Review Article Open Access
RAC1b: A New Player in the Scenario of Thyroid Tumorigenesis?
Ana Luisa Silva1*, Márcia Faria1, Liliana Capinha1 and Maria João Bugalho1,2,3
1Research Unit of Molecular Pathobiology, Portugal
2Endocrinology service, Portuguese Oncology Institute of Lisbon Francisco Gentil E.P.E , Lisbon, Portugal
3University Clinic of Endocrinology, NOVA Medical School / Faculty of Medical Sciences, New University of Lisbon, Portugal
- *Corresponding Author:
- Ana Luísa Silva
Molecular Pathobiology Research Unit
Oncology Portuguese Institute of Lisbon Francisco Gentil E.P.E
Rua Professor Lima Basto
1099-023 Lisbon, Portugal
Tel: 35121 7229818
Fax: 351217229895
E-mail: silva.r.analuisa@gmail.com
Received: Nov 27, 2015; Accepted: Mar 10, 2016; Published: Mar 15, 2016
Citation: Silva AL, Faria M, Capinha L, Bugalho MJ (2016) RAC1b: A New Player in the Scenario of Thyroid Tumorigenesis. Adv Mol Diag 1:103.
Copyright: © 2016 Silva AL, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction H2O in any medium, provided the original author and source are credited.
Visit for more related articles at Advances in Molecular Diagnostics
Abstract
Tumor-associated RAC1b overexpression has been recently highlighted as one promising target for therapeutic intervention in pancreatic, breast, colon and lung cancer. Recent data documenting RAC1b overexpression in a subset of papillary thyroid carcinomas, carrying the activating mutation BRAFV600E and associated with an unfavorable outcome, support the inclusion of thyroid cancer to the previous list thus opening new therapeutic avenues to thyroid cancer patients.
Herein, we will focus on the potential of RAC1b, a hyperactive variant of the small GTPase RAC1, as a molecular player in the development of thyroid malignancies.
Keywords
Thyroid cancer; Rac1b; Rac1 splice variant; Clinical outcome; Molecular marker
In the last years, advances in cancer research led to the identification of a group of underlying mechanisms common to all cancers - hallmarks of cancer [1,2]. Nevertheless, there are significant differences between different types of cancer in terms of biological and molecular behavior. Moreover, phenotypic and functional heterogeneity can arise among cancer cells within the same tumor [3]. During many years, the clinical behavior of human cancer has been predicted based on histological features. However, different biological evolution and clinical responses were observed for the same histological type of tumors [4]. The pursuit for new ways to characterize each tumor as a particular entity, tailoring case management to individual risk level, has been persistent. One widely used approach is related to genetic alterations linked to several types of cancer. Genetic analysis has been performed in order to study how mutations identified in cancer-critical genes can affect crucial pathways, and consequently modify tumor cell properties and behavior.
Thyroid Malignancies
Thyroid cancer is the most common endocrine malignancy, accounting for 90% of all endocrine malignancies [5]. Thyroid carcinomas comprise two groups based on cell type from which they develop: thyroid cancers arising from follicular cells (thyroid hormone-producing) represent approximately 95% of all tumors; carcinomas arising from “C” cells (calcitonin-producing) are known as medullary thyroid carcinomas (MTCs) and represent 3-5% of cases [6,7]. Papillary thyroid carcinoma (PTC) is the most prevalent form from the follicular cell-derived subgroup and accounts for 75-80% of cases, followed by the follicular thyroid carcinoma (FTC), which represents approximately 10-15% of all thyroid cancers [7,8]. PTCs and FTCs are collectively designated well differentiated thyroid carcinomas (DTCs). A few cases show a poorly differentiated (PDTC) or undifferentiated (ATC) phenotype [7,8].
The standard of care for thyroid carcinoma is surgery. In the case of DTCs, therapy include thyroidectomy, TSH suppressive therapy and eventually radioiodine treatment for selected patients accordingly to individual risk level. Around 10% of patients with advanced forms of cancer are refractory to radioiodine and the survival at 10 years is of 10% comparative to 60% for those responsive to radioiodine [9].
The identification of molecular markers with a reliable prognostic value may greatly improve the management of patients with DTCs. At present, stratification risk of these patients mainly relies on clinical and histological criteria proved to be insufficient to tailor case management to individual risk levels [10].
Activating alterations in the canonical Ras/Raf/MEK/ERK pathway (mitogen-activated protein kinase-MAPK pathway) are considered to have key role in thyroid carcinogenesis [8,11]. In fact, the BRAFV600E activating mutation is the most frequent genetic alteration in PTCs, while oncogenic alterations in either HRAS, KRAS or NRAS are most prevalent in FTCs (mutations involving codon 61 in NRAS and HRAS are the most common) [8]. Although a single oncogenic alteration in MAPK pathway might be sufficient to drive thyroid cell neoplastic transformation, further supportive molecular events are likely to be associated with thyroid malignancy progression leading to more aggressive phenotypes and poorer clinical outcomes.
