Ratanasampil Suppresses the Hypoxia-Related Inflammatory Responses by Inhibiting Oxidative Stress and NF-kB Activation in Microglia
Received: 10-Oct-2018 / Accepted Date: 22-Oct-2018 / Published Date: 29-Oct-2018 DOI: 10.4172/2161-0460.1000451
Keywords: RNSP; A Tibetan medicine; Alzheimer’s disease; Microglia; Neuroinflammation; Oxidative stress; NF-κB activation
Introduction
Neuroinflammation has been generally accepted as a hallmark in Alzheimer's Disease (AD), and microglia, the resident innate immune cells in the brain, closely mediate neuroinflammation [1,2]. Recently, two research groups have provided evidence that neuroinflammation is a key driver of AD [3,4].
Hypoxia is a known neuroinflammatogen in the brain, as the brain requires highly oxygen supply [5]. Hypoxic conditions activate microglia to produce and secrete pro-inflammatory mediators, including interleukin-1β (IL-1β) and Tumor Necrosis Factor-α (TNF-α) [6,7], which may promote cognitive decline in aged people and AD patients [8,9]. Previous studies, including our own, have found that hypoxia can drive microglia to generate Reactive Oxygen Species (ROS) [10-13], which further activates nuclear factor- κB (NF-κB), resultant in the expression of pro-inflammatory mediators [11,14]. Therefore, targeting regulating neuroinflammation is a major approach for the prevention and early intervention of AD.
Ratanasampil (RNSP), Tibetan for “70-taste pearl-balls,” clinically used in the treatment of hypoxia-related diseases, such as stroke. RNSP comprises around 32 kinds of plants ingredients (Saffron, Lignum Dalbergiae Odoriferae, Lagotis brachystachya, Licorice, Myrobalan, Nepeta hemsleyana etc.) 8 kinds of animal ingredients (musk, bezoar et al.,), 20 kinds of mineral ingredients (Pearl and Opal etc.) and 10 kinds of mental ingredients (Gold, Silver and Copper etc.). The clinical effects of RNSP were focused on the components from plants including crocin from saffron and glycyrrhetinic acid from glycyrrhiza uralensis [15,16]. The possible mechanisms of RNSP are considered by the antioxidant effects of crocin (Figure 1A) [17,18] and anti-inflammatory effects of glycyrrhetinic acid (Figure 1B) [19,20]. In previous studies, we have shown that RNSP improves the learning and memory and reduces β-amyloid (Aβ) protein levels in mouse AD models [21,22]. Furthermore, RNSP was also found to improve the cognitive function and decrease the serum levels of Aβ42 and pro-inflammatory mediators in mild-to-moderate AD patients, who living at high altitude. These findings suggest the utility of RNSP in clinical applications for AD [23]. However, the molecular mechanisms of the effects need to be clarified.
Our current findings concerning the neuroprotective effects of RNSP encouraged us to investigate further targets of RNSP [24]. In this study, we examined the effects of RNSP on microglia and its molecular mechanisms using MG6 microglia under Hypoxia/Reoxygenation (H/R) conditions.
Materials and Methods
Reagents
RNSP (Zhunzi Z63020062) was purchased from Qinghai Jinke Tibetan Medicine Pharmaceutical Co., Ltd. (Xining, China). In order to eliminate the interference caused by the methanol solvent, a suitable methanol concentration for cell culture was titrated. Antibodies against mouse anti-phospho-IκBα, rabbit anti-IκBα, mouse antiphospho-p65, P65 antibody and 8-oxo-dG antibody were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA).
Microglia cell culture
The MG6 cell line (Riken Cell Bank, Tsukuba, Japan) was cultured in DMEM supplemented with 1% penicillin-streptomycin (Invitrogen, Grand Island, NY, USA), 100 μmol/L β-mercaptoethanol, 4500 mg/L glucose (Invitrogen),10 μg/mL of insulin, and 10% FBS according to previously described methods.
Assays for cell viability
MG6 cells were seeded in 96-well plates at density of 5×103 cells/ well overnight and then exposed to hypoxic conditions (1% O2) for 6 h, after which they were returned to normoxic conditions (20% O2) for various durations. The Cell-Counting Kit (Dojindo, Kumamoto, Japan) was used to examine relative cell viability. The cell viability was analyzed by dividing the optical density of the H/R group by the control group. MG6 cells were treated with of RNSP ranging from 1 to 500 μg/mL for 24 h. The cell viability was analyzed by dividing the optical density of the RNSP-treated group by that of the no-treatment group. RNSP upto 10 μg/mL had no significant toxic effect on MG6 cells, so 10 μg/mL RNSP was used in subsequent experiments.
Real-time quantitative polymerase chain reaction (qRT-PCR)
RNA extraction, cDNA synthesis and qRT-PCR were performed as previously described [14]. The sequences of primer pairs were as follows: IL-1β: 5’- CAACCAACAAGTGATATTCTCCATG- 3’ and 5’-GATCCACACTCTCAGCTGCA-3’;inducible nitric oxide synthase (iNOS): 5’-GCCACCAACAATGGCAAC-3’ and 5’- CGTACCGGATGAGCTGTGAATT-3’;TNF-α: 5’-ATGGCCTCCCTCTCAGTTC- 3’ and 5’ TTGGTGGTTTGCTACGACGTG- 3’;IL-6:5’-CCTCTGGTCTTCTGGAGTACC-3’and5’ ACTCCTTCTGTGACTCCAGC-3’;arginase1:5’-CGCCTT TCTCAAA AGGACAG-3’ and 5-CCAGCTCTTCATTGGCTTTC-3; IL-4: 5’-TGGGTCTCAACCC CCAGCTAGT-3’ and 5-TGCATGGCGTCCCTTCTC CTGT-3’; IL-10: 5’-GACCAGCT GGACAACATACTGCTAA- 3’ and 5’-GATAAGGATTGGCAACCCAAGTAA-3’; TGF-β: 5’-CCTGCAAGACCATCGACATG-3’ and 5’-TGTTGTACAAAGCGAGCACC- 3’. Expression for all experiments was normalized to Actin.
