Research Article Open Access
Raman Microspectroscopy Demonstrates Alterations in Human Mandibular Bone after Radiotherapy
Singh SP1,2, Parviainen I1, Dekker H3, Schulten EAJM3, ten Bruggenkate CM3, Bravenboer N3, Mikkonen JJ2, Turunen MJ4, Koistinen AP2 and Kullaa AM1,5,6*
1Institute of Dentistry, University of Eastern Finland, Kuopio Campus, Yliopistonranta 1, Kuopio, Finland
2SIB Labs, University of Eastern Finland, Yliopistonranta 1, Kuopio, Finland
3Department of Oral and Maxillofacial Surgery and Oral Pathology, VU University Medical Center/Academic Centre for Dentistry Amsterdam (ACTA), Amsterdam, The Netherlands
4Department of Applied Physics, Faculty of Science and Forestry, University of Eastern Finland, Kuopio, Finland
5Research Group of Oral Health Sciences, Faculty of Medicine, University of Oulu, Oulu, Finland
6Educational Dental Clinic, Kuopio University Hospital, Kuopio, Finland
- *Corresponding Author:
- Arja Kullaa
Institute of Dentistry
University of Eastern Finland
Kuopio campus, Yliopistonranta 1
Kuopio, Finland
Tel: +358-44-5150452
E-mail: arja.kullaa@uef.fi/Arja.Kullaa@oulu.fi
Received date: August 21, 2015; Accepted date: September 07, 2015; Published date: September 14, 2015
Citation: Singh SP, Parviainen I, Dekker H, Schulten EAJM, ten Bruggenkate CM, et al. (2015) Raman Microspectroscopy Demonstrates Alterations in Human Mandibular Bone after Radiotherapy. J Anal Bioanal Tech 6:276. doi:10.4172/2155-9872.1000276
Copyright: © 2015 Singh SP, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Analytical & Bioanalytical Techniques
Abstract
Quality and alterations in the biochemical composition of bones used for dental implantation after radiotherapy in cancer patients is always a critical and debatable factor. Clinically the irradiated bone is similar to control bone. The aim of this study was to verify any compositional alterations in human mandible bone after irradiation using Raman microspectroscopy. A total of 36 bone biopsies (21-control, 4-cancer and 11-irradiated) were investigated. Data acquisition points were determined under histopathological supervision. Both mineral and matrix constituents were analyzed by computing area associated with of phosphate (958 cm-1), carbonate (1070 cm-1) and matrix (amide I) bands. Unpaired Student’s t-test was employed to measure level of significance. Absolute mineral contents (phosphate and carbonate) were highest in cancerous specimens. Spectral profile and band-intensity calculations suggest proximity of irradiated specimens with control specimens. Significant differences in both matrix and mineral contents were observed when control/irradiated samples were compared against cancerous specimens. However, no significant differences were observed between control and irradiated groups. Irradiated bone is similar to control and cause of implant loss could be related to osteocytes of the surrounding tissue.
Keywords
Raman spectroscopy; Oral cancer; Mandible; Radiotherapy; Bone
Introduction
Lower disease free survival rates associated with oral cancers have been primarily attributed to late detection and postoperative complications [1]. Following successful diagnosis treatment of oral cancer subjects often include surgery followed by chemo/radiotherapy or combination of both [1,2]. Oral rehabilitation of cancer patients is a meaningful procedure to increase the quality of life [2]. Implant therapy is widely performed to improve masticatory function after tumour surgery. In oral cancer patients, tumour resection is usually combined with irradiation, which locally impairs bone quality and weakens the bone density [2,3]. Radiotherapy of the planned implant site is known to be an important factor in the etiology of implant loss [2-4]. Bone tissue exhibits numerous changes because of radiation, such as diminished vascularization, reduced remodeling capacity and increased risk of osteoradionecrosis [5-7]. Other side effects of radiation therapy of oral cavity area may include severe complications such as xerostomia (drying of the mucsoa), mucositis, reduced mouth opening etc. [5].
Even though radiotherapy plays an important role in the treatment of head and neck malignancies, its concomitant effects may have a major impact on the final outcome of oral rehabilitation. Placing dental implants in essential irradiated tissue is a clinical challenge: periimplantitis and osteoradionecrosis (ORN) are frequently observed, resulting in implant loss in up to 22% of patients [5-7]. Therefore it is pertinent to explore possible prognostic factors which can predict the occurrence of peri-implantitis or implant loss preferably in a noninvasive manner.
