Radiological - Pathological Correlation in a Late Diagnosis of Oochronosis
Received: 04-Mar-2024 / Manuscript No. roa-24-129781 / Editor assigned: 06-Mar-2024 / PreQC No. roa-24-129781 / Reviewed: 20-Mar-2024 / QC No. roa-24-129781 / Revised: 25-Mar-2024 / Manuscript No. roa-24-129781 / Published Date: 29-Apr-2024
Abstract
Alkaptonuria is a rare autosomal recessive multisystem disorder of phenylalanine/tyrosine metabolism which occurs due to lack of homogentisic acid oxidase, which causes homogentisic acid deposition in the tissues. Alkaptonuria is characterized by a triad of homogentisic aciduria, oochronosis, and precious degenerative arthropathy. Oochronosis is blue-blackish discoloration of skin and cartilage involving multiple sites like ear pinna, cheeks, palms, soles, etc as well as urine that turns black upon standing.
Keywords
CT- computed tomogram; HPE - Histopathological examination; HGA - Homogentisic acid
Case Report
This is a case report of oochronosis secondary to alkaptonuria. A female patient in her 60’s patient who had chief complaints of acute severe chest pain radiating to left arm, chronic back pain, and bilateral hip joint pain for the last 6 years.
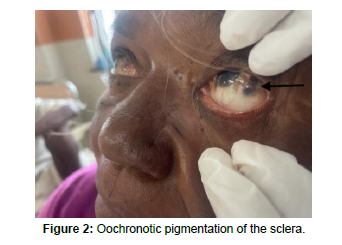
On physical examination, the Patient had bluish-blackish subcutaneous discoloration involving multiple sites like bilateral palms, soles, and cheeks. Patches of bluish-blackish ocular pigmentation were also noted in bilateral sclera (Figure 1 and Figure 2).
The patient also had lumbar spinal stiffness and restricted range of motion of the knee joint (Figure 3).
Extensive disc calcification involving the whole spine, vacuum discal phenomenon, and osteophytic bridges were demonstrated on standard radiograph and computer tomography scans.
Acute myocardial infarction was diagnosed on further work up with 2D Echo cardiography and Echocardiography.
She was born of a 3rd-degree consanguineous marriage and was 4th in birth order. There was no history of any chronic drug intake, exogeneous use of phenol products, or any type of addiction. Clinical, radiograph, and histopathological criteria were used to make the diagnosis of oochronosis.
Imaging findings
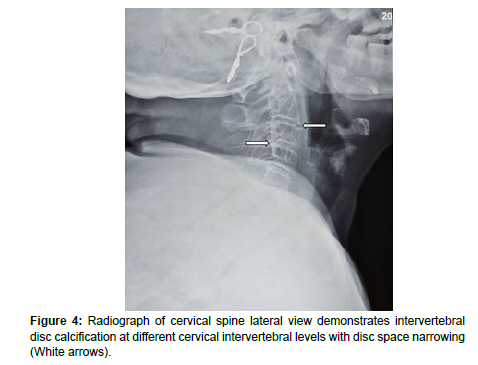
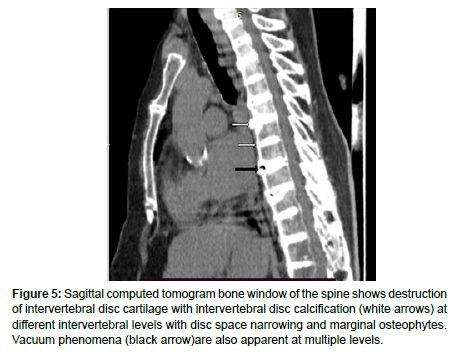
Radiograph images and CT scans images of the spine revealed widespread reduction of the intervertebral disc spaces (Figure 4 and Figure 5) with the presence of discal calcification and vacuum phenomenon (Figure 5) at multiple levels. Marginal osteophytes were also noted involving multiple levels.
Figure 5: Sagittal computed tomogram bone window of the spine shows destruction of intervertebral disc cartilage with intervertebral disc calcification (white arrows) at different intervertebral levels with disc space narrowing and marginal osteophytes. Vacuum phenomena (black arrow)are also apparent at multiple levels.
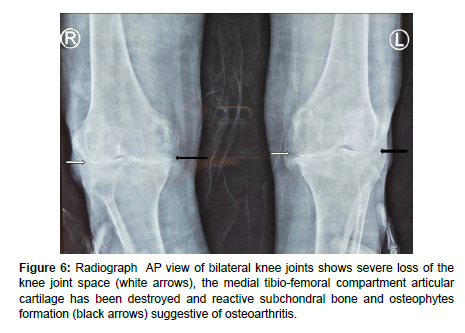
Radiographs of knees showed severe loss of the knee joint space, as well as reactive subchondral bone and osteophytes formation (Figure 6) which is characteristic of osteoarthritis.