Detection of mitochondrial ROS
Mitochondrial ROS was examined using Mito SOX Red (Invitrogen), which is alive-cell permeant and rapidly and selectively targets mitochondria. The MG6 microglia cells were exposed to normoxia and H/R in the presence or absence of RNSP (10 μg/mL) pretreatment. The cells were incubated in Hank’s Balanced Salt Solution (HBSS) containing 5 mM Mito SOX Red for 10 min at 37°C after H/R. After washing twice by PBS, the cells were mounted in warm buffer for imaging.
NO2-/NO3-assay
MG6 were cultured at a density of 5×105 cells/mL. After treatment with RNSP (10 μg/L) for 24 h, microglia were exposed to hypoxic conditions (1% O2) for 6 h, after which they were returned to normoxic conditions (20% O2) for various durations and then collected. The amounts of NO2- and NO3- were measured by NO2-/NO3- assay kits (R&D Systems, Minneapolis, USA).
Western blotting
24 h after treatment with RNSP (10 μg/ml), MG6 microglia cells were exposed to hypoxic conditions (1% O2) for 6 h, after which they were returned to normoxic conditions (20% O2) for various durations. The cell lysates were collected at various time points. Western blotting was performed using an SDS-PAGE electrophoresis system. Protein samples (30 μg) were re-suspended in sample buffer and then electrophoresed on a 15% or 12% Tris gel, followed by blotting to a PVDF membrane. After blocking by 5% nonfat dry milk, the membranes were incubated at 4°C overnight with first antibody: mouse anti- phospho- IκBα (1:1000) and rabbit anti-IκBα (1:1000) antibodies. After washing, the membranes were incubated with corresponding second antibodies for 2 h at room temperature followed by ECL (Amersham Pharmacia Biotech). Immunoreactive bands were visualized using ImageQuant (LAS-4000; Fuji Photo Film, Tokyo, Japan).
Immunofluorescence imaging
The MG6 cells were fixed with 4% paraformaldehyde after H 6 h/R 1 h stimulation in presence or absence of RNSP pretreatment. They were then incubated with mouse anti-p65 (1:500) or mouse anti-8-oxo-dG (1:500) overnight at 4°C. The sections were incubated with donkey anti-mouse Alexus 488 (1:500; Jackson ImmunoResearch, West Grove, PA, USA) after washing by PBS followed by Hoechst (1:200) and mounted in Vectashield anti-fading medium (Vector Laboratories, Burlingame, CA, USA). Images were obtained using a confocal laser-scanning microscope (CLSM; 2si Confocal Laser Microscope, Nikon, Tokyo, Japan). The line plot profile and fluorescence intensity were analyzed using the Image J software program.
Statistical analyses
The independent experiments and statistical analysis used (Oneway ANOVA with a post hoc Tukey’s test and a two-tailed unpaired Student’s t-test) were all indicated in the figure legends. All values are presented as the mean ± SEM with p values using the GraphPad Prism 7 Software (GraphPad Software Inc., San Diego, CA, USA). A value of p<0.05 was considered to indicate statistical significance.
Results
The effects of RNSP on the H/R-induced toxicity in microglia
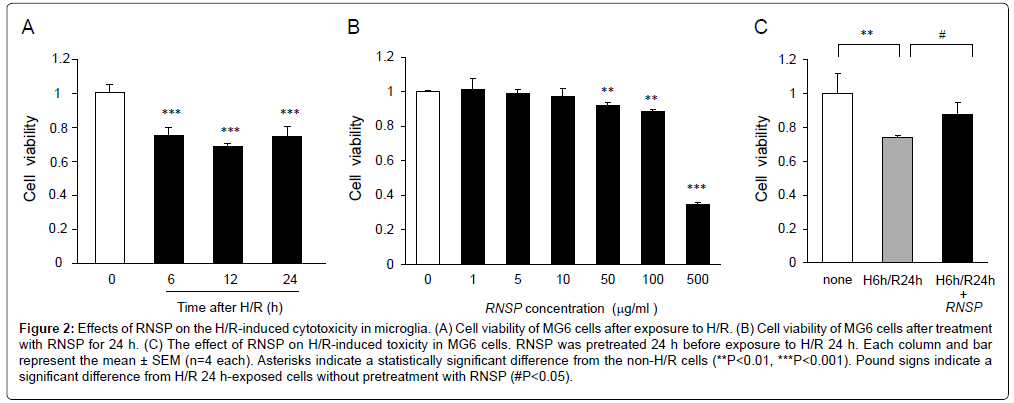
We first examined the MG6 microglia viability under H/R condition using a Cell Counting Kit-8 wherein the cells were exposed to hypoxic conditions (1% O2, H) for 6 h, after which they were returned to normoxic conditions (20% O2, reoxygenation, R) for various durations. The MG6 cell viability was markly reduced from H 6 h/R 6 h to H 6 h/R 24 h (Figure 2A). To determine the most suitable concentration of RNSP for the treatment of MG6 microglia, cells were treated by RNSP at concentrations from 1 to 500 μg/mL for 72 h. The cell viability was not significantly changed after treatment with 1-10 μg/mL RNSP for 72 h (Figure 2B). However, it was significantly decreased after pretreatment with over 50 μg/mL RNSP (89% of viable cells). We therefore used RNSP at 10 μg/mL and H 6 h/R 24 h to examine the effects of RNSP on the H/R-induced cytotoxicity of MG6 microglia in the subsequent experiments.
Figure 2: Effects of RNSP on the H/R-induced cytotoxicity in microglia. (A) Cell viability of MG6 cells after exposure to H/R. (B) Cell viability of MG6 cells after treatment with RNSP for 24 h. (C) The effect of RNSP on H/R-induced toxicity in MG6 cells. RNSP was pretreated 24 h before exposure to H/R 24 h. Each column and bar represent the mean ± SEM (n=4 each). Asterisks indicate a statistically significant difference from the non-H/R cells (**P<0.01, ***P<0.001). Pound signs indicate a significant difference from H/R 24 h-exposed cells without pretreatment with RNSP (#P<0.05).
