Research Article Open Access
Randomized Controlled Trial versus Real-World Study in Postherpetic Neuralgia
| Nalamachu S1* and Bucior I2 | |
| 1International Clinical Research Institute, Overland Park, KS, USA | |
| 2Depomed, Inc., Newark, CA, USA | |
| Corresponding Author : | Nalamachu S International Clinical Research Institute 8675 College Blvd., Suite 150 Overland Park, KS 66210, USA Tel: 913-599-2440 Fax: 913559-5252 E-mail: nalamachu@yahoo.com |
| Received May 10, 2014; Accepted August 22, 2014; Published August 25, 2014 | |
| Citation: Nalamachu S, Bucior I (2014) Randomized Controlled Trial versus Real-World Study in Postherpetic Neuralgia. J Pain Relief 3:154. doi: 10.4172/2167-0846.1000154 | |
| Copyright: 2014 Nalamachu S, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited | |
Visit for more related articles at Journal of Pain & Relief
Abstract
Background: Potential flaws in the design of randomized controlled trials (RCTs) and their low generalizability to clinical practice are being increasingly discussed. As some recent RCTs in neuropathic pain, including postherpetic neuralgia (PHN), demonstrated moderate or no treatment effect, better understanding of factors that may improve trial design, effectiveness, and data interpretation is needed. Objective: To compare RCTs and a real-world study of gastroretentive gabapentin (G-GR) in PHN Methods: Data from two RCTs (Phase 3; n=359) and one real-world study (Phase 4; n=197) of patients with PHN who received G-GR 1800 mg once-daily. The Visual Analog Scale (VAS) and Brief Pain Inventory (BPI) were completed at baseline and the end of study. Patients’ Global Impression of Change (PGIC) was completed at the end of study. Results: Main differences in patient characteristics included higher baseline pain intensity on the VAS and BPI and no use of concomitant neuropathic pain medication in Phase 3. Reductions from baseline in the VAS (p=0.0201) and BPI pain scores (all p<0.05) were significantly greater in Phase 3 compared with Phase 4. In contrast, more patients reported “Very Much” or “Much” improvement on the PGIC in Phase 4 (p=0.0446). Similar proportion of patients experienced ≥1 AE (Phase 3, 54.6%; Phase 4, 50.8%), and AE incidence decreased rapidly to steady low levels after the 2-week titration. More patients discontinued treatment due to AEs during titration in Phase 4 (12.2% vs. 3.1%). Conclusion: To support evidence relevant to clinical practice in PHN and other neuropathic pain syndromes, real-world studies should be a standard complement to RCTs. Based on the RCT vs. real-world study comparison, management of AEs during titration seems important for achieving optimal treatment in clinical practice. For better trial design, measures of overall improvement (e.g., PGIC) should be considered as co-primary efficacy endpoints, along with pain intensity.
| Keywords |
| Randomized controlled trial; Real-world study; Neuropathic pain; Postherpetic neuralgia; Gabapentin |
| Introduction |
| Neuropathic pain arises from neural tissue injury that affects the somatosensory system and manifests as a collection of abnormal sensations and/or pain symptoms that can last for weeks or even years, and which may have a debilitating impact on quality of life [1-3]. Almost any pathological process that creates damage or dysfunction in neural tissue can potentially cause neuropathic pain. This includes a complication of the varicella zoster reactivation (herpes zoster, HZ, or shingles) that leads to postherpetic neuralgia (PHN) [1,4,5]. The effective management of PHN and other neuropathic pain syndromes remains an ongoing challenge, all current treatments are symptomatic, and patients may be left undertreated [6,7]. |
