS device.
The primary objective of the multivariable regression model was to determine if distal pain moderated the FS-TENS dose-response association. Pain ratings are intrinsically variable with fluctuations of several points occurring in otherwise stable pain [65]. Nevertheless, the multivariable regression models accounted for 25%-30% of the variance in baseline to follow-up changes in the pain measures. The strongest predictors of improvement in pain outcomes were an elevated baseline pain measure corresponding to the pain outcome and greater utilization. Higher baseline pain has been shown to predict better outcomes for some chronic pain treatments [66] and worse outcomes for others [67]. Two studies that examined the impact of baseline pain characteristics on TENS treatment for chronic pain found that elevated baseline levels predicted worse outcomes [68,69]. The reasons for the inconsistent relationship between baseline pain and outcomes are unclear. In the present study, an elevated baseline mood interference was associated with smaller improvements in pain intensity, sleep interference and activity interference. The negative impact of mood on the efficacy of chronic pain treatments is well established [67,70].
Dose parameters, including stimulation intensity and frequency of use influence TENS efficacy [17,40,43,49]. The adequacy of the stimulation intensity in the present study was supported by the device calibration procedure and further instructions to titrate to a “strong but comfortable” level. The present study did not find an association between stimulation intensity and FS-TENS effectiveness. This result was surprising given the relationship between intensity and outcomes in acute and experimental pain [36,40-44]. Rao and colleagues [21], demonstrated a positive correlation between higher intensity stimulation and pain relief for some types of chronic pain, including radiculopathy and peripheral nerve injury. However, other studies have failed to demonstrate a dose-response relationship between TENS intensity and chronic pain outcomes [17,45]. The therapeutic stimulation intensity is affected by many factors, including age, BMI and medical conditions that may have obscured an association to outcomes. In this study, the absolute stimulation intensity was used, whereas a more appropriate indicator may have been the intensity normalized to the participant’s sensation threshold. Future studies should consider these factors when evaluating the role of stimulation intensity in regulating chronic pain outcomes.
The present study demonstrated that higher utilization, a measure of frequency of TENS use, was associated with improved chronic pain outcomes. Each 10% increase in utilization was associated with a 0.2 to 0.3-point improvement in pain measures over the assessment period. This result re-enforces the importance of regular TENS use [32,49]. Distal pain moderated the association between FS-TENS utilization and changes in sleep and activity interference. The direction of moderation was such that distal pain decreased the strength of the associations. This suggests that the dose-response association between utilization and sleep and activity interference was attenuated by distal pain. Unlike sleep and activity interference, distal pain did not moderate the association between utilization and pain intensity or mood interference. Irrespective of the outcome measure, the dose-response association was as strong (i.e., pain intensity, mood interference) or stronger (i.e., sleep interference, activity interference) in the proximal pain group as in the distal pain group. This result is the opposite of what would be expected if FS-TENS effects were limited to the immediate area of stimulation.
A dose-response association does not prove causality [71], however it is supportive of a direct effect of FS-TENS on pain outcomes [72]. Moreover, the fact that the strength of the association was similar in participants with distal and proximal pain is consistent with FS-TENS having widespread effects. FS-TENS likely provided pain relief in participants with distal pain through peripheral, spinal and supraspinal means [12,73]. In participants without distal pain, the effect may have resulted primarily from supraspinal mechanisms, [11,12,24] although spinal mechanisms may have been involved with pain in stimulated dermatomes, such as in the lower back. The fact that the outcomes and dose-response association were similar in the two groups suggests that the benefits of peripheral, spinal and supraspinal analgesia may not be additive. This may be analogous to the non-additive effects of descending pain inhibition activated by TENS and conditioned pain modulation (CPM) [74].