Pretreatment with RNSP for 24 h significantly inhibited the H/R-induced cytotoxicity in MG6 microglia (Figure 2C). It strongly demonstrates that pretreatment with RNSP protects MG6 microglia from H/R-induced cytotoxicity. Therefore, pretreatment with RNSP (10 μg/mL) for 24 h was used in the subsequent experiments.
The effects of RNSP on the H/R-induced expression of proinflammatory mediators in microglia
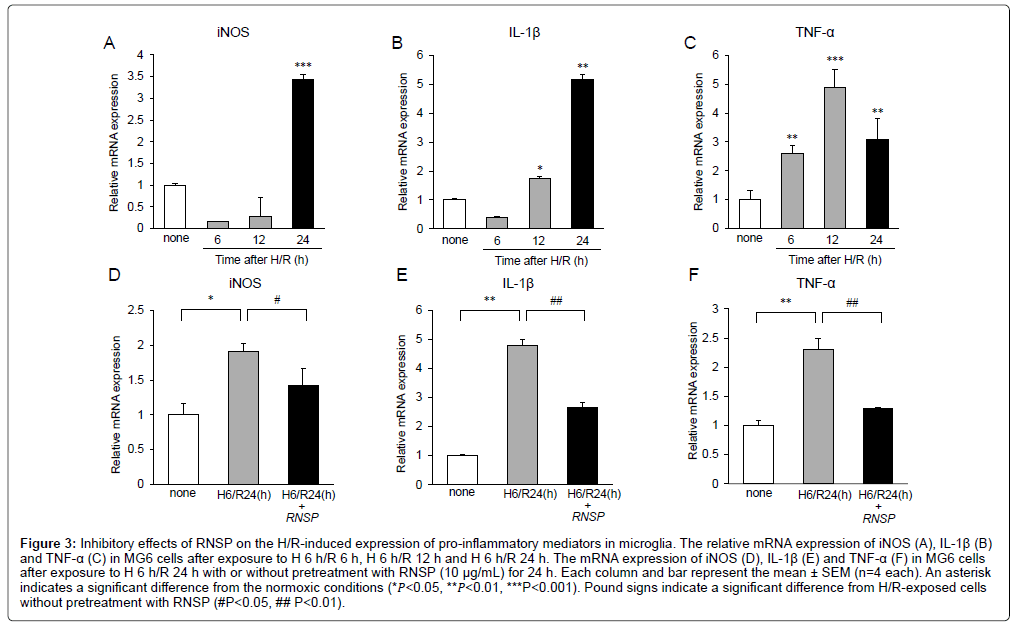
Microglia-related neuroinflammation is involved in the cognitive deficits seen in AD patients [2-4]. Therefore, we next examined the effects of RNSP (10 μg/mL) on the H/R- induced expression of proinflammatory mediators by microglia. The mean mRNA expression of iNOS, IL-1β and TNF-α during H/R exposure were assessed using quantitative RT-PCR. The mean mRNA expression of iNOS was slightly decreased from H/R 6 h to H/R 12 h but significantly increased at H 6 h/R 24 h (Figure 3A). The mean mRNA expression of IL-1β were slightly decreased at H/R 6 h but significantly increased from H/R 12 h to H/R 24 h (Figure 3B). The mean mRNA expression of TNF-α were significantly increased from H 6/R 6 h to H 6/R 24 h (Figure 3C). These observations indicate that the pro-inflammatory mediators are significantly increased at the lately times (H/R 24 h) during H/R exposure. It was noted that RNSP (10 μg/mL) significantly inhibited the mean mRNA expression of iNOS, IL-1β and TNF-α in MG6 microglia at H 6/R 24 h (Figures 3D-3F).
Figure 3: Inhibitory effects of RNSP on the H/R-induced expression of pro-inflammatory mediators in microglia. The relative mRNA expression of iNOS (A), IL-1β (B) and TNF-α (C) in MG6 cells after exposure to H 6 h/R 6 h, H 6 h/R 12 h and H 6 h/R 24 h. The mRNA expression of iNOS (D), IL-1β (E) and TNF-α (F) in MG6 cells after exposure to H 6 h/R 24 h with or without pretreatment with RNSP (10 μg/mL) for 24 h. Each column and bar represent the mean ± SEM (n=4 each). An asterisk indicates a significant difference from the normoxic conditions (*𝑃<0.05, **𝑃<0.01, ***P<0.001). Pound signs indicate a significant difference from H/R-exposed cells without pretreatment with RNSP (#P<0.05, ## P<0.01).