| As new or improved treatment options are developed, randomized controlled trials (RCTs) remain the standard for assessing their effectiveness and safety [8]. However, as potential flaws resulting from the strict design and implementation of RCTs are being increasingly discussed, it is apparent that better understanding of factors that may improve the design of neuropathic pain trials is needed [9,10]. It has also become clear that since RCTs are designed to test a therapeutic hypothesis under an optimal setting, several factors may comprise their strict and controlled conditions and thus restrict their application to real-world clinical practice [11]. For example, certain study characteristics (such as larger sample size or parallel-study groups) may be important for decreasing placebo response rates in RCTs and thus increasing the likelihood of positive outcomes [12]. Likewise, strict population inclusion and exclusion criteria in RCTs may result in the elimination of important segments of the population, narrowing the relevance of the treatment [10]. Also, as the demand for study participants has grown, clinical trials have become a way for individuals to have access to medical treatment or earn income. Thus, ready-torecruit individuals may enter trials with certain expectations, which ultimately may affect the response to placebo and/or active treatments [13-15]. |
| In contrast to RCTs, real-world studies measure the effectiveness and safety of an intervention in clinical practice [11]. They are not as strictly designed, are open-label, and patient exclusion criteria are limited to those in the product label. Therefore, such studies can be an important complement to RCTs, and comparing the conclusions of RCTs and real-world studies can improve interpretation of each other’s results. For example, some recent RCTs in PHN and other neuropathic pain syndromes were characterized by high placebo rates and thus showed diminished or no drug efficacy over placebo [12,16]. Comparisons between medical evidence established in RCTs and real-world studies could help evaluate possible factors associated with positive vs. negative treatment outcomes and guide better design of future neuropathic pain trials. Furthermore, as existing treatment options may not be satisfactory for some patients [6,7] , comprehensive analyses of complementary real-world studies may lead to more effective treatment of neuropathic pain in clinical practice. |
| For management of neuropathic pain associated with PHN, gabapentin was the first oral medication approved by the Food and Drug Administration (FDA) [17-19]. A novel formulation of gabapentin utilizing gastroretentive technology (gastroretentive gabapentin, G-GR) resulted in more efficient drug absorption, improved bioavailability, and reduced dosing frequency from thrice daily to once daily [20,21]. Efficacy and safety of G-GR in management of PHN has been established in two RCTs, and further examined in a real-world study (the only real-world study for any formulation of gabapentin) [16,22,23]. For other first-line treatment options, one realworld study of pregabalin and one of lidocaine patch 5% in treatment of PHN have been performed [23-25]. Therefore, as comparative analyses between RCTs and real-world studies in PHN are lacking, the current analysis compares the study designs as well as the efficacy and safety outcomes between two RCTs and one real-world study of G-GR in treatment of PHN. Potential factors that may improve the design and the quality of medical evidence of clinical trials in PHN and other neuropathic pain syndromes are discussed. |
| Methods |
| Study design and treatment |
| Integrated data from two RCTs (Phase 3, double-blind, randomized, placebo-controlled studies 81-0045 and 81-0062; clinicaltrials.gov identifiers NCT00335933 and NCT00636636) [16,22] and one realworld study (Phase 4, open-label, single-arm study 81-0067) [23] were compared in this analysis. All studies shared a similar G-GR treatment schedule, which included a 2-week titration period, a stable-dose treatment period (8 weeks for the Phase 3 studies and 6 weeks for the Phase 4 study), and a 1-week dose tapering period. The 2-week titration period used a set schedule—Day 1: 300 mg; Day 2: 600 mg; Days 3â��6: 900 mg; Days 7â��10: 1200 mg; Days 11â��14: 1500 mg; Day 15: 1800 mg. During the stable-dose treatment period, patients received 1800 mg G-GR once daily with the evening meal. The schedule for the 1-week dose tapering was 2x600 mg for 3 days and 1x600 mg for the last 4 days |
| Patient selection |