Study strengths
This study had several strengths. First, the analyses were based on a large, heterogeneous sample of chronic pain. This supports generalization of the results to real-world use of FS-TENS. Second, moderated multivariable regression modeling was used to measure the dose-response association and to evaluate the impact of distal pain on pain outcomes, while controlling for common covariates of therapeutic efficacy such as demographics, pain characteristics and baseline pain measures [65]. Third, device usage parameters including utilization and stimulation intensity were objectively tracked. Electronic tracking of TENS usage addresses overestimation bias from subjective recall [45].
Study limitations
This study had several limitations that may impact interpretation of the results. First, most participants used FS-TENS for a majority of the two-month assessment period, therefore the study cohort was likely enriched with responders. It is possible that inclusion of participants who stopped using their device or who chose not to provide pain assessments would yield different results. Second, the distal pain group was comprised of participants with foot or leg pain but was not limited to these sites. This allocation was necessary because isolated foot and leg pain was rare. As a result it is not possible to completely assign the benefits of FS-TENS in the distal pain group to pain relief in the feet or legs. However, additional pain relief in proximal sites would strengthen the study conclusions. Third, there was a large discrepancy between the number of participants with proximal pain (18%) and those with distal pain (82%). This difference is not surprising given the high prevalence of chronic lower extremity pain. However, it may suggest that the absence of distal pain is an atypical variant of chronic pain and may limit the generalizability of the study results. Fourth, although the regression analyses controlled for available demographic and clinical variables, it is possible that unaccounted covariates would alter the results. For example, pain catastrophizing has been shown to influence health outcomes [75]. Fifth, pain outcomes were limited to baseline to follow-up changes in pain measures assessed by an 11-point numerical rating scale. Although these are recommended outcome measures for pain therapy trials [76], other outcomes such as global impressions, quality of life scales, and change in analgesic use may have yielded different results. Sixth, the study was retrospective which may increase the potential for bias. It may be beneficial to confirm the key findings with a prospective design. Finally, the results were obtained with a specific TENS device, electrode configuration, and stimulation parameters, and therefore the conclusions may not be generalizable to other TENS instruments and methods.
Conclusion
TENS produces pain relief by decreasing central excitability and increasing central inhibition [11,12]. FS-TENS is a form of TENS where the device is designed to be applied in a fixed location rather than for co-localization with the patient’s pain. In this large-scale study of FS-TENS users with heterogenous chronic pain, participants with distal and proximal pain had similar pain outcomes and dose-response associations. These results are consistent with FS-TENS having widespread effects beyond the site of stimulation.
Data Availability
The study authors will make the study data available to researchers interested in its use for academic, non-commercial purposes. Please contact the study authors.
Funding
This study was funded by NeuroMetrix, Inc. The company manufactures the FS-TENS device used in this study.
Conflicts of Interest
Drs. Gozani and Kong are employees and shareholders of NeuroMetrix, Inc., which funded this study.
References
- Johannes CB, Le TK, Zhou X, Johnston JA, Dworkin RH (2010) The prevalence of chronic pain in United States adults: Results of an Internet-based survey. J Pain11:1230-1239.
- Gaskin DJ, Richard P (2012) The economic costs of pain in the United States. J Pain13: 715-724.
- Toblin RL, Mack KA, Perveen G, Paulozzi LJ (2011) A population-based survey of chronic pain and its treatment with prescription drugs. Pain152:1249-1255.
- Turk DC, Wilson HD, Cahana A (2011) Treatment of chronic non-cancer pain. Lancet 377: 2226-2235.
- Krebs EE, Gravely A, Nugent S, Jensen AC, DeRonne B, et al. (2018) Effect of opioid vs nonopioid medications on pain-related function in patients with chronic back pain or hip or knee osteoarthritis pain: The SPACE randomized clinical trial. JAMA319:872-882.
- van Walsem A, Pandhi S, Nixon RM, Guyot P, Karabis A, et al. (2015) Â Relative benefit-risk comparing diclofenac to other traditional non-steroidal anti-inflammatory drugs and cyclooxygenase-2 inhibitors in patients with osteoarthritis or rheumatoid arthritis: A network meta-analysis. Arthritis Res Ther17:66.