The effects of RNSP on the H/R induced expression of antiinflammatory mediators in microglia
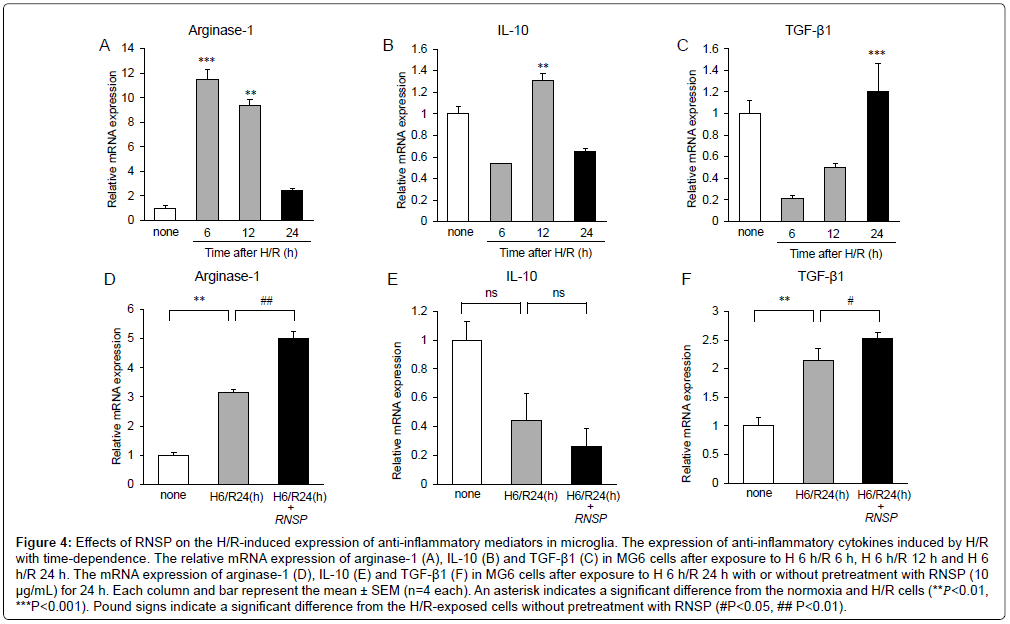
To further explore the effects of RNSP on microglia-related neuroinflammation, the effects of RNSP (10 μg/mL) on the H/Rinduced expression of anti-inflammatory mediators by MG6 microglia were examined under H/R exposure. The mean mRNA expression of arginase-1 was significantly increased from H/R 6 h to H/R 12 h but sharply decreased at H/R 24 h (Figure 4A). The mean mRNA expression of IL-10 was significantly increased at H/R 12 h and decreased at H/R 24 h (Figure 4B). However, the mean mRNA expression of TGF-β1 was slightly decreased from H/R 6 h to H/R 12 h but significantly increased at H/R 24 h (Figure 4C). These observations indicate that the antiinflammatory mediators were significantly decreased at later times during H/R exposure. It was noted that RNSP (10 μg/mL) significantly increased the mean mRNA expression of arginase-1 and TGF-β1 in MG6 microglia at H 6 h/R 24 h; however, the effect of RNSP on IL-10 has not been determined (Figures 4D-4F).
Figure 4: Effects of RNSP on the H/R-induced expression of anti-inflammatory mediators in microglia. The expression of anti-inflammatory cytokines induced by H/R with time-dependence. The relative mRNA expression of arginase-1 (A), IL-10 (B) and TGF-β1 (C) in MG6 cells after exposure to H 6 h/R 6 h, H 6 h/R 12 h and H 6 h/R 24 h. The mRNA expression of arginase-1 (D), IL-10 (E) and TGF-β1 (F) in MG6 cells after exposure to H 6 h/R 24 h with or without pretreatment with RNSP (10 μg/mL) for 24 h. Each column and bar represent the mean ± SEM (n=4 each). An asterisk indicates a significant difference from the normoxia and H/R cells (**𝑃<0.01, ***P<0.001). Pound signs indicate a significant difference from the H/R-exposed cells without pretreatment with RNSP (#P<0.05, ## P<0.01).
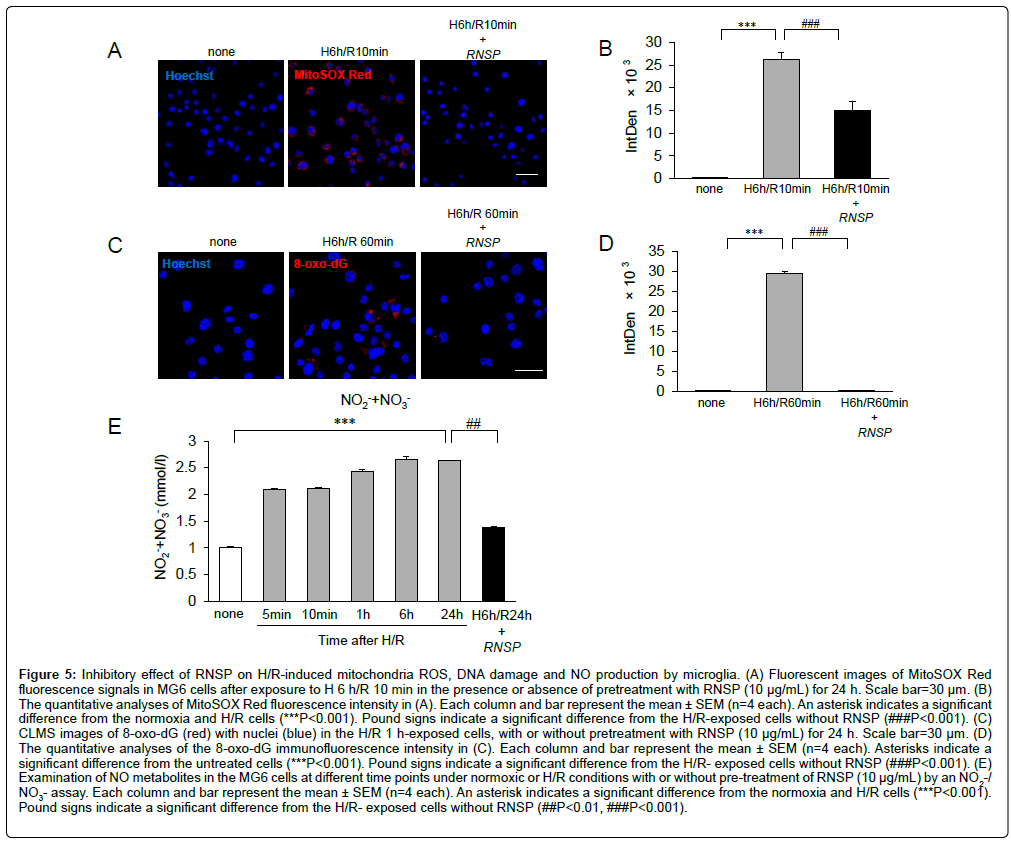
The effects of RNSP on the H/R-induced oxidative stress in microglia
Our previous study showed that hypoxia-induced mitochondrial oxidant generation was involved in oxidative stress in microglia [14]. These observations prompted us to examine the effects of RNSP on H/R-induced oxidative stress in microglia using two approaches: MitoSOX Red probe was used to examine mitochondria-derived ROS generation [25] and immunofluorescence imaging for 8-oxo-dG was to examine the DNA oxidation [26]. Compared to the untreated MG6 microglia, the immunofluorescence intensity of MitoSOX Red was significantly increased in MG6 microglia at H/R10 min (Figures 5A and 5B), suggesting the ROS under H/R conditions were derived from mitochondria. Pretreatment with RNSP (10 ug/mL) significantly inhibited the mean fluorescent intensity of MitoSOX Red in microglia at H/R 10 min (Figures 5A and 5B), thus suggesting the early antioxidant effects of RNSP on microglia. Immunofluorescence imaging showed 8-oxo- dG was significantly increased comparing to the cells without exposure to H/R 1 h (***p<0.001). The H/R-induced NO production in microglia was further examined. Compared to the untreated MG6 cells, the mean levels of NO2-/NO3- were significantly increased from H/R 5 min to H/R 24 h in the in the culture medium of MG6 cells, and pretreatment with RNSP (10 μg/mL) markly inhibited the mean levels of NO2-/NO3- at H/R 24 h in MG6 cells (Figures 5C-5E). These findings confirm that RNSP inhibits the H/R- induced oxidative stress in microglia.