| Detailed patient selection criteria for individual studies have been previously published [16,22,23]. Briefly, men or women aged ≥18 years were eligible to enter the Phase 3 or 4 studies. In the Phase 3 studies, the main inclusion criteria included patients who experienced PHN for at least 3 (Study 81-0045) or 6 (Study 81-0062) months after the healing of a HZ skin rash, with an average daily pain score at the end of a 1-week pre-treatment baseline period of ≥4 based on an 11-point Likert numerical rating scale (NRS), where 0=no pain and 10=worst possible pain. In the Phase 4 study, patients with active PHN were eligible to enroll, regardless of their baseline pain scores, or how long had elapsed since healing of a HZ rash. For the exclusion criteria, the Phase 3 studies included several of those criteria, including no use of concomitant medication. Exclusion criteria in the Phase 4 study were limited to those in the product label and included pregnant women or nursing mothers, patients with hypersensitivity to gabapentin, and patients who had an estimated creatinine clearance levels of <30 mL/min or were on hemodialysis. There were no restrictions on the use of prior medications, and their continued use was permitted. |
| Efficacy and safety assessments |
| Only patients who received G-GR 1800 mg once daily were included in the current efficacy and safety analyses (placebo groups from the Phase 3 studies were not included). Efficacy assessments included pain intensity evaluated on the Visual Analog Scale (VAS), pain intensity and various pain interference measures evaluated using the Brief Pain Inventory (BPI), and overall improvements evaluated using the Patients’ or Clinical Global Impression of Change (PGIC/CGIC). For pain scores on the BPI, worst, least, average, and current pain were assessed. For the BPI interference scores, general activity, mood, walking ability, normal work, relationships, sleep, and enjoyment of life, as well as the average of the 7 interference scores were assessed. The VAS and BPI scores were based on the 11-point NRS (where 0=no pain/no interference item, and 10=worst possible pain/worst possible interference item), and both were completed at the end of the baseline week and at the end of study (Week 10 for Phase 3 and Week 8 for Phase 4). The P/CGIC was completed at the end of study. Safety assessments included the incidence and severity of adverse events (AEs) and serious AEs (SAEs), and analysis of discontinuations due to AEs. |
| Statistical methods |
| In the Phase 3 and 4 studies, efficacy analyses were performed on all patients who received ≥1 dose of G-GR, completed VAS at baseline, and completed ≥1 post-baseline VAS assessment. Mean changes from baseline in the VAS and BPI scores were estimated with an analysis of covariance (ANCOVA) model that included treatment, study centers, and the baseline value as the covariate. Last observation carried forward (LOCF) methodology was used to impute missing efficacy data. For the PGIC and CGIC, the proportion of patients “Very Much” or “Much” improved at the end of the study was determined. Differences between continuous efficacy endpoints in the Phase 3 and 4 studies were calculated using the two-tailed t-test. For differences between dichotomous efficacy endpoints, the two-tailed Z test for the difference in proportions between two groups was calculated. Statistical tests were based on the significance level of α=0.05. Probabilities of being “Very Much” or “Much” improved on the PGIC for patients with any reduction from baseline in the VAS or the average of 7 BPI interference scores were calculated |
| Safety analyses were performed on all patients who received ≥1 dose of G-GR. All AEs were linked to system organ class (SOC) and preferred term (PT) using the Medical Dictionary for Regulatory Activities coding (MedDRA®; Version 9.0 in Phase 3 and Version 14.0 in Phase 4). Patients were counted under multiple SOCs and PTs, but for each SOC and PT, a patient was only counted once. Because AEs were collected until Week 12 in Phase 3 and until Week 9 in Phase 4, the analysis of AEs by week included common data until Week 9. |
| Results |
| Study, patient, and disease characteristics |
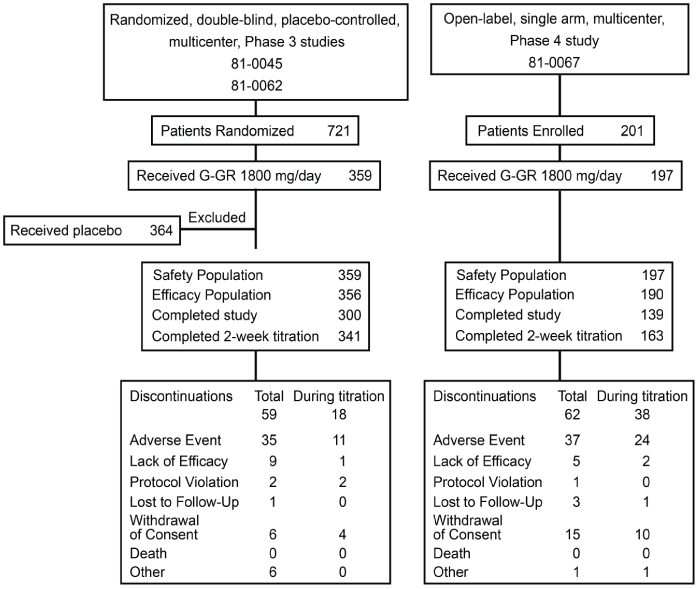