- Toth C (2013) Pregabalin: Latest safety evidence and clinical implications for the management of neuropathic pain. Ther Adv Drug Saf5: 38-56.
- Chiappini S, Schifano F (2016) A decade of gabapentinoid misuse: An analysis of the european medicines agency's 'suspected adverse drug reactions' database. CNS Drugs30: 647-654.
- Simon LS (2011) Relieving pain in America: A blueprint for transforming prevention, care, education, and research. J Pain Palliat Care Pharmacother 26:197-198.
- Radhakrishnan R, Sluka KA (2005) Deep tissue afferents, but not cutaneous afferents, mediate transcutaneous electrical nerve stimulation-Induced antihyperalgesia. J Pain6: 673-680.
- Vance CG, Dailey DL, Rakel BA, Sluka KA (2014) Using TENS for pain control: The state of the evidence. Pain Manag4:197-209.
- DeSantana JM, Walsh DM, Vance C, Rakel BA, Sluka KA (2008) Effectiveness of transcutaneous electrical nerve stimulation for treatment of hyperalgesia and pain. Curr Rheumatol Rep10: 492-499.
- Johnson M, Martinson M (2007) Efficacy of electrical nerve stimulation for chronic musculoskeletal pain: A meta-analysis of randomized controlled trials. Pain130:157-165.
- Johnson MI, Bjordal JM (2011) Transcutaneous electrical nerve stimulation for the management of painful conditions: Focus on neuropathic pain. Expert Rev Neurother11:735-753.
- Jin DM, Xu Y, Geng DF, Yan TB (2010) Effect of transcutaneous electrical nerve stimulation on symptomatic diabetic peripheral neuropathy: A meta-analysis of randomized controlled trials. Diabetes Res Clin Pract 89:10-15.
- Sluka KA, Bjordal JM, Marchand S, Rakel BA (2013) What makes transcutaneous electrical nerve stimulation work? Making sense of the mixed results in the clinical literature. Phys Ther 93:1397-1402.
- Resende L, Merriwether E, Rampazo EP, Dailey D, Embree J, et al. (2018) Meta-analysis of transcutaneous electrical nerve stimulation for relief of spinal pain. Eur J Pain22: 663-678.
- Jauregui JJ, Cherian JJ, Gwam CU, Chughtai M, Mistry JB, et al. (2016) A meta-analysis of transcutaneous electrical nerve stimulation for chronic low back pain. Surg Technol Int 28: 296-302.
- Lee JE, Anderson CM, Perkhounkova Y, Sleeuwenhoek BM, Louison RR (2018) Transcutaneous electrical nerve stimulation reduces resting pain in head and neck cancer patients: a randomized and placebo-controlled double-blind pilot study. Cancer Nurs.
- MacPherson F, Colvin L (2015) Transcutaneous electrical nerve stimulation (TENS) : Research to support clinical practice. Oxford: Oxford University Press 114: 711-712.
- Rao VR, Wolf SL, Gersh MR (1981) Examination of electrode placements and stimulating parameters in treating chronic pain with conventional transcutaneous electrical nerve stimulation (TENS). Pain 11: 37-47.
- Brown L, Tabasam G, Bjordal JM, Johnson MI (2007) An investigation into the effect of electrode placement of transcutaneous electrical nerve stimulation (TENS) on experimentally induced ischemic pain in healthy human participants. Clin J Pain23: 735-743.
- Neto MLP, Maciel LYS, Cruz KML, Filho VJS, Bonjardim LR, et al. (2017) Does electrode placement influence tens-induced antihyperalgesia in experimental inflammatory pain model? Braz J Phys Ther21: 92-99.
- Dailey DL, Rakel BA, Vance CGT, Liebano RE, Amrit AS, et al. (2013) Transcutaneous electrical nerve stimulation reduces pain, fatigue and hyperalgesia while restoring central inhibition in primary fibromyalgia. Pain 154: 2554-2562.