Variety of invasive and non-invasive techniques can be utilized to assess radiation induced effects. Histopathology is considered as gold standard, but it is invasive and repetitive sampling for response monitoring is a major concern. Micro-computed tomography (micro-CT) is a non-invasive technique that can be used for bone analysis. It can provide superficial information such as micro architecture. However, minor compositional alterations such collagen mineralisation is difficult to monitor. Optical spectroscopy methods offer an alternate approach for bone analysis. Methods based on fluorescence, infra-red and Raman spectroscopy are being widely explored for non-invasive disease diagnosis [8-11]. Greatest benefit of these techniques lies in their ability to provide objective and detailed information about biochemical changes associated with disease onset or therapeutic interventions in a non-invasive manner and within short period of time. Among these, diagnosis based on Raman effect is being considered as clinically implementable as it is not influenced with water and requires no sample preparation [10,11]. Numbers of studies have successfully demonstrated its efficacy in disease diagnosis and therapeutic response monitoring [11]. In case of bone specimens it offers additional advantages over existing methods as a snapshot of variations in different biochemical parameters such as mineral, crystallinity, carbonate and collagen can be obtained in an objective and non-invasive manner [12-14]. These parameters can serve as direct or indirect representative of the progress of therapeutic interventions and mechanical competence of the bone tissue. Raman spectroscopy has been utilized to study different bone related abnormalities such as osteoporosis, fracture risk, metastasis and drug induced reversal of bone resorption [14,15].
The changes in composition, mechanical properties and the remodelling rate of the bone are critical factors for dental implantation after radiotherapy in cancer patients. An exact understanding of the features of bone quality and bone formation is important in clinical practice for an optimal surgical technique. Irradiated bone presents a challenging environment for implant placement. Implant loss or osteoradionecrosis are common post-treatment complications in oral cancer patients. The reason behind this debatable and can be attributed to either radiation induced changes in the bone or changes in surrounding tissues. Clinically the irradiated bone is considered similar to control. The present study aims at determining minor alterations in the irradiated bone using Raman microspectroscopy.
Materials and Methods
Bone specimens
A total of 36 bone samples obtained during the dental implant surgery were used. Biopsies of the alveolar bone of the mandible approximately 10 × 3.5 mm were obtained with trephine drills from 4 patients with non-radiated oral cancer (OSCC), from 11 patients after radiotherapy and from 21 healthy controls. Bone specimens were fixed in 70% ethanol.
All patients gave their consent to participate in the study, and this work was approved by the ethical committee (Medisch Ethische Toetsingscommissie, VU University medical center, Amsterdam, The Netherlands; 2011/220).
Preparation of specimens
The undecalcified bone specimens were dehydrated in increasing concentrations of ethanol and embedded into Poly (methyl methacrylate) (PMMA). Thereafter, thin sections were cut using a microtome (Polycut S; Reichert-Jung, Wien, Austria). Fresh surface of bone revealed from PMMA block were used for acquiring Raman spectra. For histological examination 10 μm sections were stained using standard protocols with Masson-Goldner trichrome. An optical microscope (AxioImager M2; Carl Zeiss GmbH, Jena, Germany) was used for histological study.
Raman microspectroscopy
Remaining bone blocks were used for acquiring spectra with a dispersive Raman microscope (Senterra 200LX, Bruker Optics GmbH, Ettlingen, Germany). The wavelength of 785 nm at 100 mW power (source) was used for excitation. A 20X objective (NA-0.5) was chosen to minimize polarization effects. Three point measurements with an exposure time of 60 s and 5 co-additions were performed. All the data acquisition points were selected in correlation with histopathology and under pathological supervision. The spectra were acquired for 4000-127 cm-1 range. On z-axis the beam was focused ~10 μm below the surface to ensure spectral acquisition only from bone sections. A background spectrum of the embedding medium i.e., PMMA was also obtained under similar conditions. Both background and bone spectra were interpolated to the finger-print range (1800-800 cm-1) and cosmic peaks were removed. This was followed by normalization of bone spectra using PMMA spectrum. Subsequently to minimize the influence of the background the embedding medium spectrum was mathematically subtracted from the bone spectra [16-18]. Baseline corrections to remove the fluorescence background were performed by fitting a 5th order polynomial function.