| Studies shared similar treatment schedule, but there were major differences in the design of the two Phase 3 vs. one Phase 4 study (Table 1). These differences included the requirement for the minimum length of PHN (≥3 months) and for the pain intensity of ≥4 on the NRS for patients entering the Phase 3 studies, whereas in the Phase 4 study, any patient with active PHN regardless of the baseline pain intensity could enroll (Table 1) Also, patients enrolled in the Phase 3 studies were not allowed to use concomitant medications, whereas there were no such restrictions in the Phase 4 study. In total, 359 patients received G-GR 1800 mg once daily in the two Phase 3 studies (Figure 1). Almost all patients (95%) completed the 2-week titration period, and 84% completed the Phase 3 studies. In the Phase 4 study, 197 patients received G-GR 1800 mg once daily, 83% completed the 2-week titration, and 71% completed the study (Figure 1). AEs were the most common reason for discontinuation in all studies. |
| Demographics (i.e., age, gender, and race) of patients treated with G-GR 1800 mg/day were similar between the Phase 3 and 4 studies (Table 2). The duration of PHN prior to entry into the Phase 3 studies was calculated as months between the resolution of HZ and study entry, whereas in the Phase 4 study, it was calculated as months between the date of PHN diagnosis and the date of informed consent. Despite these differences, the mean duration of PHN was similar between the two clinical programs (27 months in Phase 3 and 29 months in Phase 4) (Table 2). A total of 84 (42.6%) patients were taking a concomitant neuropathic pain medication at baseline in the Phase 4 study, mostly opioids (28.9%) and anticonvulsants (13.2%). |
| There were significant differences in disease characteristics between the two clinical programs. At study entry, the mean pain intensity on the VAS and BPI was significantly higher in the Phase 3 studies compared with the Phase 4 study (65.1 vs. 56.9, p<0.0001) (Table 3). Also, the baseline measurements of pain intensity on the VAS in the Phase 3 studies showed smaller variation from the average value, which was reflected by the smaller standard deviation (SD) and the range of baseline values (Phase 3: SD, 15.4; range 26�100 vs. Phase 4: SD, 22.9; range 2�100). For mean BPI interference scores at baseline, mood (p=0.0051), sleep (p=0.0002), and the average of 7 BPI interferences scores (p=0.0483) were significantly greater in the Phase 3 studies (Table 3). |
| Efficacy |
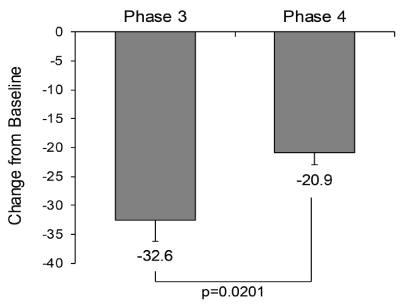
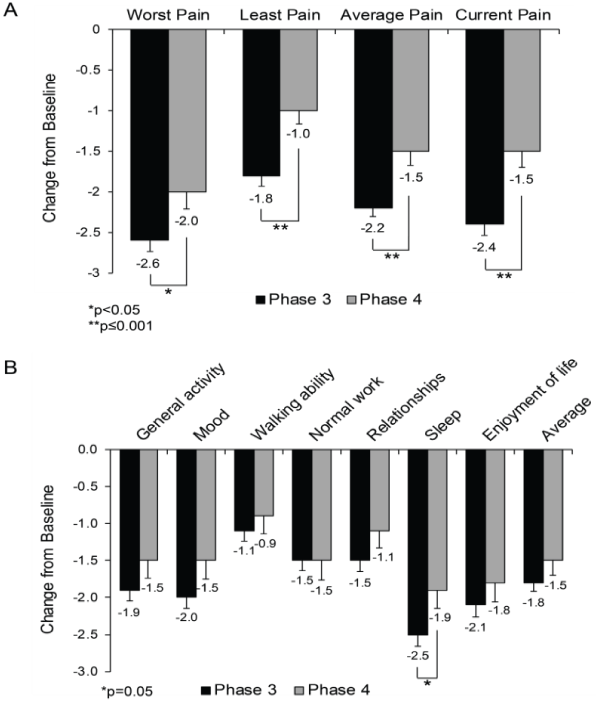
| For pain intensity measured on the VAS, patients reported significant reductions from baseline in the integrated Phase 3 studies (-32.6) and in the Phase 4 study (-20.9) (Figure 2). The difference between the two clinical programs was statistically significant (p=0.0201). For the measurement of pain intensity within the last 24 hours on the BPI (Phase 3/Phase 4), the mean reductions from baseline in the worst (-2.6/- 2.0), least (-1.8/-1.0), average (-2.2/-1.5), and current (-2.4/-1.5) pain were also significantly different between the Phase 3 and 4 studies (Figure 3A). In contrast, for the measurement of quality-of-life components on the BPI interference scores, reductions in individual items and the average score were not significantly different between the Phase 3 and 4 studies, except for sleep interference (p=0.0474) (Figure 3B). |
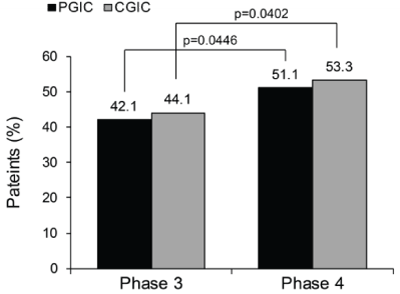
| In total, 42.1% of patients reported feeling “Very Much” or “Much” improved on the PGIC in the Phase 3 studies, whereas 51.1% felt “Very Much” or “Much” improved in the Phase 4 study (Figure 4). The difference in the proportion of patients was significant (p=0.0446). Also, the difference in the proportion of patients “Very Much” or “Much” improved on the CGIC was significant when the Phase 3 studies were compared with the Phase 4 study (44.1% vs. 53.3%, p=0.0402) (Figure 4). |
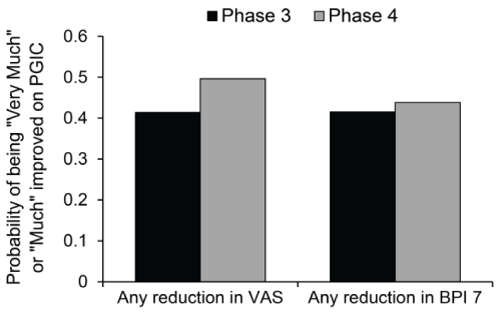
| Additional exploratory analyses were performed to calculate the probabilities of being “Very Much” or “Much” improved on the PGIC for patients with any reduction from baseline in the VAS or the average of 7 BPI interference scores (Figure 5). For patients with any reduction in the VAS score, the probability to also report “Very Much” or “Much” improvement on the PGIC was similar between the Phase 3 and Phase 4 studies (0.41 and 0.50, respectively). Likewise, for patients with any reduction in the average of 7 BPI interference scores, the probability to also report improvements on the PGIC was similar between the two clinical programs (0.42 in Phase 3 and 0.44 in Phase 4). |
