Research Article Open Access
Rapid Measurement of Adenosine Concentration in Human Blood Using Fixed Potential Amperometry: Comparison with Mass Spectrometry and High- Performance Liquid Chromatography
Marion Marlinge1, Donato Vairo2, Viviana Marolda2, Laurie Bruzzese2, Nabil Adjriou2, Claire Guiol2, Nathalie Kipson2, Anna Bonnardel2, Marguerite Gastaldi1, François Kerbaul3, Pierre Michelet3, Jean Claude Deharo4, Giovanna Mottola2, Patrick Mace1, Mohamed Chefrour1 and Regis Guieu1,2*1Laboratory of Biochemistry, Timone Hospital 264 Rue Saint-Pierre, 13385 Marseille, France
2UMR MD2, Aix Marseille University, Bd Pierre Dramard, 13015 Marseille, France
3RUSH Department, Timone 2, 264 Rue Saint-Pierre, 13385 Marseille, France
4Department of Cardiology, Timone Hospital 264 Rue Saint-Pierre, 13385 Marseille, France
- *Corresponding Author:
- Régis Guieu
UMR MD2, Aix Marseille University
Bvd P Dramard 13015, Marseille
France
Tel: +33491396500
E-mail: guieu.regis@orange.fr
Received date: July 11, 2017; Accepted date: July 17, 2017; Published date: July 20, 2017
Citation: Marlinge M, Vairo D, Marolda V, Bruzzese L, Adjriou N, et al. (2017) Rapid Measurement of Adenosine Concentration in Human Blood Using Fixed Potential Amperometry: Comparison with Mass Spectrometry and High-Performance Liquid Chromatography. J Anal Bioanal Tech 8: 371. doi: 10.4172/2155-9872.1000371
Copyright: © 2017 Marlinge M, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Analytical & Bioanalytical Techniques
Abstract
Background: Adenosine is a nucleoside that impacts the cardiovascular system during cardiovascular or inflammatory diseases. The rapid determination of adenosine in blood may be useful in emergency medicine especially in syncope diagnose or septic shock. We compare its measurement in blood using fixed potential amperometry (FPA), with usual methods: mass spectrometry (LC-MS/MS) or high performance liquid chromatography (HPLC). Methods: Twenty healthy subjects (14 men and 6 women) and ten patients suffering from vasovagal syncope (VVS, 6 women and 4 men) were included. Blood samples were collected by vein puncture for plasma adenosine assay and in the same time using finger puncture for direct FAP measurement and on blotting paper for LC-MS/MS. Results: Mean plasma adenosine concentration was 26% higher using HPLC compared with LC-MSMS; p<0.01. In whole blood, adenosine concentration was 35% higher using FPA compared with LC-MS/MS. We found a good correlation between adenosine values measured by FAP and LC-MS/MS in whole blood and between LCMS/ MS and HPLC in plasma. Mean adenosine concentration was higher in patients whatever the method used. Conclusion: Adenosine measurement to the patient’s bed, using FPA may be useful in some cases where high adenosine is associated with pejorative outcome.
Keywords
Adenosine; Adenosine plasma concentration; Cardiovascular diseases; Fixed potential amperometry; Mass spectrometry; High performance liquid chromatography
Introduction
Adenosine is an ATP derivative that is implicated in a lot of cardiovascular and neurological diseases including neurocardiogenic syncope [1-3], coronary artery diseases [4,5], neuropathic pain [6], septic shock [7] and severe systemic inflammatory response [8]. Thus, measurig adenosine in blood may be helpful for some pathologies. As example measuring adenosine in blood help to distinguish patient’s vasovagal syncope (VVS) that have high adenosine plasma concentration (APC) from patients with sudden syncope that have low APC and more pejorative prognostic [2].
Due to its short half-life in the blood, the sample collection and measurment of adenosine is not easy. We have developed a method using a stop solution to inhibit adenosine degradation in body fluid. After plasma deproteinization, adenosine concentration was evaluated using high performance liquid chromatography [9-12]. Some teams have developed a tandem mass spectrometry method that require no stop solution for adenosine measurement in whole blood collected on blotting paper in the perspective of adenosine deaminase deficiency screening [13]. Finally, some have developed a specific fixed potential amperometry (FPA) method using specific electrode to measure adenosine in extracellular cortical slice in real time in the perspective of seizure studies [14].