Data analysis
All the data pre-processing and analysis were performed using MATLAB (MATLAB 7.5.0, The Mathworks, Inc., Natick, MA) using locally written scripts. Mean spectrum for control, irradiated and cancerous specimens was calculated by averaging the variations at X axis keeping the Y axis constant. Curve fitting was performed to compute area associated with Raman bands using a custom MATLAB based in-house program. In this method, first locations of sub-peaks were identified by minima in a second derivative spectrum. The sum of the squared differences between observed and computed spectra are minimized to obtain the best fit. These peaks were modelled using Gaussian function and areas of selected bands were measured for band intensity. Different parameters shown in Table 1 were computed using this algorithm. Briefly, mineral components were computed by intensity of Phosphate (958 cm-1) and Carbonate bands (1070 cm-1) [14,19]. Mineral crystallinity, inversely proportional to full width at half maximum (FWHM) of the phosphate band was computed [14,19]. Mineral to matrix ratio was calculated by dividing intensity of phosphate band by amide I band (1660 cm-1) [14,19]. Carbonate substitution rate was determined by generating carbonate to phosphate ratio [14,19]. The data are expressed as the mean ± standard deviation (SD), and statistical comparisons were performed with unpaired Student's t-test (Graph Pad Prism, version 6.1). p<0.05 was considered significant, p<0.01 as highly significant, and p<0.001 as very highly significant.
| S No | Raman Parameter | Control (40) | Cancer (19) | Irradiated (26) |
|---|---|---|---|---|
| 1 | Phosphate content | 4.25 ± 0.09 | 4.32 ± 0.10 | 4.27 ± 0.04 |
| 2 | Carbonate content | 1.00±0.17 | 1.16±0.15 | 1.02±0.17 |
| 3 | Carbonate substitution | 0.23±0.04 | 0.27 ±0.03 | 0.24±0.04 |
| 4 | Mineral Crystallinity | 0.039±0.001 | 0.038±0.0008 | 0.039±0.001 |
| 5 | Mineral to matrix ratio-Phosphate | 2.49±0.38 | 2.27±0.30 | 2.46±0.31 |
| 6 | Mineral to matrix ratio-Carbonate | 0.59± 0.15 | 0.62± 0.11 | 0.59± 0.10 |
Table 1: Summary of different biochemical parameters analyzed with Raman spectroscopy. Data is presented as mean ± standard deviation. Numbers in bracket are number of spectra used in each case.
Results and Discussion
Radiation-induced effects on structural and biochemical composition in human mandibular bone was explored with histopathology and Raman spectroscopy. In the following sub-sections a summary of important findings of the study is provided.
Histological features of control, irradiated and unradiated cancerous bones
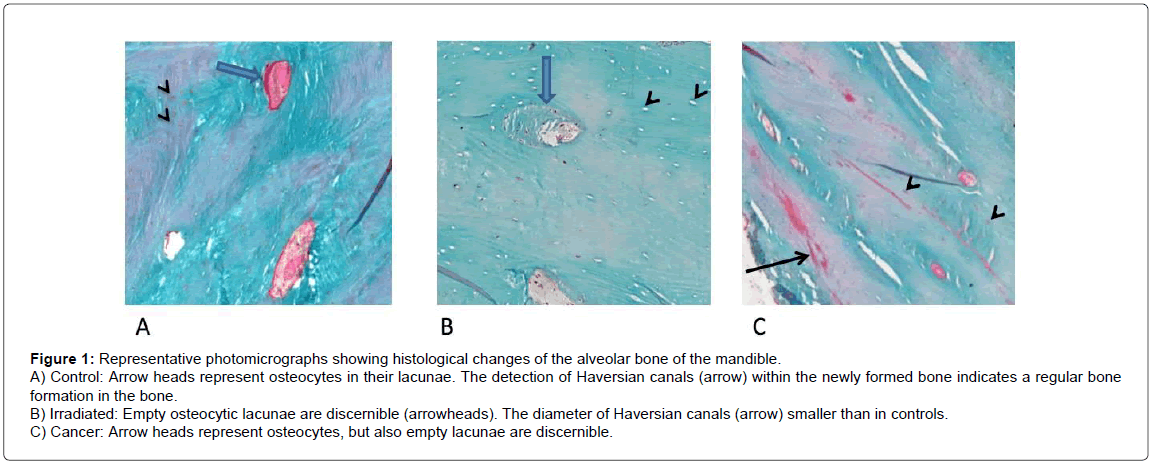
Representative histological sections of control, irradiated and cancerous bone specimens is shown in Figure 1. In control bone, osteoblasts and newly formed bone are recognizable (Figure 1A) indicating the capacity of bone remodeling. Previous studies have shown that bone irradiation is characterized by loss of osteocytes and lamellar structure [20]. As can be seen from Figure 1B, irradiated specimens exhibit significant amount of empty lacunae as a sign of osteocyte deaths. The bone of cancer patients without radiotherapy shows newly formed bone, but the density of osteocytes has decreased (Figure 1C). Corroborating earlier observations, reduction in vascularization and apoptosis of bone cells were also observed in irradiated specimens [21-24].