| Safety |
| A total of 196 (54.6%) patients in the Phase 3 studies and 100 (50.8%) patients in the Phase 4 study experienced an AE (Table 4). The most common AEs occurring in ≥4% of patients (Phase3 vs. Phase 4) were dizziness (10.9% vs. 13.7%), somnolence (4.5% vs. 5.6%), and headache (4.2% vs. 3.6%). In both clinical programs, the prevalence of all AEs as well as AEs most common for gabapentinoids (dizziness and somnolence) decreased rapidly during the 2-week titration period and reached sustained low levels after 2 week soft treatment (Figure 6). In the Phase 3 studies, 9.7% of patients experienced AEs that led to study discontinuation, and 3.1% of patients discontinued during the titration period (Table 4). In contrast, more patients (18.8%) discontinued the Phase 4 study due to AEs, and most of these discontinuations occurred during the 2-week titration (12.2%). Discontinuations due to AEs most common for gabapentinoids (dizziness and somnolence) were more frequent in the Phase 4 vs. Phase 3 (dizziness, 6.1% vs. 2.2%; somnolence, 4.1% vs. 0.3%). |
| No patients treated with G-GR died and the incidence of SAEs was low in both clinical programs (Table 4). In the Phase 3 studies, SAEs reported by patients treated with G-GR included ventricular hypertrophy, pneumonia, dehydration, chronic pancreatitis, upper limb fracture, osteochondrosis, and Pancoast's tumour. Only chronic pancreatitis was judged by the investigator to be related to G-GR. SAEs reported in the Phase 4 study included coronary artery disease, duodenal ulcer, gout, pneumonia, hematuria, and confusional state; only confusional state was judged by the investigator to be probably related to G-GR. |
| Discussion |
| Development of novel therapies for neuropathic pain relies on a linear model, with RCTs used to establish the effectiveness of studied intervention. RCTs, however, have their limitations and some recent RCTs in neuropathic pain, including in PHN, showed only moderate treatment effects or even failed [12,16,26]. Therefore, there is a growing concern about the quality of RCTs, and their inability to detect a positive signal in an efficacious analgesic [27]. Also, different approaches to establishing and interpreting medical evidence in neuropathic pain should become more common, and comparisons between RCTs and complementary real-world studies can guide better design of clinical trials and better treatment of neuropathic pain in clinical practice [11]. |
| There are notable differences in the design and characteristics of RCTs vs. real-world studies. The strict, controlled conditions under which RCTs are conducted, and the range of potential biases that favor active-treatment groups (including enriched populations selected through inclusion/exclusion criteria) may hinder RCTs and limit the quality of evidence with regard to clinical practice [28,29]. Conversely, the design of real-world studies is simpler and more pragmatic, but these studies may have low internal validity [11]. For example, in the current analysis, there were several differences in the design and baseline disease characteristics between two RCTs and one real-world study, including different pain intensity scores at study entry (which were higher for the RCTs) and the use of concomitant medications (which was only allowed in the real-world study). As these factors may differently affect the outcomes of clinical trials in neuropathic pain, real-world studies should be performed more often to complement classical RCTs [11,30]. |
| To better understand the design of clinical trials in PHN in particular and neuropathic pain in general, and characterize potential factors important for successful transition of the treatment to clinical practice, the current analysis described and analyzed the differences between two Phase 3 RCTs and one real-world Phase 4 study for the treatment of PHN with G-GR. For efficacy measurements, assessments of improvements in various measures encompassing the quality of life showed no significant differences between the two programs (except for significantly greater improvement in sleep quality observed in the Phase 3 studies compared with the Phase 4 study). However, for measurements of pain intensity, treatment with G-GR provided significantly greater relief in pain intensity evaluated