- Kawamura H, Ito K, Yamamoto M, Yamamoto H, Ishida K, et al. (1997) The transcutaneous electrical nerve stimulatoin applied to contralateral limbs for the phantom limb pain. J Phys Ther Sci9:71-76.
- Katz J, France C, Melzack R (1989) An association between phantom limb sensations and stump skin conductance during transcutaneous electrical nerve stimulation (TENS) applied to the contralateral leg: a case study. Pain 36: 367-377.
- Saxena KN, Shokeen S, Taneja B (2016) Comparative evaluation of efficacy of transcutaneous electrical nerve stimulation administered by dermatomal stimulation versus acupuncture points stimulation. Northern Journal of ISA 1: 29‑34.
- Tsang HHY (1986) Diffuse Inhibition of flexion reflex by transcutaneous electrical nerve stimulation (tens) in man.
- Yarnitsky D, Volokh L, Ironi A, Weller B, Shor M, et al. (2017) Nonpainful remote electrical stimulation alleviates episodic migraine pain. Neurology 88:1250-1255.
- Garrison DW, Foreman RD (2002) Effects of transcutaneous electrical nerve stimulation (tens) electrode placement on spontaneous and noxiously evoked dorsal horn cell activity in the cat. Neuromodulation 5: 231-237.
- Gozani SN (2016) Fixed-site high-frequency transcutaneous electrical nerve stimulation for treatment of chronic low back and lower extremity pain. J Pain Res 9: 469-479.
- Kong X, Gozani SN (2018) Effectiveness of fixed-site high-frequency transcutaneous electrical nerve stimulation in chronic pain: A large-scale, observational study. J Pain Res11: 703-714.
- Gewandter J, Chaudari J, Kitt R, Ibegbu C, Serventi J, et al. (2017) Wearable TENs band for chemotherapy-induced peripheral neuropathy (CIPN): A feasibility study. The Journal of Pain 18: S89.
- Winkelman JW, Mei LA, Platt S, Schoerning L (2016) Pilot open-label trial of transcutaneous electrical nerve stimulation (TENS) below the knee for the treatment of restless legs syndrome (RLS). Sleep39(Abstract Supplement).
- Ainsworth L, Budelier K, Clinesmith M, Fiedler A, Landstrom R, et al. (2006) Transcutaneous electrical nerve stimulation (TENS) reduces chronic hyperalgesia induced by muscle inflammation. Pain120: 182-187.
- Aarskog R, Johnson MI, Demmink JH, Lofthus A, Iversen V, et al. (2007) Is mechanical pain threshold after transcutaneous electrical nerve stimulation (TENS) increased locally and unilaterally? A randomized placebo-controlled trial in healthy subjects. Physiother Res Int12: 251-263.
- Pallett EJ, Rentowl P, Johnson MI, Watson PJ (2014) Implementation fidelity of self-administered transcutaneous electrical nerve stimulation (TENS) in patients with chronic back pain: An observational study. Clin J Pain30: 224-231.
- Shenker NG, Haigh RC, Mapp PI, Harris N, Blake DR (2008) Contralateral hyperalgesia and allodynia following intradermal capsaicin injection in man. Rheumatology (Oxford) 47:1417-1421.
- Cleeland CS, Ryan KM (1994) Pain assessment: Global use of the brief pain inventory. Ann Acad Med Singapore 23:129-138.
- Moran F, Leonard T, Hawthorne S, Hughes CM, Johnson MI, et al. (2011) Hypoalgesia in response to transcutaneous electrical nerve stimulation (TENS) depends on stimulation intensity. J Pain12: 929-935.
- Claydon LS, Chesterton LS, Barlas P, Sim J (2011) Dose-specific effects of transcutaneous electrical nerve stimulation (TENS) on experimental pain: a systematic review. Clin J Pain 27: 635-647.
- Rakel B, Frantz R (2003) Effectiveness of transcutaneous electrical nerve stimulation on postoperative pain with movement. J Pain 4: 455-464.