Figure 5: Inhibitory effect of RNSP on H/R-induced mitochondria ROS, DNA damage and NO production by microglia. (A) Fluorescent images of MitoSOX Red fluorescence signals in MG6 cells after exposure to H 6 h/R 10 min in the presence or absence of pretreatment with RNSP (10 μg/mL) for 24 h. Scale bar=30 μm. (B) The quantitative analyses of MitoSOX Red fluorescence intensity in (A). Each column and bar represent the mean ± SEM (n=4 each). An asterisk indicates a significant difference from the normoxia and H/R cells (***P<0.001). Pound signs indicate a significant difference from the H/R-exposed cells without RNSP (###P<0.001). (C) CLMS images of 8-oxo-dG (red) with nuclei (blue) in the H/R 1 h-exposed cells, with or without pretreatment with RNSP (10 μg/mL) for 24 h. Scale bar=30 μm. (D) The quantitative analyses of the 8-oxo-dG immunofluorescence intensity in (C). Each column and bar represent the mean ± SEM (n=4 each). Asterisks indicate a significant difference from the untreated cells (***P<0.001). Pound signs indicate a significant difference from the H/R- exposed cells without RNSP (###P<0.001). (E) Examination of NO metabolites in the MG6 cells at different time points under normoxic or H/R conditions with or without pre-treatment of RNSP (10 μg/mL) by an NO2-/ NO3- assay. Each column and bar represent the mean ± SEM (n=4 each). An asterisk indicates a significant difference from the normoxia and H/R cells (***P<0.001). Pound signs indicate a significant difference from the H/R- exposed cells without RNSP (##P<0.01, ###P<0.001).
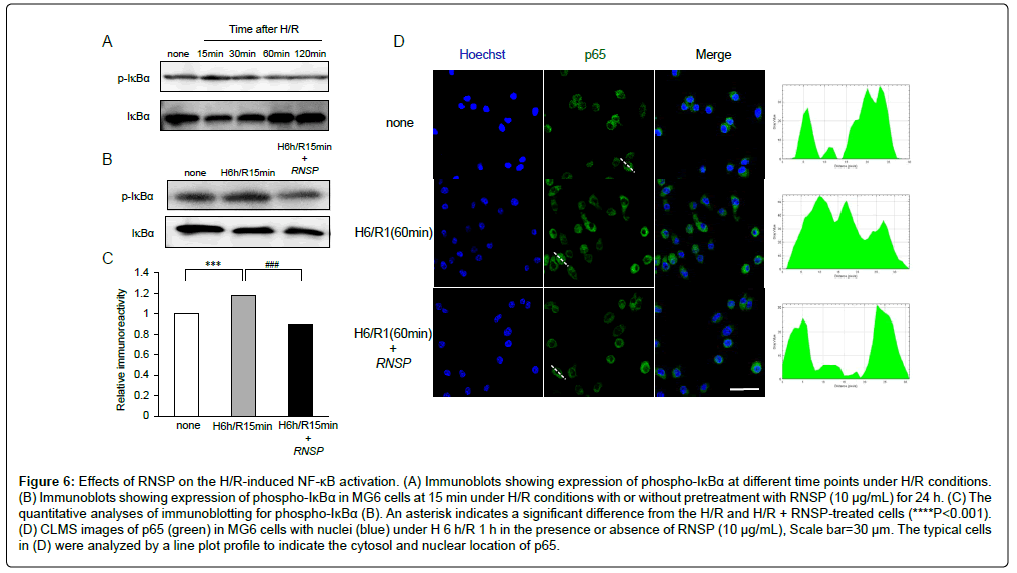
The effects of RNSP on the H/R-induced NF-κB activation in microglia
Finally, the effects of RNSP on the activation of NF-κB during H/R exposure were examined, as NF-κB regulates most of inflammatory molecules. Compared with the untreated cells, the phosphorylation of IκBα in MG6 microglia was significantly increased from H/R 15 min to H/R 30 min and gradually recovered to the base levels at H/R 2 h (Figure 6A). Pretreatment with RNSP (10 μg/mL) significantly inhibited the H/R- induced phosphorylation of IκBα in microglia (Figure 6B and 6C). Furthermore, p65 nuclear translocation was induced in MG6 microglia at H/R 60 min, and pretreatment with RNSP (10 μg/mL) significantly inhibited the H/R-induced p65 nuclear translocation in microglia (Figure 6D). These findings confirm that RNSP suppresses the H/R-induced NF-κB activation in microglia.