Figure 1: Representative photomicrographs showing histological changes of the alveolar bone of the mandible.
A) Control: Arrow heads represent osteocytes in their lacunae. The detection of Haversian canals (arrow) within the newly formed bone indicates a regular bone formation in the bone.
B) Irradiated: Empty osteocytic lacunae are discernible (arrowheads). The diameter of Haversian canals (arrow) smaller than in controls.
C) Cancer: Arrow heads represent osteocytes, but also empty lacunae are discernible.
Raman spectral features of control, irradiated and unradiated cancerous bones
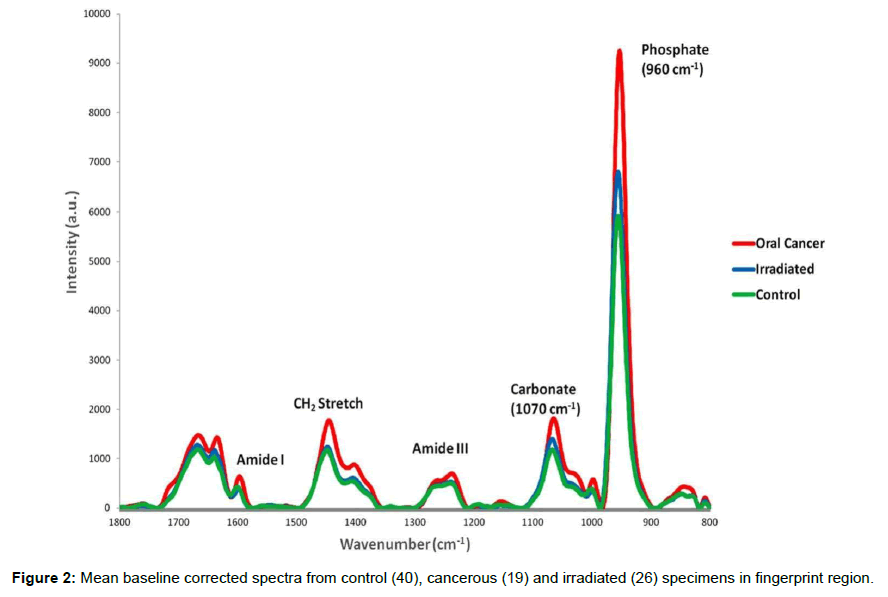
Bone is a biological tissue with complex molecular structure. It can be divided into three parts. First is the organic component which mainly consists of collagen and provides bone with its shape and form. The hydroxyapatite (HA) comprises most of the second part which provides bone its strength and rigidity. Third part is comprised of small blood vessels, cells and water etc. Raman spectroscopy can provide detailed information about biochemical composition in a non-destructive manner under a very short span of time. It has been utilized in orthopaedic research for studying changes associated with disease, fracture and aging [12-15]. Mean Raman spectra of control, cancerous and irradiated specimens in 1800-800 cm-1 range are shown in Figure 2. Major spectral features can be assigned to bone mineral and matrix components. The major inorganic constituent of bone are present in form of hydroxyapatite (Ca10[PO4]6[OH]2) crystals between the collagen fibers. They also contain carbonate and hydrogen phosphate groups (~5-8%). Raman bands around 958 and 1070 cm-1 correspond to primary vibrational modes of phosphate (v1PO43-) and carbonate (CO32-), respectively, the major mineral constituent of bone [25]. The origin of phosphate band at 958 cm-1 has been assigned to symmetrical stretching of υ1 band of PO43-. This band is influenced to a minor extent by environmental factors hence it is considered as the most appropriate band to evaluate phosphate level among the four vibrational modes of PO43− [25,26]. Intensity of both carbonate and phosphate bands were highest in cancerous specimens, suggesting higher level of mineralization with respect to control or irradiated specimens. In addition to