- Bjordal JM, Johnson MI, Ljunggreen AE (2003) Transcutaneous electrical nerve stimulation (TENS) can reduce postoperative analgesic consumption. A meta-analysis with assessment of optimal treatment parameters for postoperative pain. Eur J Pain 7: 181-188.
- Johnson MI, Paley CA, Howe TE, Sluka KA (2015) Transcutaneous electrical nerve stimulation for acute pain. Cochrane Database Syst Rev 6: CD006142.
- Johnson MI, Ashton CH, Thompson JW (1991) An in-depth study of long-term users of transcutaneous electrical nerve stimulation (TENS). Implications for clinical use of TENS. Pain 44: 221-229.
- Law PP, Cheing GL (2004) Optimal stimulation frequency of transcutaneous electrical nerve stimulation on people with knee osteoarthritis. J Rehabil Med 36: 220-225.
- Marchand S, Charest J, Li J, Chenard JR, Lavignolle B, et al. (1993) Is TENS purely a placebo effect? A controlled study on chronic low back pain. Pain 54: 99-106.
- Cheing GL, Luk ML (2005) Transcutaneous electrical nerve stimulation for neuropathic pain. J Hand Surg Br 30: 50-55.
- Bennett MI, Hughes N, Johnson MI (2011) Methodological quality in randomised controlled trials of transcutaneous electric nerve stimulation for pain: low fidelity may explain negative findings. Pain 152: 1226-1232.
- Jensen MP, Hu X, Potts SL, Gould EM (2013) Single vs composite measures of pain intensity: relative sensitivity for detecting treatment effects. Pain 154: 534-538.
- Dworkin RH, Turk DC, Wyrwich KW, Beaton D, Cleeland CS, et al. (2008) Interpreting the clinical importance of treatment outcomes in chronic pain clinical trials: IMMPACT recommendations. J Pain 9:105-121.
- Coggon D, Ntani G, Palmer KT, Felli VE, Harari R, et al. (2013) Patterns of multisite pain and associations with risk factors. Pain 154: 1769-1777.
- Janke EA, Collins A, Kozak AT (2007) Overview of the relationship between pain and obesity: What do we know? Where do we go next? J Rehabil Res Dev 44: 245-262.
- Carnes D, Parsons S, Ashby D, Breen A, Foster NE, et al. (2007) Chronic musculoskeletal pain rarely presents in a single body site: Results from a UK population study. Rheumatology 46: 1168-1170.
- Dragioti E, Larsson B, Bernfort L, Levin LA, Gerdle B (2017) A cross-sectional study of factors associated with the number of anatomical pain sites in an actual elderly general population: results from the PainS65+ cohort. J Pain Res 10: 2009-2019.
- Wolfe F, Clauw DJ, Fitzcharles MA, Goldenberg DL, Katz RS, et al. (2010) The American College of Rheumatology preliminary diagnostic criteria for fibromyalgia and measurement of symptom severity. Arthritis Care Res 62: 600-610.
- Vij B, Whipple MO, Tepper SJ, Mohabbat AB, Stillman M, et al. (2015) Frequency of migraine headaches in patients with fibromyalgia. Headache 55: 860-865.
- Fishbain DA, Chabal C, Abbott A, Heine LW, Cutler R (1996) Transcutaneous electrical nerve stimulation (TENS) treatment outcome in long-term users. Clin J Pain 12: 201-214.
- DeSantana JM, Da Silva LF, De Resende MA, Sluka KA (2009) Transcutaneous electrical nerve stimulation at both high and low frequencies activates ventrolateral periaqueductal grey to decrease mechanical hyperalgesia in arthritic rats. Neuroscience 163: 1233-1241.
- Sluka KA, Walsh D (2003) Transcutaneous electrical nerve stimulation: Basic science mechanisms and clinical effectiveness. J Pain 4: 109-121.