The aim of this study was to measure adenosine concentration in blood using fixed potential amperometry (FAP) and to compare the results with those obtained with high performance liquid chromatography (HPLC) or Mass spectrometry (LC-MS/MS).
Materials and Methods
Blood samples
Blood samples were collected from 20 healthy subjects (14 men and 6 women, mean age 49 ± 12, recruited among the medical staff) and from 10 patients suffering from VVS (6 women and 4 men, mean age 36 ± 7 years). Their VVS state was well documented by previous head-up tilt test (a test that reproduce the symptomatology), [15] and were recruited in the department of cardiology of the Timone hospital. Patients were included to have a broad range of APC range, because VVS patients are known to have basal high APC [16]. Healthy subjects and patients gave their written inform consent for blood collection. Whole blood was collected through a cubital vein using special tubes (5 mL) under vacuum containing 2 mL of cold stop solution as previously described [11,12]. The stop solution was composed of dipyridamole 0.2 mmol/l, ethylene diamine tetracetic acid disodium (4 mmol/l), erythro 9-2- hydroxy-3-nonyl adenine (5 mmol/l), α,β -methyleneadenosine 5’ diphosphate (79 mmol/l); coformycine 10 μg/mL, and heparin sulfate 1 IU/mL (SIGMA Aldrich). In the same time, whole blood was collected using finger puncture followed by deposit a drop of blood (20 μL) using finnpipette®, on a blotting paper (Whatman 903 protein saver cards™) and dried over night at room temperature to obtain dried blood spot (DBS).
High performance liquid chromatography
The method has been previously described [9-12] Briefly, after collection, blood samples were put in ice then centrifuged (4°C, 1500 g), and deproteinized (perchloric acid, 5%, 0.25 ml/ml of plasma), again centrifuged (1500 g for 10 min) and the supernatant was pipetted off and stored at -80°C until analysis. Defreezed samples were analyzed by chromatography. A modular system with a diode array detector (Chrom System®, Germany) was used. Samples were dissolved in 1 mL of phosphate buffer and eluted with a methanol gradient (0 to 35% in 60 min) on a Merck LiChrospher C18 column (Nottingham, UK). Adenosine was identified by its elution time and by spectrum (pic absorption 254 nm) and quantified by comparison of peak areas with those of known quantities of adenosine. The sensitivity threshold was 5 pmol/ml of plasma matrix. The intra- and inter-assay coefficients of variation ranged from 3% to 6%.
LC-MS/MS
Extraction
Plasma: Internal standard solution with 2-Chloroadenosine was prepared at a concentration of 300 nM in water. One hundred microliters of plasma sample were transferred into a microfuge tube. Each sample was spiked with 50 μl internal standard solution and 300 μl methanol and vortexed for 1 min. Samples were then centrifuged for 10 min at 13300 × g at 4 °C. Supernatant was then evaporated to dryness at 60°C under nitrogen, 150 μl of 0.1% formic acid in water were added and quickly vortexed before transferring into an HPLC auto sampler vial.
Whole blood spot: Six millimeters of dried blood spot (DBS) were cut out followed by extraction with mixture consisting of methanol (400 μl) and internal standard (50 μl) in 2 mL microfuge tubes then mixed for 90 min at 45°C. After extraction, an aliquot of 350 μL were transferred into a new 2-mL safe-lock tube and evaporated to dryness at 60°C under nitrogen, 150 μl of 0.1% formic acid in water were added and quickly vortexed before transferring into an HPLC auto sampler vial.
LC-MS/MS assay: Samples were analyzed using a Shimadzu UFLC XR system consisting of two LC-20ADXR binary pumps, a DGU- 20A5R vacuum degasser, and a CT0-20AC thermostated column oven and a SIL-20ACXR cooled auto sampler (Shimadzu, Marne la Vallée, France). The LC system was interfaced with an ABSciex 4500 triple quadrupole mass spectrometer (Les Ulis, France) operating with an electrospray ionization source (ESI) using nitrogen (purity: 99.99%). Ten microliters of the extracted sample were injected onto a 2.1 × 100 mm, 3 μm Atlantis® T3 column, Waters (Guyancourt, France). The starting mobile phase consisted of 3% methanol and 97% acidified water (0.1% formic acid) with a flow of 0.7 ml/min for 3.5 min. Then, the gradient of methanol was increased to 30% for 3 min. The column was re- equilibrated for 2 min to starting conditions.