this, blue-shift was also observed in PO43− band of the cancer spectrum with respect to control and irradiated spectrum. Earlier studies have shown that positioning of the phosphate band is influenced by concentrations of carbonate and monohydrogen phosphate (HPO42-) [27]. In the present study there is a possibility that in cancerous specimens, due to large number of dividing cells, might cause increase in HPO42- content by newly deposited mineral which in turn can influence the position of phosphate band. The organic constituent of the bone mainly consists of collagen in a dense fibrous structural arrangement. Major bands related to the matrix components include 1003 cm-1 (ring vibrational modes of amino acid phenylalanine), bands in amide III (collagen and protein structural changes), 1447 cm-1 (stretching modes of CH groups of lipids and proteins) and 1660 cm-1 (amide I, collagen and protein content) [28]. Variations in term of relative intensity were observed in all these bands. These differences were further explored by computing band intensity and ratios using curve-fitting methods and the findings are discussed below.
Curve fitting analysis of Raman spectra
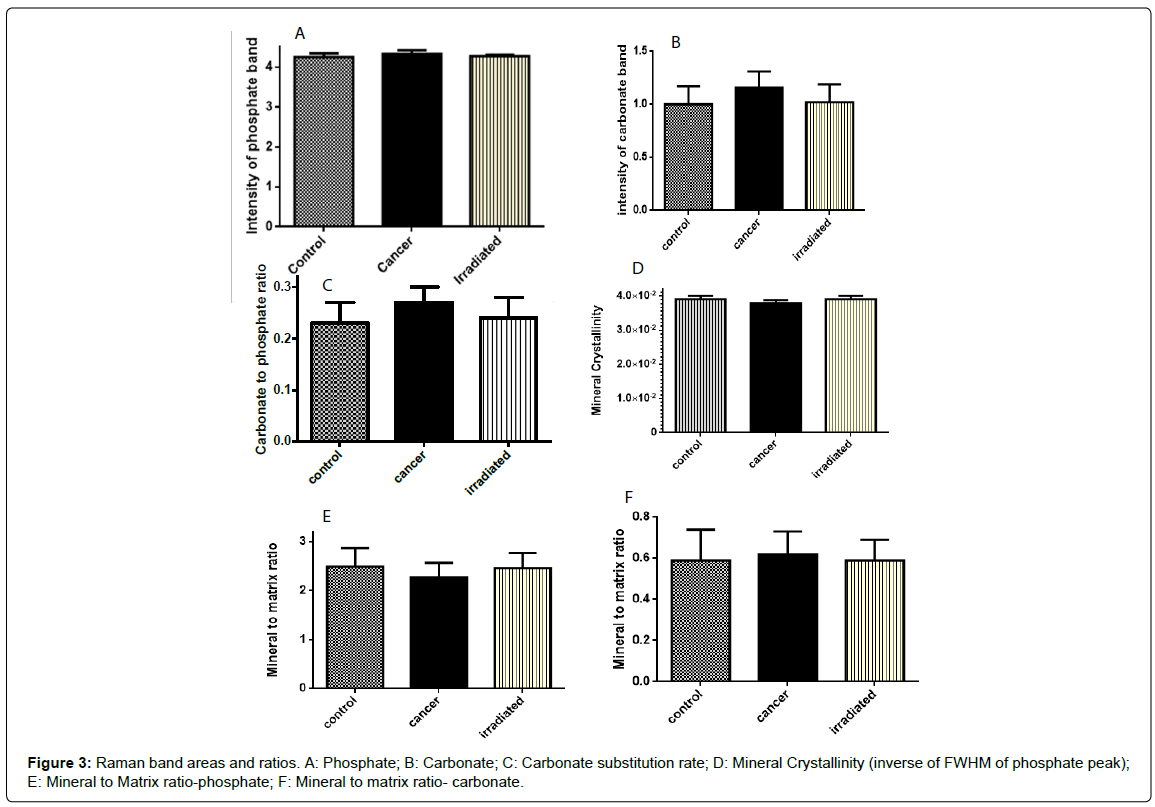
Variation among different parameters is shown in Figure 3A-3F and Table 2. Phosphate and carbonate contents in all three conditions i.e., control irradiated and cancers were measured by computing intensity associated with 958 and 1070 cm-1 band, respectively. Highest phosphate content was observed in cancerous specimens followed by irradiated and control samples (Table 1). This can be due to high proliferation and deposition of new minerals by cancerous cells. Unpaired Student’s t-test was used to evaluate significance of difference between the groups. As shown in Figure 3A and Table 2, differences between control and cancerous specimens and cancerous and irradiated specimens were significant (p=0.0084 and p=0.0225). However, no significant difference was observed between control and irradiated specimens. Similar trend was observed for carbonate bands (Figure 3B). Intensity of this band was highest in cancerous specimens and it was significantly different from control (p=0.0009) and irradiated (p=0.0064) samples, Table 2. No significant difference was observed between control and irradiated specimens, Table 2.