on the VAS and BPI scores in the Phase 3 studies when compared with the Phase 4 study. In contrast, the analysis of patients’ impression of overall improvement showed that significantly more patients in the Phase 4 study reported feeling “Very Much” or “Much” improved on the PGIC. Finally, the probability to report feeling “Very Much” or “Much” improved on the PGIC by patients with any reduction in pain intensity or in the average of pain-interference scores was similar between the Phase 3 and 4 studies. These results suggest that, although measurements of pain intensity are the only primary efficacy endpoints in trials of PHN and other neuropathic pain syndromes, pain control is not the only element that contributes to patients feeling overall improvement at the end of treatment. Therefore, measures of overall improvement (e.g., PGIC) should be considered as co-primary efficacy endpoints, which could potentially improve detection of positive efficacy signals in neuropathic pain trials. |
| Most patients with PHN are over 60 years of age, often have medical comorbidities, and are likely to already be taking several medications [5,31-33]. Therefore, in contrast to selected patient population in RCTs, patients in clinical practice are often taking various concomitant medications and may find it more difficult to tolerate and adjust to AEs associated with yet another therapeutic. Current assessments of G-GR safety and tolerability in the Phase 3 RCTs vs. the Phase 4 real-world study showed no differences in the incidence and profile of reported AEs. However, more patients who enrolled in the Phase 4 study discontinued G-GR treatment due to AEs compared with patients enrolled in the Phase 3 studies, primarily due to more discontinuations during the 2-week titration period. Consequently, titration to therapeutically efficacious dosages may be limited by AEs, and patients may either discontinue treatment or have dosages adjusted to lower levels, leaving them undertreated [7,34]. A retrospective analysis of administrative claims found that an immediate-formulation of gabapentin and pregabalin were not used effectively in the treatment of PHN, as only a small fraction of patients reached therapeutic dosages and were thus left with suboptimal treatment [35]. Thus, it is important to note that, in a number of different studies, all AEs associated with G-GR decreased rapidly and reached low levels after the 2-week titration period. This has been shown in the RCTs and the real-world study for the treatment of PHN [23,36], as well as in the RCTs for the treatment of hot flashes in post-menopausal women [37]. Thus, as long as patients are aware of the tolerability profile and much lower incidence of AEs after titration, they may be able to manage the titration better and be more successful in reaching therapeutically effective dosages of G-GR. As other gabapentinoids share similar AE profile and also require titration [19,34], this observation may be generally important for achieving optimal treatment of PHN in clinical practice. |
| Conclusion |
| The combined evidence from two RCTs and one real-world study in neuropathic pain can address clinical questions that cannot be answered by either study alone, and real-world studies should be a standard complement to RCTs [30]. The current comparison of study characteristics and clinical results between the RCTs and the real-world study of G-GR in treatment of PHN provides potentially important information for better design of primary efficacy endpoints in clinical trials, with measures of overall improvement as potential coprimary efficacy endpoints in addition to standard measures of pain intensity. Also, the current analysis contributes to better understanding of factors important for quality evidence and its relevance to clinical practice, with reduction in AE incidence over time having potential impact on the management of the titration to therapeutic dosages. In summary, these observations can contribute to better optimization of clinical trials and improve treatment of neuropathic pain in real-world clinical practice. |
| Acknowledgements |
| The study was supported by Depomed, Inc. |
References
- Jay GW, Barkin RL (2014) Neuropathic pain: Etiology, pathophysiology, mechanisms, and evaluations. Dis Mon 60: 6-47.