Reagents: LC-MS grade methanol and water were purchased from VWR (Fontenay-sous-Bois, France). Formic acid, Adenosine and 2-Chloroadenosine were from Sigma Chemical (Saint-Quentin Fallavier, France). Whatman 903 protein saver cards™ for sample collection and preparation were acquired from GE Healthcare (Cardiff, UK).
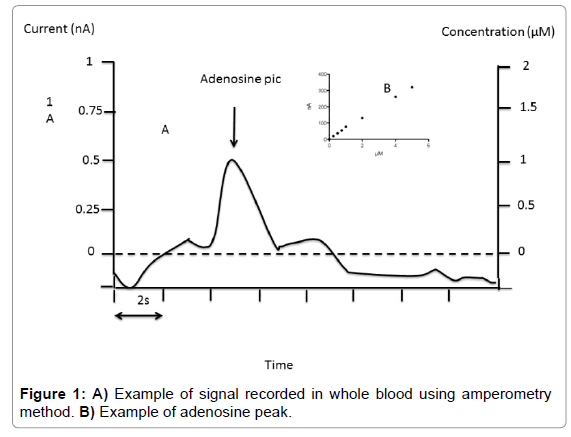
Fixed potential amperometry: We used fixed potential amperometry (FPA) described by Van Gompel et al. [14], with some modifications. FPA uses carbon-based microelectrodes to detect the current associated with electroactive compounds. Adenosine measurement was performed with commercial Pinnacle Biosensor (Pinnacle Biosensors 7001, Sarissa-Biomedical Ltd, Coventry UK). Amperometry biosensors were stored at 4°C until their use. Rehydratation of biosensors was performed using special phosphate buffer (NaCl 100 mM, MgCl2 1 mM, glycerol 2 mM, pH 7.4) for 10 min as suggested by Sarissa- Biomedical. Biosensors were used only once. Calibrations were performed in 50 ml of Phosphate Buffered Saline (PBS, pH 7.4) relative to the internal reference. The biosensors were then polarized to +500 mV and again allowed to asymptote for 10 minutes. Averaged response to 1 μM of analyte was used for pre-in vivo calibration. In vivo data were not used if the sensor showed no post-in vivo calibration responsivity. After calibration procedure, using 7 increasing concentration points (Figure 1) whole blood collected on the finger was mixed with buffer (v/v). Height of the pic was chosen for adenosine quantification (Figure 1). Sensitivity threshold was 0.1 μM and inter assay CV was < 10% in the range of 0.1 to 10 μM.
Statistical analysis: Data were expressed as means and standard deviation or median and interquartile range. A variance analysis (ANOVA two ways analysis) was used for comparison between adenosine level as a function of method used. We used the Pearson’r correlation coefficient for correlation studies.