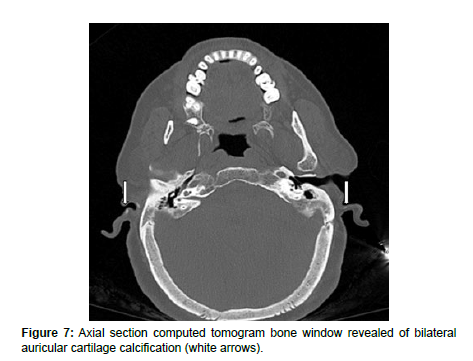
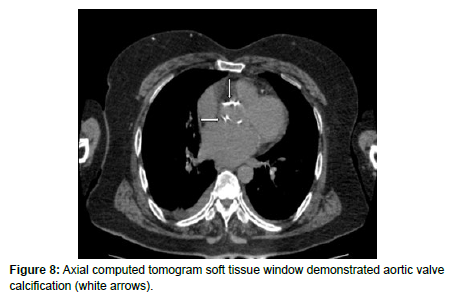
In addition, there was calcification of bilateral auricular cartilage (Figure 7) and aortic valve calcification (Figure 8) supporting the diagnosis of oochronosis.
Pathological finding
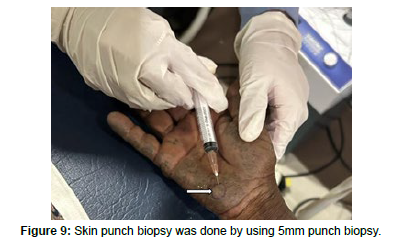
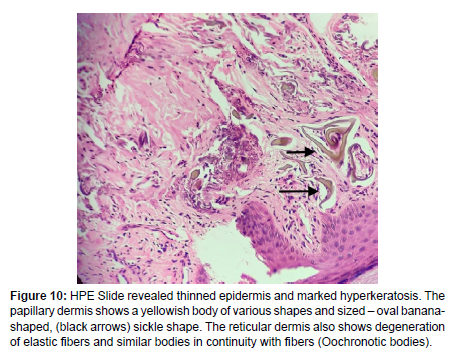
Skin punch biopsy from the palmer surface of the right hand (Figure 9) was done and the slide revealed a thinned epidermis and marked hyperkeratosis. The papillary dermis shows a yellowish body of various shapes and sized – oval banana-shaped, sickle shape (Figure 10). The reticular dermis also shows degeneration of elastic fibers and similar bodies in continuity with fibers (Oochronotic bodies).
Figure 10: HPE Slide revealed thinned epidermis and marked hyperkeratosis. The papillary dermis shows a yellowish body of various shapes and sized – oval bananashaped, (black arrows) sickle shape. The reticular dermis also shows degeneration of elastic fibers and similar bodies in continuity with fibers (Oochronotic bodies).
Urine analysis and biochemical examination
On urine analysis, the patient’s urine demonstrated dark pigmentation (Figure 11) on long standing (which occur due to the oxidation and polymerization of homogentisic acid).
When urine was heated with Benedict reagent a dark supernatant and a yellow precipitate of cuprous oxide was formed which suggested the presence of HGA.
When ammoniacal silver nitrate was added to urine the urine immediately produced a black precipitate indicating the presence of HGA.
These are the basic qualitative tests that supported the clinical suspicion of alkaptonuria.
Semi quantitative analysis of urine by gas chromatography / mass spectrometry performed in a reference laboratory in India revealed elevated levels of HGA which helped to confirm the diagnosis of alkaptonuria.
Discussion
Alkaptonuria is an autosomal-recessive disorder that was first described by Garrod in 1902. A deficient enzyme was identified by La Du et al. in 1958. Pollak et al. mapped the alkaptonuria gene to the chromosome 3q2 [1].
Alkaptonuria occurs due to a deficiency of homogentisic acid oxidase in the kidney and liver, which leads to the accumulation of homogentisic acid [2,3], which is a metabolite of phenylalanine, and tyrosine metabolism [1,4]. As homogentisic acid accumulates both intracellularly and extracellularly, it is oxidized to benzoquinone acetate, which further polymerizes to form a melanin-like polymer, that results in the deposition of polymer, a dark yellow pigment or ‘ochre’ occurs in the cartilage and other connective tissue [1].
The urine of the alkaptonuric patients darkens on standing, due to oxidation and polymerization of HGA to a melanin-like product [2-5]. In patients of alkaptonuria, fresh urine does not have an abnormal color and may not darken for many hours if it remains at an acid pH2 [6]. As a result, in alkaptonuric patients, this finding may be overlooked. During childhood, alkaptonuria may be asymptomatic until the third or fourth decade [7].
During adulthood, they may present with blackish pigmentation of connective tissues namely the sclera and cartilage of the ears, palms soles, etc [2,6]. Skin pigmentation becomes more obvious in 4th decade. The discoloration tends to be most pronounced on sun-exposed sites, cartilage of the ears and nose, and areas of high eccrine sweat gland density, such as axillae, palms soles, and genitalia [1].
Alkaptonuric patients may develop complications due to multiorgan involvement associated with rupture of tendons and ligaments, prostatitis, renal stones, generalized arteriosclerosis, and calcification in the heart valves leading to myocardial infarction [2, 5]. Although alkaptonuria causes morbidity in these patients, they have a normal life span.