Figure 6: Effects of RNSP on the H/R-induced NF-κB activation. (A) Immunoblots showing expression of phospho-IκBα at different time points under H/R conditions. (B) Immunoblots showing expression of phospho-IκBα in MG6 cells at 15 min under H/R conditions with or without pretreatment with RNSP (10 μg/mL) for 24 h. (C) The quantitative analyses of immunoblotting for phospho-IκBα (B). An asterisk indicates a significant difference from the H/R and H/R + RNSP-treated cells (****P<0.001). (D) CLMS images of p65 (green) in MG6 cells with nuclei (blue) under H 6 h/R 1 h in the presence or absence of RNSP (10 μg/mL), Scale bar=30 μm. The typical cells in (D) were analyzed by a line plot profile to indicate the cytosol and nuclear location of p65.
Discussion
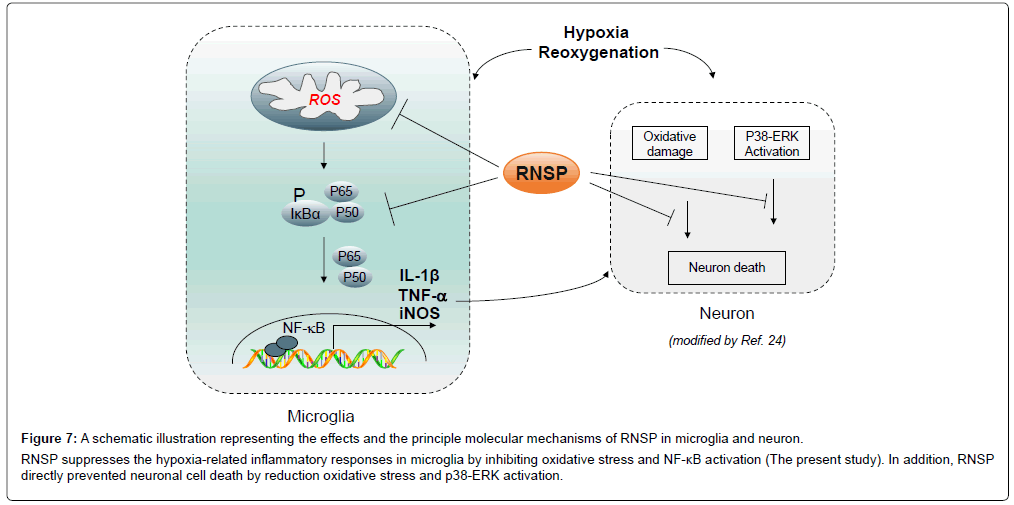
The present study indicates that RNSP protects against H/Rinduced cytotoxicity and regulates the H/R-induced inflammatory responses in MG6 microglia by reducing the oxidative stress and NF-κB activation (summarized in Figure 6). This is first to describe the principle molecular mechanisms underlying the clinical benefits of RNSP in AD patients. Oxidative stress was proved to damage the cellular components, including DNA, resulting in subsequent cell death [27]. Present observations found the intensity of MitoSOX Red, a marker for mitochondria-derived ROS generation, increased as quickly as at H/R 10 min, which involved 10 min of reoxygenation after hypoxia, and the fluorescent intensity of 8-oxo-dG, a biomarker for oxidative stress-damaged DNA [26] was significantly increased from H/R 1 h, which involved 1 h of reoxygenation after hypoxia in the MG6 microglia. These findings indicated that mitochondria are the origin of ROS generation, thus inducing oxidative stress in microglia. In addition, the increased levels of NO2-/NO3-, which are metabolic agents for NO production, persisted through H/R 24 h, indicating the continuative ROS overproduction due to oxidative stress in microglia under H/R conditions. Of note, pretreatment with RNSP significantly inhibited the H/R-induced mitochondrial ROS generation, 8-oxo-dG expression and NO production (Figure 5), resulting in the protection against subsequent cell death in microglia (Figure 2). This indicated that RNSP was able to reduce oxidative stress in microglia (Figure 7).
Figure 7: A schematic illustration representing the effects and the principle molecular mechanisms of RNSP in microglia and neuron. RNSP suppresses the hypoxia-related inflammatory responses in microglia by inhibiting oxidative stress and NF-κB activation (The present study). In addition, RNSP directly prevented neuronal cell death by reduction oxidative stress and p38-ERK activation.
Therefore, the clinical effects of RNSP on improving the cognitive functions in mild-to- moderate AD patients living at high altitude may due to a reduction in oxidative stress [23]. NF-κB activation is rapidly and transiently induced by oxidative stress [28]. In the present study, phosphorylation of IκBα in MG6 was detected after H/R 15 min, and p65 nuclear translocation was induced at H/R 60 min, which suggests that NF-κB activation is associated with an increased intracellular redox state during H/R 60 min [29]. NF-κB activation polarizes microglia into the neurotoxic phenotype, as NF-κB is a transcription factor that encodes the genes of the pro-inflammatory (neurotoxic) mediators, such as IL-1β, TNF-α and iNOS [30]. In the present study, the increased expression of neurotoxic mediators (IL-1β, TNF-α and iNOS) paralleled the decreased expression of neuroprotective (antiinflammatory) mediators (arginase-1 and IL-10) at H/R 24 h in MG6 microglia, indicating that microglia are shifted to the neurotoxic phenotype at the later phases under H/R conditions. Indeed, the lasting expression of neurotoxic mediators establishes a feedforward loop for NF-κB activation, as pro- inflammatory mediators such as IL-1β promote NF-κB activation [31]. Pretreatment with RNSP significantly decreases the H/R-induced NF-κB activation and the expression of pro-inflammatory mediators but reverses the H/Rdecreased expression of the anti- inflammatory mediator TGFβ1 in microglia (Figures 3-6), suggesting that RNSP may be able to ameliorate the microglia-mediated neuroinflammation and shift activated microglia to neuroprotective phenotypes. The effects of RNSP on ameliorating microglia-mediated neuroinflammation will guide its clinical usage in the early intervention of AD, as microgliamediated neuroinflammation is widely accepted as an early hallmark in AD [2-4,32]. The beneficial effects of RNSP on microglia-mediated neuroinflammation may depend on its anti-pro-inflammatory components, such as glycyrrhiza uralensis [19,20].