Results
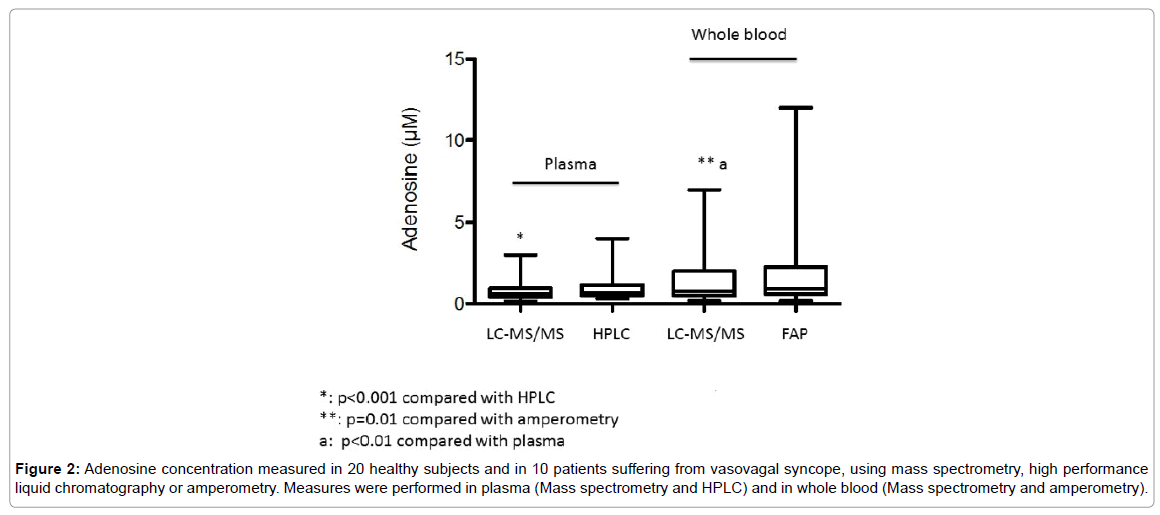
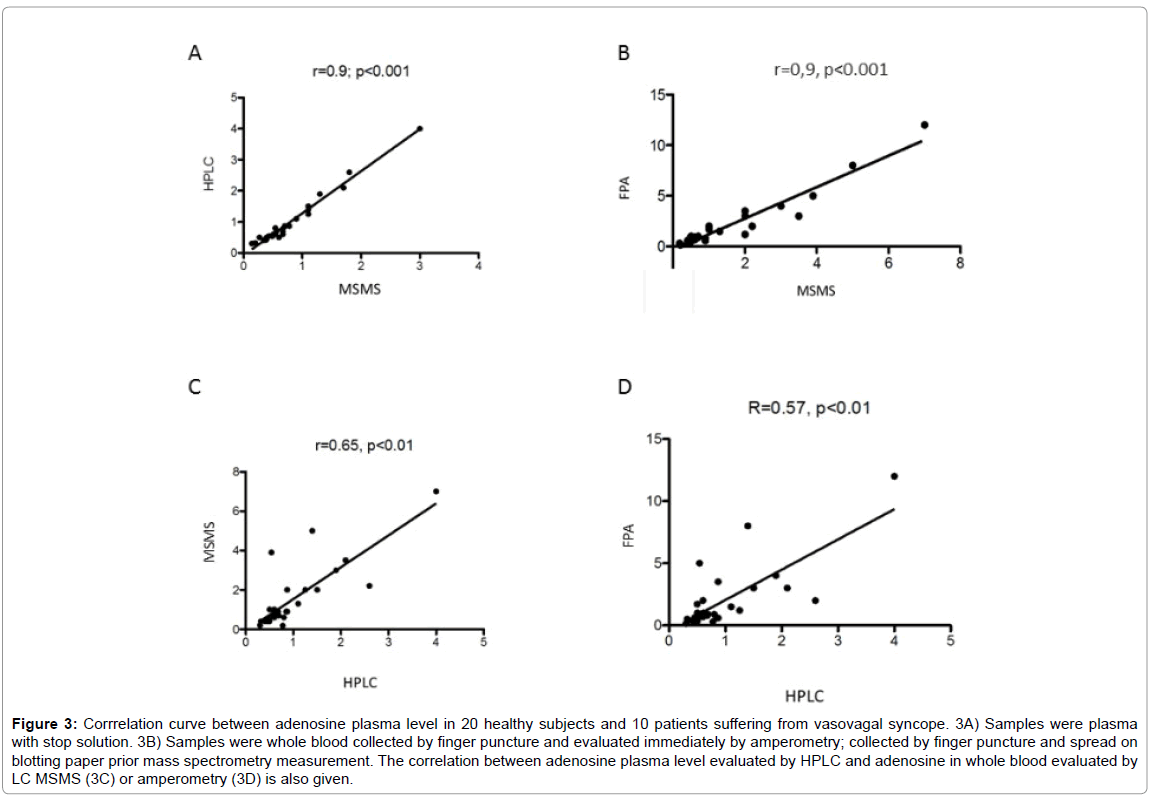
In plasma, mean adenosine concentration was significantly higher using HPLC compared with LC-MS/MS; 0.96 ± 0.79 μM vs 0.76 ± 0.58 μM, mean +26%, p< 0.01 (Figure 2). However, a good correlation was found between the results obtained with these two methods (Figure 3A). In whole blood, mean adenosine concentration was significantly higher using FPA compared with LC-MS/MS: 1.9 ± 2.5 μM vs 1.4 ± 1.5 μM, +35%, p=0.01 (Figure 2). As previously, a good correlation was found between the values obtained with these two methods (Figure 3B).