- Pickering G, Leplege A (2011) Herpes zoster pain, postherpetic neuralgia, and quality of life in the elderly. Pain Pract 11: 397-402.
- Smith BH, Torrance N (2012) Epidemiology of neuropathic pain and its impact on quality of life. Curr Pain Headache Rep 16: 191-198.
- Johnson RW, Wasner G, Saddier P, Baron R (2007) Postherpetic neuralgia: epidemiology, pathophysiology and management. Expert Rev Neurother 7: 1581-1595.
- Tontodonati M, Ursini T, Polilli E, Vadini F, Di Masi F, et al. (2012) Post-herpetic neuralgia. Int J Gen Med 5: 861-871
- Backonja M, Woolf CJ (2010) Future directions in neuropathic pain therapy: closing the translational loop. Oncologist 15 Suppl 2: 24-29
- Sacks GM (2013) Unmet need in the treatment of postherpetic neuralgia. Am J Manag Care 19: S207-213
- Meldrum ML (2000) A brief history of the randomized controlled trial. From oranges and lemons to the gold standard. HematolOncolClin North Am 14: 745-760
- Grossman J, Mackenzie FJ (2005) The randomized controlled trial: gold standard, or merely standard? PerspectBiol Med 48: 516-534.
- Simon SD (2001) Is the randomized clinical trial the gold standard of research? J Androl 22: 938-943.
- Saturni S, Bellini F, Braido F, Paggiaro P, Sanduzzi A, Scichilone N, Santus PA, Morandi L, Papi A (2014) Randomized controlled trials and real life studies. Approaches and methodologies: a clinical point of view. PulmPharmacolTher 27: 129-138
- Katz J, Finnerup NB, Dworkin RH (2008) Clinical trial outcome in neuropathic pain: relationship to study characteristics. Neurology 70: 263-272.
- Fisher JA (2007) "Ready-to-Recruit" or "Ready-to-Consent" Populations?: Informed Consent and the Limits of Subject Autonomy. QualInq13: 875-894.
- Steinkopf L (2012) Enhancing drug compliance and the placebo effect by raising subjective expectations. Med Hypotheses 79: 698-700.
- Finniss DG, Kaptchuk TJ, Miller F, Benedetti F (2010) Biological, clinical, and ethical advances of placebo effects. The Lancet 375: 686-695.
- Wallace MS, Irving G, Cowles VE (2010) Gabapentin extended-release tablets for the treatment of patients with postherpetic neuralgia: a randomized, double-blind, placebo-controlled, multicentre study. Clin Drug Investig 30: 765-776.
- Rowbotham M, Harden N, Stacey B, Bernstein P, Magnus-Miller L (1998) Gabapentin for the treatment of postherpetic neuralgia: a randomized controlled trial. JAMA 280: 1837-1842.
- Rice ASC, Maton S, Group PNS (2001) Gabapentin in posterpetic neuralgia: a randomised, double blind, placebo controlled study. Pain 94: 215-224.
- Harden RN, Kaye AD, Kintanar T, Argoff CE (2013) Evidence-based guidance for the management of postherpetic neuralgia in primary care. Postgrad Med 125: 191-202.
- Argoff CE, Chen C, Cowles VE (2012) Clinical development of a once-daily gastroretentive formulation of gabapentin for treatment of postherpetic neuralgia: an overview. Expert Opin Drug Deliv 9: 1147-1160
- Chen C, Han CH, Sweeney M, Cowles VE (2013) Pharmacokinetics, efficacy, and tolerability of a once-daily gastroretentive dosage form of gabapentin for the treatment of postherpetic neuralgia. J Pharm Sci 102: 1155-1164.
- Sang CN, Sathyanarayana R, Sweeney M, Investigators DMS (2013) Gastroretentive gabapentin (G-GR) formulation reduces intensity of pain associated with postherpetic neuralgia (PHN). Clin J Pain 29: 281-288.