Hypoxia is known to worsen the cognitive function, as evidenced by hypoxia-suffering mountaineers having a deteriorated memory [33,34] and healthy individuals living in high altitude having greater cognitive dysfunctions than those at lower altitudes [35]. The brain requires oxygen, which is largely dependent on the cerebral blood flow [36,37], and the cerebral blood flow is slowed with aging and further decreased in AD patients [38,39]. This low cerebral oxygen availability results in more cognitive defects [40]. Therefore, chronic hypoxia may contribute to the cognitive decline in aging individuals as well as AD patients [40,41]. The microglial proliferation and activation associated with neuronal loss could be histologically observed in human AD brain [9,32]. It is well known that hypoxia activated microglia induce neuronal death by producing IL-1β, TNF- α as well as and IL-6 [2,6,9]. And hypoxia shifts microglia into neurotoxic phenotype in ROS-dependent [14]. In the present cultured cell study, we give the first evidence that RNSP inhibiting the H/R induced productions of ROS and neurotoxic mediators in microglia, thus demonstrate the anti-inflammation and antioxidant effects of RNSP on microglia. Taking together with the effects on mitigating microglia-related neuroinflammation and the directly neuroprotective effects on neurons [24], RNSP could be used to prevent or treat for delaying pathophysiology of AD and other neurodegenerative diseases. These findings along with the observation of the direct roles of RNSP in neuroprotection and microglia regulation prove the clinical benefits of RNSP in the prevention and management of AD [23].
Conclusion
The present study provides the first evidence of the potential protective effects of RNSP on the hypoxia-related neuroinflammatory responses in microglia. The effects were dependent on reducing the oxidative stress and NF-κB activation, highlighting a new molecular target for RNSP in the clinical intervention of AD.
Acknowledgement
This work was supported by funding from the National Natural Science Foundation of China (No. 81560711), Qinghai Province Natural Science Foundation (No.2012-Z913) and the China Scholarship Council (No. 201608635002) to Aiqin Zhu and Grants-in-Aid for Scientific Research (16K11478, 16H0848) from MEXT (Japan) to Zhou Wu.
References
- McGeer PL, McGeer E, Rogers J, Sibley J (1990) Anti-inflammatory drugs and Alzheimer disease. Lancet 335: 1037.
- McGeer PL, McGeer EG (2013) The amyloid cascade-inflammatory hypothesis of Alzheimer disease: Implications for therapy. Acta Neuropathol 126: 479-497.
- Olmos-Alonso A, Schetters ST, Sri S, Askew K, Mancuso R, et al. (2016) Pharmacological targeting of CSF1R inhibits microglial proliferation and prevents the progression of Alzheimer's-like pathology. Brain 139: 891-907.
- Spangenberg EE, Lee RJ, Najafi AR, Rice RA, Elmore MR, et al. (2016) Eliminating microglia in Alzheimer's mice prevents neuronal loss without modulating amyloid-beta pathology. Brain 139: 1265-1281.
- Carlson BW, Carlson JR, Neelon VJ ,Hartman M (2008) Tailoring protocols to successfully recruit and retain older adults in a longitudinal study of sleep and cognition. Res Gerontol Nurs 1: 232-237.
- Kaur C, Sivakumar V, Zou Z, Ling EA (2014) Microglia-derived proinflammatory cytokines tumor necrosis factor-alpha and interleukin-1beta induce Purkinje neuronal apoptosis via their receptors in hypoxic neonatal rat brain. Brain Struct Funct 219: 151-170.
- Sivakumar V, Foulds WS, Luu CD, Ling EA, Kaur C (2011) Retinal ganglion cell death is induced by microglia derived pro-inflammatory cytokines in the hypoxic neonatal retina. J Pathol 224: 245-260.
- Heneka MT, O'Banion MK, Terwel D, Kummer MP (2010) Neuroinflammatory processes in Alzheimer's disease. J Neural Transm (Vienna) 117: 919-947.
- McGeer PL, Rogers J, McGeer EG (2006) Inflammation, anti-inflammatory agents and Alzheimer disease: The last 12 years. J Alzheimers Dis 9: 271-276.
- Kaur C, Ling EA (2009) Periventricular white matter damage in the hypoxic neonatal brain: Role of microglial cells. Prog Neurobiol 87: 264-280.
- Nakanishi H, Wu Z (2009) Microglia-aging: roles of microglial lysosome- and mitochondria-derived reactive oxygen species in brain aging. Behav Brain Res 201: 1-7.
- Kaur C, Sivakumar V, Yip GW, Ling EA (2009) Expression of syndecan-2 in the amoeboid microglial cells and its involvement in inflammation in the hypoxic developing brain. Glia 57: 336-349.
- Rathnasamy G, Ling EA, Kaur C (2011) Iron and iron regulatory proteins in amoeboid microglial cells are linked to oligodendrocyte death in hypoxic neonatal rat periventricular white matter through production of proinflammatory cytokines and reactive oxygen/nitrogen species. J Neurosci 31: 17982-17995.
- Wu Z, Zhu AQ, Takayama F, Okada R, Liu Y, et al. (2013) Brazilian green propolis suppresses the hypoxia-induced neuroinflammatory responses by inhibiting NF- kappaB activation in microglia. Oxid Med Cell Longev; 906726.
- Li SH, Hu H (2005) Research advances in Tibetan Medicine Seventy-taste-pearl- balls. Xinjiang Journal of Traditional Chinese Medicine 5: 82-83.
- Soeda S, Ochiai T, Paopong L, Tanaka H, Shoyama Y, et al. (2001) Crocin suppresses tumor necrosis factor-alpha-induced cell death of neuronally differentiated PC-12 cells. Life Sci 69: 2887-2898.
- Papandreou MA, Kanakis CD, Polissiou MG, Efthimiopoulos S, Cordopatis P, et al. (2006) Inhibitory activity on amyloid-beta aggregation and antioxidant properties of Crocus sativus stigmas extract and its crocin constituents. J Agric Food Chem 54: 8762-8768.