| S No | Raman bands | p-value (Unpaired Student’s t-test) |
|---|---|---|
| 1 | Phosphate contentControl and Cancer | p=0.0084 (VS) |
| 2 | Phosphate contentControl and Irradiated | p=0.2749 (NS) |
| 3 | Phosphate contentCancer and Irradiated | p=0.0225 (S) |
| 4 | Carbonate contentControl and Cancer | p=0.0009 (VS) |
| 5 | Carbonate contentControl and Irradiated | p=0.6588 (NS) |
| 6 | Carbonate contentCancer and Irradiated | p=0.0064 (VS) |
| 7 | Carbonate substitution Control and Cancer | p=0.0006 (VS) |
| 8 | Carbonate substitution Control and Irradiated | p=0.3247 (NS) |
| 9 | Carbonate substitution Cancer and Irradiated | p=0.0163 (S) |
| 10 | Mineral crystallinityControl and Cancer | p=0.0003 (VS) |
| 11 | Mineral crystallinityControl and Irradiated | p=1.00(NS) |
| 12 | Mineral crystallinityCancer and Irradiated | p=0.0008 (VS) |
| 16 | Mineral to matrix ratio-phosphateControl and Cancer | p=0.0309 (S) |
| 17 | Mineral to matrix ratio-phosphateControl and Irradiated | p=0.7379 (NS) |
| 18 | Mineral to matrix ratio-phosphateCancer and Irradiated | p=0.0457 (S) |
| 19 | Mineral to matrix ratio-CarbonateControl and Cancer | p=0.4464(NS) |
| 20 | Mineral to matrix ratio-CarbonateControl and Irradiated | p=0.9829(NS) |
| 21 | Mineral to matrix ratio-CarbonateCancer and Irradiated | p=0.3666(NS) |
Table 2: Statistical analysis (Unpaired Student’s t-test) of different biochemical parameters identified by Raman spectroscopy (VS: Very significant; S: Significant; NS: Not significant).
In case of bone specimens, absolute intensities of individual bands are influenced by variation in cross-section and losses due to elastic scattering therefore it is advised to study the band ratios [29]. As mentioned earlier, minerals in bone are present in hydroxyapatite crystal form. It has been shown that with time these crystals can undergo carbonate substitutions either by hydroxide (type A) or phosphate (type B) site. This substitution rate has been correlated with bone maturity and aging. Carbonate substitution rates can be computed by peak area ratio of carbonate and phosphate bands [14,19]. Therefore, in the next step phosphate to carbonate ratios were generated. As can be seen from Table 2, the carbonate to phosphate ratio was highest in cancerous specimens. Significant differences were observed when cancerous specimens were analyzed against control (p=0.0006) and irradiated (p=0.0163) samples. Similar to earlier observations no significant differences were observed between control and irradiated cases. These findings are in accordance with an earlier study on metastatic bone cancerous specimens [19]. In the cited study, authors have suggested that the increased carbonate content or substitution rates in tumorbearing bones can be attributed to acid-base imbalance in the bone microenvironment in the extracellular fluid [19].