Ochronotic arthropathy is the most troublesome feature and appears around the 4th decade. There is involvement of weight-bearing joints like the spine and knees as well as shoulders, with narrowing of joint spaces and calcifications. Arthritis is the only disabling effect of this condition and occurs in almost all patients as age advances [1,7,8].
In the radiology setting, ochronosis can present with various imaging findings depending on the organ systems involved. In the musculoskeletal system, ochronosis can cause severe osteoporosis, multilevel intervertebral disc calcification, disc space narrowing, syndesmophyte formation, symmetrical or asymmetrical joint space narrowing, subchondral sclerosis, ossification and calcification of tendons and ligaments, leading to joint stiffness and pain. These changes may be visible on plain radiographs as well as on MRI or CT.
The diagnosis of alkaptonuria is confirmed by the identification and quantification of homogentisic acid in urine. The oxidation property of HGA reduces the copper reagent in Benedict solution yielding a yellow-orange precipitate with a dark supernatant [5]. The addition of silver nitrate and ammonium hydroxide to urine also produces a black color [9]. Quantification of homogentisic acid in urine can be done using gas-liquid chromatography [2,5]. which reveals high urinary excretion of HGA in patients of alkaptonuria. Screening for mutations is done after extracting the genomic DNA from whole blood and subjecting it to PCR [1,2,10].
At present there is no effective therapy available for the treatment of alkaptonuria. Treatment of alkaptonuria is based on restricting the intake of tyrosine and phenylalanine-containing protein foods items like milk, meat, poultry, egg, cheese, and nuts. Diet may prevent further progression of arthropathy. Eva et al. (2003) report that the improvement in clinical symptoms and reversal of radiological evidence of joint involvement with these treatment strategies are possible [11]. Vitamin C (ascorbic acid), an antioxidant given in a dose of 500 mg twice daily, inhibits the polymerization of homogentisic acid and can reduce tissue damage, but its efficacy has not been proven [1,7]. Regular long-term follow-up is needed to monitor growth and complications
Conclusion
Alkaptonuria is a autosomal recessive multisystem disorder that causes degenerative arthropathy that leads to reduction of functional ability. The use of molecular analysis and genetic research is very useful.
References
- Tharini G, Ravindran V, Hema N, Prabhavathy D, Parveen B (2011) Alkaptonuria. Indian J Dermatol 56: 194-196.
- Phornphutkul C, Introne WJ, Perry MB, Bernardini I, Murphey MD, et al. (2002) Natural history of alkaptonuria. N Engl J Med 347: 2111-2121.
- Garrod AE (1902) The incidence of alkaptonuria: A study of chemical individuality. Lancet 2: 1616-1620.
- Dogra A, Bajwa GS, Bajwa N, Khurana S (2001) Alkaptonuria. Indian J Dermatol Venereol Leprol 67: 271-272.
- La Du BN (2001) Alkaptonuria. In: Scriver CR, Beaudet AL, Sly WS, et al. The Metabolic and Molecular Bases of Inherited Disease. New York: Mcgraw-Hill 2109- 2123.
- Rezvani I (2007) Defects in Metabolism of Amino Acids. In: Kliegman RM, Behrman RE, Jenson HB, Stanton BF. Nelson Text Book of Paediatrics. USA: Elsvier, Saunders Co. 529-566.
- Verma SB (2005) Early detection of alkaptonuria. Indian J Dermatol Venereol Leprol 71: 189-191.
- Mahajan VK, Sharma NL, Yadav RS (2004) Precocious degenerative arthropathy and bluish patches on ears: Ochronosis and alkaptonuria. Indian J Dermatol 49: 149-153.
- Strasinger SK, Di Lorenzo MS (2001) Urine screening for metabolic disorders. In: Strasinger SK, Di Lorenzo MS. Urine analysis and body fluids. USA 135-148.
- Hill A, Hoag GN, Zaleski WA (1972) The investigation of aromatic acids in phenylketonuria, alkaptonuria and tyrosinosis using gas-liquid chromatography. Clin Chim Acta 37: 455-462.
- Eva M, György K, Udo FHE, Ron AW (2003) Reversal of clinical symptoms and radiographic abnormalities with protein restriction and ascorbic acid in alkaptonuria. Annals of Clinical Biochemistry 40: 108-111.
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Citation: Simmi K, Mirza G, Chitra S, Rahul S, Ankita C, et al. (2024) Radiological- Pathological Correlation in a Late Diagnosis of Oochronosis. OMICS J Radiol13: 545.
Copyright: © 2024 Simmi K, et al. This is an open-access article distributed underthe terms of the Creative Commons Attribution License, which permits unrestricteduse, distribution, and reproduction in any medium, provided the original author andsource are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Open Access Journals
Article Usage
- Total views: 1213
- [From(publication date): 0-2024 - Nov 15, 2025]
- Breakdown by view type
- HTML page views: 915
- PDF downloads: 298