Figure 2: Adenosine concentration measured in 20 healthy subjects and in 10 patients suffering from vasovagal syncope, using mass spectrometry, high performance liquid chromatography or amperometry. Measures were performed in plasma (Mass spectrometry and HPLC) and in whole blood (Mass spectrometry and amperometry).
Adenosine concentration evaluated with LC-MS/MS was significantly higher in whole blood compared with plasma; whole blood vs plasma: 1.4 ± 1.5 μM vs 0.76 ± 0.58 μM, p < 0.01 (Figure 2). A correlation was also found even less strong between whole blood using LC-/MSMS or FPA and HPLC. LC-MS/MS vs HPLC: r=0.65, p < 0.01; FPA vs HPLC: r=0.57, p< 0.01 (Figures 3C and 3D).
Figure 3: Corrrelation curve between adenosine plasma level in 20 healthy subjects and 10 patients suffering from vasovagal syncope. 3A) Samples were plasma with stop solution. 3B) Samples were whole blood collected by finger puncture and evaluated immediately by amperometry; collected by finger puncture and spread on blotting paper prior mass spectrometry measurement. The correlation between adenosine plasma level evaluated by HPLC and adenosine in whole blood evaluated by LC MSMS (3C) or amperometry (3D) is also given.
Mean adenosine concentration was significantly higher in VVS patients whatever the method and the medium (plasma or whole blood) used. In plasma: LC-MS/MS; Patients vs Controls: 1.32 ± 0.71 μM vs 0.48 ± 0.16 μM, mean +275% p< 0.001. HPLC; 1.7 ± 1 vs 0.57 ± 0.16 μM, +298%, p< 0.001. In whole blood: MS/MS; 3.1 ± 1.7 vs 0.6 ± 0.24; +516%, p< 0.001. FPA: 4.3 ± 3.3 vs 0.74 ± 0.45 μM, + 610%, p< 0.001.
Finally, FPA method gives the higher concentration in adenosine whatever the medium. We choose FPA rather than fast scan cyclic voltametry (FSCV) because FPA is able to distinguish between adenosine and hypoxanthine [14].
Discussion
The mains result of this work is that amperometry (FPA) may be a quick and useful method to measure adenosine concentration in whole blood collected after finger puncture. Indeed, an acceptable correlation was found between results obtained with FPA compared with mass spectrometry or HPLC. The use of spotting paper for whole blood analysis, without stop solution does not alter the results with the condition of placing quickly the drop of blood on the blotter. Adenosine concentration was higher in whole blood than in plasma whatever the method used. This may be explained by a release of adenosine by red blood cells.
Adenosine in plasma was higher using HPLC compared with LCMS/ MS suggesting that mass spectrometry is more specific than HPLC which cannot separate coelution in spite of the use of diode array detector. The most sensitive method remains LC-MS/MS, while FPA was the fastest method, since adenosine concentration may be obtained in less than 10 minutes after blood collection. In this context, we do not evaluate APC using FAP because the goal of this method is to evaluate adenosine in whole blood so to save time.
We found a high adenosine concentration in blood of VVS patients whatever the method and the medium (plasma or whole blood). High APC was previously repeorted in VVS [3,16]. This high APC is responsible for the drop in blood pressure and loss of consciousness. Conversely, low adenosine is associated with sudden syncope [2,3]. This last kind of syncope has a more pejorative prognosis because lacking prodromes and because the cause of loss of consciousness is often atrio-ventricular block [3]. Because syncope accounts for more than 1% of admissions in emergency department, (HAS data: http) the use of a quick measurment of adenosine using FPA in an emergency department, may help to distinguish VVS from others kind of syncope.