Mineral crystallinity reflects the mineral crystal size and associated with stoichiometric perfection of apatite crystal [14]. Optimal distribution of crystals can serve as an additional parameter to assess the bone strength. Previous studies have shown a negative correlation between carbonate level and mineral crystal size and distribution [19,29]. With increase in the carbonate content there is replacement of stoichiometric phosphate locations in apatite crystal. It leads to imperfection and decrease in crystallinity. Corroborating earlier observations in the present study the lowest level of crystallinity was observed for cancerous specimens, Table 1 [19]. Very highly significant differences were observed between control-cancerous (p=0.0003) and cancer-irradiated samples (p=0.0008), Table 2. Similar to earlier observations no significant differences were observed between control and irradiated specimens, Table 2.
Mineral to matrix ratio, or level of collagen mineralization, is another major compositional property which is related to bone mechanical strength. It is measured by computing the amount of phosphate and carbonate with respect to collagen [19]. The changes in collagen secondary structure is manifested by deformations in the amide I band (1660 cm-1). Both Raman and IR spectroscopy methods have been used to study collagen cross-linking [14]. These methods work on the principle of measuring two major enzymatic cross links, namely non-reducible pyridinoline (PYD), and reducible dehydrodihydroxynorleucine (deH-DHLNL). As shown in Table 1, collagen mineralization with respect to phosphate content was highest in control specimens followed by irradiated and cancerous samples. This can be attributed to multiple alterations such as loss of lamellar structure or deformation in collagen structure due to cancer and irradiation. The differences between control-cancerous (p=0.0309) and irradiated-cancerous (p=0.0457) specimens was found to be statistically significant, Table 2. No significant difference was observed between control and irradiated specimens. Mineralization level with respect to carbonate content was also computed, however no significant differences were observed. Minor differences between control and irradiated bones are suggestive of reversal of cancer associated changes in the bone composition. These results need to be validated on larger sample size and other gold standard methods. Other aspects such as radiation doses and time lag and their influence on bone quality should also be analyzed.
Conclusion
Overall findings of the study further support applicability of Raman spectroscopic approaches for non-invasive disease diagnosis and treatment response monitoring. Minor differences between organic and inorganic component of control, cancerous and irradiated mandible bones can be identified. Major differences in both mineral and matrix components were observed between control-cancerous or cancerous-irradiated cases. Phosphate and carbonate content was highest in cancerous specimens. Similar to clinical observations, control and irradiated specimens show no differences in the mineral and organic matrix composition, suggesting cause of implant loss could be primarily associated with minor changes in the vasculature, osteocytes and surrounding tissues. Extrinsic factors such as timing of implant surgery after irradiation could also have influence. Future application of these techniques for routine clinical practice will help in online monitoring of bone quality and could help in reducing postoperative complications.
References
- Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, et al. (2015) Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 136: E359-386.
- Argiris A, Karamouzis MV, Raben D, Ferris RL (2008) Head and neck cancer. Lancet 371: 1695-1709.
- Brennan MT, Elting LS, Spijkervet FK (2010) Systematic reviews of oral complications from cancer therapies, Oral Care Study Group, MASCC/ISOO: methodology and quality of the literature. Support Care Cancer 18: 979-984.
- Alsaadi G, Quirynen M, Komárek A, van Steenberghe D (2008) Impact of local and systemic factors on the incidence of late oral implant loss. Clin Oral Implants Res 19: 670-676.
- Jereczek-Fossa BA, Orecchia R (2002) Radiotherapy-induced mandibular bone complications. Cancer Treat Rev 28: 65-74.
- Chen JA, Wang CC, Wong YK, Wang CP, et al. (2014) Osteoradionecrosis of mandible bone in patients with oral cancer-associated factors and treatment outcomes. Head Neck.
- O'Dell K, Sinha U (2011) Osteoradionecrosis. Oral Maxillofac Surg Clin North Am 23: 455-464.
- Tamura M (1997) Biomedical Optical Spectroscopy and Diagnostics. Measurement Science and Technology 8.
- Kendall C, Isabelle M, Bazant-Hegemark F, Hutchings J, Orr L, et al. (2009) Vibrational spectroscopy: a clinical tool for cancer diagnostics. Analyst 134: 1029-1045.