- Freeman R, Emir B, Parsons B (2015) Predictors of placebo response in peripheral neuropathic pain: Insights from pregabalin clinical trials. J Pain Res 8: 257-268.
- Vase L, Vollert J, Finnerup NB, Miao X, Atkinson G, et al. (2015) Predictors of the placebo analgesia response in randomized controlled trials of chronic pain: A meta-analysis of the individual data from nine industrially sponsored trials. Pain 156: 1795-1802.
- Price DD, Milling LS, Kirsch I, Duff A, Montgomery GH, et al. (1999) An analysis of factors that contribute to the magnitude of placebo analgesia in an experimental paradigm. Pain 83: 147-156.
- Oosterhof J, Wilder-Smith OH, Oostendorp RA, Crul BJ (2012) Different mechanisms for the short-term effects of real versus sham transcutaneous electrical nerve stimulation (TENS) in patients with chronic pain: A pilot study. J Pain Palliat Care Pharmacother 26: 5-12.
- Dobscha SK, Lovejoy TI, Morasco BJ, Kovas AE, Peters DM, et al. (2016) Predictors of improvements in pain intensity in a national cohort of older veterans with chronic pain. J Pain 17: 824-835.
- Emir B, Johnson K, Kuhn M, Parsons B (2017) Predictive modeling of response to pregabalin for the treatment of neuropathic pain using 6-week observational data: A spectrum of modern analytics applications. Clin Ther 39: 98-106.
- Ang DC, Bair MJ, Damush TM, Wu J, Tu W, et al. (2010) Predictors of pain outcomes in patients with chronic musculoskeletal pain co-morbid with depression: Results from a randomized controlled trial. Pain Med 11: 482-491.
- Oosterhof J, Samwel HJ, de Boo TM, Wilder-Smith OH, Oostendorp RA, et al. (2008) Predicting outcome of TENS in chronic pain: A prospective, randomized, placebo controlled trial. Pain 136: 11-20.
- Koke AJ, Smeets RJ, Perez RS, Kessels A, Winkens B, et al. (2014) Can we "predict" long-term outcome for ambulatory transcutaneous electrical nerve stimulation in patients with chronic pain? Pain Pract 1-9.
- Dworkin RH, Richlin DM, Handlin DS, Brand L (1986) Predicting treatment response in depressed and non-depressed chronic pain patients. Pain 24: 343-353.
- Rosenbaum PR (2003) Does a dose-response relationship reduce sensitivity to hidden bias? Biostatistics 4: 1-10.
- Hill AB (1965) The Environment and disease: Association or causation? Proc R Soc Med 58: 295-300.
- Melzack R, Wall PD (19650 Pain mechanisms: A new theory. Science 150: 971-979.
- Liebano RE, Vance CG, Rakel BA, Lee JE, Cooper NA, et al. (2013) Transcutaneous electrical nerve stimulation and conditioned pain modulation influence the perception of pain in humans. Eur J Pain 17: 1539-1546.
- Sullivan MJ, Thorn B, Haythornthwaite JA, Keefe F, Martin M, et al. (2001) Theoretical perspectives on the relation between catastrophizing and pain. Clin J Pain 17: 52-64.
- Dworkin RH, Turk DC, Farrar JT, Haythornthwaite JA , Jensen MP, et al. (2005) Core outcome measures for chronic pain clinical trials: IMMPACT recommendations. Pain 113: 9-19.
Citation: Gozani SN, Kong X (2018) Real-World Evidence for the Widespread Effects of Fixed-Site High-Frequency Transcutaneous Electrical Nerve Stimulation in Chronic Pain. J Pain Relief 7: 329. DOI: 10.4172/2167-0846.1000329
Copyright: © 2018 Gozani SN, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Conferences
42nd Global Conference on Nursing Care & Patient Safety
Toronto, CanadaRecommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 3406
- [From(publication date): 0-2018 - Apr 04, 2025]
- Breakdown by view type
- HTML page views: 2624
- PDF downloads: 782