Due to the small size of the apparatus which is easily transportable, quick adenosine measurement to the patient’s bed in critical care unit, using FPA may be also useful in the case of septic shock [7] where high adenosine predict outcome or during systemic inflammation response syndrome where high adenosine level is associated with vasoplegia [8]. We concluded that adenosine measurement using FPA may be useful in some cases where high adenosine is associated with pejorative outcome.
Financial Support
Amidex Foundation, France.
References
- Brignole M, Guieu R, Tomaino M, Iori M, Ungar A, et al. (2017) Mechanism of syncope without prodromes with normal heart and normal electrocardiogram. Heart Rhythm 14: 234-239.
- Guieu R,Deharo JC, Ruf J,Mottola G,Kipson N,et al. (2015) Adenosine and Clinical Forms of Neurally-Mediated Syncope. J Am Coll Cardiol 66: 204-205.
- Deharo JC,Guieu R, Mechulan A,Peyrouse E,Kipson N,et al. (2013) Syncope without prodromes in patients with normal heart and normal electrocardiogram: a distinct entity. J Am Coll Cardiol 62: 1075-1080.
- RufJ,Paganelli F, Bonello L,Kipson N,Mottola G, et al. (2016) Spare Adenosine A(2a) Receptors Are Associated with Positive Exercise Stress Test in Coronary Artery Disease. Mol Med 22.
- Guieu R, Kipson N, Ruf J, Fournier N, Laine M,et al. (2015) Low basal expression of A2A adenosine receptors and increase in adenosine plasma concentration are associated with positive exercise stress testing. Int J Cardiol 180: 15-17.
- Guieu R,Peragut JC, Roussel P,Hassani H, Sampieri F, et al. (1996) Adenosine and Neuropathic pain. Pain 68: 271-274.
- Martin C, Leone M, Viviand X,AyemML,Guieu R (2000) High adenosine plasma concentration as a prognostic index for outcome in patients with septic shock. Crit Care Med 28:3198-3202.
- Kerbaul F, Collart F, Giorgi R, Ibrahim Z, Guillen JC, et al. (2006) Role of endogenous adenosine as a predictive marker of vasoplegia during cardiopulmonary bypass and postoperative severe systemic inflammatory response. Crit Care Med 34: 640-645.
- Guieu R, Paganelli F, Sampieri F,Bechis G,Levy S, et al. (1994) The use of HPLC to evaluate the variations of blood coronary adenosine levels during percutaneous transluminal angioplasty. ClinChim Acta 230:63-68.
- GuieuR,Sampieri F,Bechis G,Rochat H (1994) Use of HPLC to measure circulating adenosine levels in migrainous patients. ClinChim Acta227: 185-194.
- Guieu R,Sampieri F,Bechis G,Halimi G,Dussol B,et al. (1999) Develpement of an HPLC diodde array detector method for the determination of human plasma adenosine concentrations. J LiqChrom. Available online: 2006.
- Bonello L,LaineM,Kipson N, Mancini J,HelalO,et al. (2014) Ticagrelor increases adenosine plasma concentration in patients with an acute coronary syndrome. J Am Coll Cardiol63: 872-877.
- Azzari C, la Marca G,Resti M (2011) Neonatal screening for severe combined immunodeficiency caused by an adenosine deaminase defect: a reliable and inexpensive method using tandem mass spectrometry. J AllergyClin Immunol 127: 1394-1399.
- Van Gompel JJ, Bower MR, Worrell GA, Stead M, Chang S,et al.(2014) Increased cortical extracellular adenosine correlates with seizure termination. Epilepsia 55:233-244.
- Raviele A,Menozzi C,Brignole M,Gasparini G,Alboni P,et al. (1995) Value of head-up tilt testing potentiated with sublingual nitroglycerin to assess the origin of unexplained syncope. Am J Cardiol 76:267-272.
- Deharo JC,Mechulan A, Giorgi R,Franceschi F,Prevot S,et al. (2012) Adenosine plasma level and A2A adenosine receptor expression: correlation with laboratory tests in patients with neurally mediated syncope. Heart 98: 855-859.
Relevant Topics
Recommended Journals
Article Tools
Article Usage
- Total views: 4963
- [From(publication date):
August-2017 - Nov 21, 2024] - Breakdown by view type
- HTML page views : 4157
- PDF downloads : 806