- Markley HG, Dunteman E, Sweeney M (2014) Real-World Experience with Once-Daily Gabapentin for the Treatment of Postherpetic Neuralgia (PHN). Clin J Pain Epub ahead of print.
- Baron R, Brunnmuller U, Brasser M, May M, Binder A (2008) Efficacy and safety of pregabalin in patients with diabetic peripheral neuropathy or postherpetic neuralgia: Open-label, non-comparative, flexible-dose study. Eur J Pain 12: 850-858.
- Katz NP, Gammaitoni AR, Davis MW, Dworkin RH (2002) Lidocaine patch 5% reduces pain intensity and interference with quality of life in patients with postherpetic neuralgia: an effectiveness trial. Pain Med 3: 324-332.
- Polydefkis M, Raja SN (2008) What can we learn from failed neuropathic pain trials? Neurology 70: 250-251.
- Hoke A, Simpson DM, Freeman R (2013) Challenges in developing novel therapies for peripheral neuropathies: a summary of The Foundation for Peripheral Neuropathy Scientific Symposium 2012. J PeripherNervSyst 18: 1-6.
- Sauzet O, Williams JE, Ross J, Branford R, Farquhar-Smith P, et al. (2013) The characteristics and quality of randomized controlled trials in neuropathic pain: a descriptive study based on a systematic review. Clin J Pain 29: 591-599
- Moore RA, Derry S, Wiffen PJ (2013) Challenges in design and interpretation of chronic pain trials. Br J Anaesth 111: 38-45.
- Price D, Bateman ED, Chisholm A, Papadopoulos NG, Bosnic-Anticevich S, et al. (2014) Complementing the randomized controlled trial evidence base. Evolution not revolution. Ann Am ThoracSoc 11 Suppl 2: 92-98
- Dworkin RH, Johnson RW, Breuer J, Gnann JW, Levin MJ, et al. (2007) Recommendations for the management of herpes zoster.Clin Infect Dis 44 Suppl 1: S1-26.
- Jung BF, Johnson RW, Griffin DRJ, Dworkin RH (2004) Risk factors for postherpetic neuralgia in patients with herpes zoster. Neurology 62: 1545-1551.
- Fortin M, Bravo G, Hudon C, Vanasse A, Lapointe L (2005) Prevalence of multimorbidity among adults seen in family practice. Ann Fam Med 3: 223-228
- Massengill JS, Kittredge JL (2014) Practical Considerations in the Pharmacological Treatment of Postherpetic Neuralgia for the Primary Care Provider. J Pain Res7:124-132.
- Johnson P, Becker L, Halpern R, Sweeney M (2013) Real-world treatment of post-herpetic neuralgia with gabapentin or pregabalin. Clin Drug Investig 33: 35-44
- Gupta A, Li S (2013) Safety and Efficacy of Once-Daily Gastroretentive Gabapentin in Patients with Postherpetic Neuralgia Aged 75 Years and Over. Drugs Aging 30: 999-1008
- Pinkerton JV, Kagan R, Portman D, Sathyanarayana R, Sweeney M, et al. (2013) Phase 3 randomized controlled study of gastroretentive gabapentin for the treatment of moderate-to-severe hot flashes in menopause. Menopause 21: 567-573.
Tables and Figures at a glance
| Table 1 | Table 2 | Table 3 | Table 4 |
Figures at a glance
 |
 |
 |
 |
 |
 |
| Figure 1 | Figure 2 | Figure 3 | Figure 4 | Figure 5 | Figure 6 |
Relevant Topics
- Acupuncture
- Acute Pain
- Analgesics
- Anesthesia
- Arthroscopy
- Chronic Back Pain
- Chronic Pain
- Hypnosis
- Low Back Pain
- Meditation
- Musculoskeletal pain
- Natural Pain Relievers
- Nociceptive Pain
- Opioid
- Orthopedics
- Pain and Mental Health
- Pain killer drugs
- Pain Mechanisms and Pathophysiology
- Pain Medication
- Pain Medicine
- Pain Relief and Traditional Medicine
- Pain Sensation
- Pain Tolerance
- Post-Operative Pain
- Reaction to Pain
Recommended Journals
Article Tools
Article Usage
- Total views: 14273
- [From(publication date):
September-2014 - Apr 05, 2025] - Breakdown by view type
- HTML page views : 9730
- PDF downloads : 4543