- Papandreou MA, Tsachaki M, Efthimiopoulos S, Cordopatis P, Lamari FN, et al. (2011) Memory enhancing effects of saffron in aged mice are correlated with antioxidant protection. Behav Brain Res 219: 197-204.
- Chen HJ, Kang SP, Lee IJ, Lin YL (2014) Glycyrrhetinic acid suppressed NF-κB activation in TNF-α-induced hepatocytes. J Agric Food Chem 62: 618-625.
- Honda H, Nagai Y, Matsunaga T, Okamoto N, Watanabe Y, et al. (2014) Isoliquiritigenin is a potent inhibitor of NLRP3 inflammasome activation and diet-induced adipose tissue inflammation. J Leukoc Biol 96: 1087-1100.
- Zhu AQ, Masters CL, Li QX (2009) Tibet-medicine effects on ß-amyloid pathology in a transgenic mouse model of Alzheimer’s disease. Chinese Pharmacological Bulletin 6: 720-724.
- Corral-Debrinski M, Horton T, Lott MT, Shoffner JM, Beal MF, et al. (1992) Mitochondrial DNA deletions in human brain: regional variability and increase with advanced age. Nat Genet 2: 324-329.
- Zhu AQ, Xi AQ, Li GF, Li YL, Liao BX, et al. (2012) Ratanasampil (Tibetan medicine, RNSP) reduces ß-amyloid protein (Aß) and pro-inflammatory factor levels and improves cognitive functions in mild-to-moderate Alzheimer’s disease (AD) patients living at high altitude. Journal of Behavioral and Brain Science 2: 82-91.
- Zhu A, Wu Z, Meng J, McGeer PL, Zhu Y, et al. (2015) The neuroprotective effects of ratanasampil on oxidative stress-mediated neuronal damage in human neuronal SH-SY5Y Cells. Oxid Med Cell Longev 2015: 792342.
- Robinson KM, Janes MS, Pehar M, Monette JS, Ross MF, et al. (2006) Selective fluorescent imaging of superoxide In vivo using ethidium-based probes. Proc Natl Acad Sci USA 103: 15038-15043.
- Englander EW, Hu Z, Sharma A, Lee HM, Wu ZH, et al. (2002) Rat MYH, a glycosylase for repair of oxidatively damaged DNA, has brain-specific isoforms that localize to neuronal mitochondria. J Neurochem 83: 1471-1480.
- Simon HU, Haj-Yehia A, Levi-Schaffer F (2000) Role of reactive oxygen species (ROS) in apoptosis induction. Apoptosis 5: 415-418.
- Karin M, Greten FR (2005) NF-kappaB: linking inflammation and immunity to cancer development and progression. Nat Rev Immunol 5: 749-759.
- Toledano MB, Leonard WJ (1991) Modulation of transcription factor NF-kappa B binding activity by oxidation-reduction in vitro. Proc Natl Acad Sci USA 88: 4328-4332.
- Liu SF, Malik AB (2006) NF-kappa B activation as a pathological mechanism of septic shock and inflammation. Am J Physiol Lung Cell Mol Physiol 290: L622-622L645.
- Voronov E , Apte RN (2015) IL-1 in Colon Inflammation, Colon Carcinogenesis and Invasiveness of Colon Cancer. Cancer Microenviron 8: 187-200.
- Wu Z, Sun L, Hashioka S, Yu S, Schwab C, et al. (2013) Differential pathways for interleukin-1β production activated by chromogranin A and amyloid β in microglia. Neurobiol Aging 34: 2715-2725.
- Hornbein TF (1992) Long term effects of high altitude on brain function. Int J Sports Med 13 Suppl 1: S43-45.
- Nelson TO, Dunlosky J, White DM, Steinberg J, Townes BD, et al. (1990) Cognition and metacognition at extreme altitudes on mount everest. J Exp Psychol Gen 119: 367-374.
- Zhu AQ, Song CY, Yang LY (2002) EEG and CT changes in Alzheimer’s patients living at high altitude. Clinical Focus 22: 1313-1314.
- Raichle ME, Mintun MA (2006) Brain work and brain imaging. Annu Rev Neurosci 29: 449-476.
- Larsson A, Skoog I, Aevarsson, Arlig A, Jacobsson L, et al. (2001) Regional cerebral blood flow in normal individuals aged 40, 75 and 88 years studied by 99Tc(m)-d,l-HMPAO SPET. Nucl Med Commun 22: 741-746.
- Roher AE, Debbins JP, Malek-Ahmadi M, Chen K, Pipe JG, et al. (2012) Cerebral blood flow in Alzheimer's disease. Vasc Health Risk Manag 8: 599-611.
- Carlson BW, Neelon VJ, Carlson JR, Hartman M, Bliwise DL (2011) Cerebral oxygenation in wake and during sleep and its relationship to cognitive function in community-dwelling older adults without sleep disordered breathing. J Gerontol A Biol Sci Med Sci 66: 150-156.
- Peers C, Dallas ML, Boycott HE, Scragg JL, Pearson HA, et al. (2009) Hypoxia and neurodegeneration. Ann N Y Acad Sci 1177: 169-177.
- Gao L, Tian S, Gao H, Xu Y (2013) Hypoxia increases Aβ-induced tau phosphorylation by calpain and promotes behavioral consequences in AD transgenic mice. J Mol Neurosci 51: 138-147.
Citation: Meng J, Wu Z, Ni J, Hayashi Y, Nian W, et al. (2018) Ratanasampil Suppresses the Hypoxia-Related Inflammatory Responses by Inhibiting Oxidative Stress and NF-kB Activation in Microglia. J Alzheimers Dis Parkinsonism 8:451. DOI: 10.4172/2161-0460.1000451
Copyright: © 2018 Meng J, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 6188
- [From(publication date): 0-2018 - Nov 17, 2025]
- Breakdown by view type
- HTML page views: 5173
- PDF downloads: 1015