- Hanlon EB, Manoharan R, Koo TW, Shafer KE, Motz JT, et al. (2000) Prospects for in vivo Raman spectroscopy. Phys Med Biol 45: R1-59.
- Nijssen A, Koljenovic S, Bakker Schut TC, Caspers PJ, Puppels GJ (2009) Towards oncological application of Raman spectroscopy. J Biophotonics 2: 29-36.
- Peterson JR, Eboda ON, Brownley RC, Cilwa KE, Pratt LE, et al. (2015) Effects of aging on osteogenic response and heterotopic ossification following burn injury in mice. Stem Cells Dev 24: 205-213.
- McNerny EM, Gong B, Morris MD, Kohn DH (2015) Bone fracture toughness and strength correlate with collagen cross-link maturity in a dose-controlled lathyrism mouse model. J Bone Miner Res 30: 455-464.
- Morris MD, Mandair GS (2011) Raman assessment of bone quality. Clin Orthop Relat Res 469: 2160-2169.
- Balakrishnan B, Indap MM, Singh SP, Krishna CM, Chiplunkar SV (2014) Turbo methanol extract inhibits bone resorption through regulation of T cell function. Bone 58: 114-125.
- Paschalis EP, Shane E, Lyritis G, Skarantavos G, Mendelsohn R, et al. (2004) Bone fragility and collagen cross-links. J Bone Miner Res 19: 2000-2004.
- Gadeleta SJ, Boskey AL, Paschalis E, Carlson C, Menschik F, et al. (2000) A physical, chemical, and mechanical study of lumbar vertebrae from normal, ovariectomized, and nandrolone decanoate-treated cynomolgus monkeys (Macaca fascicularis). Bone 27: 541-550.
- Rieppo J, Hyttinen MM, Jurvelin JS, Helminen HJ (2004) Reference sample method reduces the error caused by variable cryosection thickness in Fourier transform infrared imaging. Appl Spectrosc 58: 137-140.
- Tchanque-Fossuo CN, Monson LA, Farberg AS, Donneys A, Zehtabzadeh AJ, et al. (2011) Dose-response effect of human equivalent radiation in the murine mandible: part I. A histomorphometric assessment. Plast Reconstr Surg 128: 114-121.
- Koga DH, Salvajoli JV, Alves FA (2008) Dental extractions and radiotherapy in head and neck oncology: review of the literature. Oral Dis 14: 40-44.
- Blanco AI, Chao C (2006) Management of radiation-induced head and neck injury. Cancer Treat Res 128: 23-41.
- Granström G (2005) Osseointegration in irradiated cancer patients: an analysis with respect to implant failures. J Oral Maxillofac Surg 63: 579-585.
- Ihde S, Kopp S, Gundlach K, Konstantinovic VS (2009) Effects of radiation therapy on craniofacial and dental implants: a review of the literature. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 107: 56-65.
- Awonusi A, Morris MD, Tecklenburg MM (2007) Carbonate assignment and calibration in the Raman spectrum of apatite. Calcif Tissue Int 81: 46-52.
- Carden A, Morris MD (2000) Application of vibrational spectroscopy to the study of mineralized tissues (review). J Biomed Opt 5: 259-268.
- Arnett T (2003) Regulation of bone cell function by acid-base balance. Proc Nutr Soc 62: 511-520.
- Zanyar M, Shazza R, Rehman IU (2007) Raman Spectroscopy of Biological Tissues. Appl Spectrosc Rev 42: 493-541.
- Tchanque-Fossuo CN, Gong B, Poushanchi B, Donneys A, Sarhaddi D, et al. (2013) Raman spectroscopy demonstrates Amifostine induced preservation of bone mineralization patterns in the irradiated murine mandible. Bone 52: 712-717.
- Bi X, Sterling JA, Merkel AR, Perrien DS, Nyman JS, et al. (2013) Prostate cancer metastases alter bone mineral and matrix composition independent of effects on bone architecture in mice-A quantitative study using microCT and Raman spectroscopy. Bone 56: 454-460.
Relevant Topics
Recommended Journals
Article Tools
Article Usage
- Total views: 14734
- [From(publication date):
December-2015 - Apr 04, 2025] - Breakdown by view type
- HTML page views : 10118
- PDF downloads : 4616