RAC1b is a hyperactive variant of the small GTPase RAC1 a member of the RAS superfamily of small GTP-binding proteins [12]. RAC1b overexpression has been reported in association with pancreatic, breast, colon and lung carcinogenesis [13-16]. Studies from our group [17] disclosed the possible role of RAC1b as a marker of prognosis in PTC patients.
Herein, we will review RAC1b biological and tumorigenic features focusing on its potential role in thyroid tumorigenesis.
The RAS Superfamily of Small GTPases
The RAS (for rat sarcoma virus) superfamily of small guanosine triphosphatases (GTPases) comprises over 150 members (Ras super family) and was first discovered as the transforming genes of ratderived Harvey and Kirsten murine sarcoma retrovirus in the early 1980s [18]. These members are activated by different extracellular stimuli and regulate intracellular signaling, performing a general switch function that is based on active GTP (Guanosine triphosphate) - bound state, and an inactive GDP (Guanosine diphosphate) -bound state [19]. Mutations in the cellular RAS genes, which may alter the structure of RAS protein or even increase this gene expression levels, were thereafter demonstrated in human tumor cell lines, suggesting a role in growth and development, since their alterations lead to uncontrolled growth [20].
This superfamily is divided into five major classes, according to their sequence and function similarities: Ras, Arf, Rab, Ran and Rho [21]. The Rho family, in particular, is known to play an important role in signaling networks that regulate cell cycle progression, actin cytoskeletal organization and cell polarity: Cdc42 regulates the formation of filopodia, RhoA promotes the formation of stress fibers and RAC1 is involved in lamellipodia formation [22-24].
The Small GTPase RAC1
RAC1 protein is encoded by RAC1 gene and can exist in two different conformational states-an inactive GDP-bound form and an active GTP-bound form [25-28]. The interconversion between the two states occurs through a cycle of guanine exchange and GTP hydrolysis, wherein GTP binding induces a conformational change that involves two important regulatory regions, termed Switch I and Switch II. Consequently, the switch regions provide a surface that, in the active state, enables their interaction with downstream effectors, allowing these GTPases to function as molecular switches [21]. This cycling process is tightly regulated by several groups of proteins: Rho-GEFs (Guanine Exchange Factors) which promote exchange of GDP for GTP; Rho-GAPs (GTPase Activating Protein) that enhance the hydrolysis of bound GTP to GDP and inorganic phosphate, regulating the inactivation of the GTPases; GDIs (GDP Dissociation Inhibitors) are also regulatory proteins that sequester Rho GTPases in the cytoplasm in an inactive GDP-bound state, preventing exchange of GDP to GTP [29].
RAC1 has the ability to interact with specific effectors, inducing activation of numerous signaling cascades that culminate in different physiological outcomes, namely cytoskeletal dynamics alteration, progression through the cell cycle and cell proliferation, apoptosis, migration and cell - cell adhesion, membrane trafficking and superoxide production [26,28,30]. There are several downstream effectors and signaling proteins influenced by activated RAC1, namely WAVE (WASP-family verprolin-homologous protein) complex and PAK (p21 activating kinase). For example, GTP-bound RAC1 binds to PAK1, stimulating its protein kinase activity, leading to the modulation of several biological activities such as actin reorganization [28,30,31].
The action of activated RAC1 also includes the stimulation of transcription factors such as the activation of the Jun NH2-terminal kinase (JNK) cascade or the transcription factor NF-κB (Nuclear factor kappa-light-chain-gene-enhancer of activated B cells). The RAC1- related activation of the NF-κB pathway involves the production of reactive oxygen species (ROS), and initiates an anti-apoptotic transcriptional response, leading to an increased cyclin D1 expression and consequently promoting the cell cycle progression [32,33].
The Hyper-Activatable RAC1 Splicing Variant, RAC1b
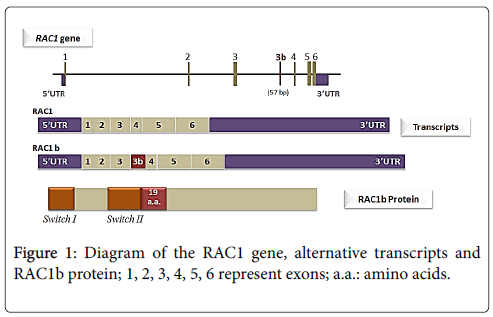
Back to 1999, Jordan and collaborators performed a RT - PCR (Reverse transcription polymerase chain reaction) based assessment of RAC1 expression in colorectal samples (tumors and normal mucosa) that lead to the identification of a new RAC1 splicing variant, termed RAC1b [25]. This variant was also found to be expressed in breast carcinomas [14]. The RAC1b isoform results from an alternative splicing event that leads to the inclusion of an additional exon (exon 3b). This additional exon is inserted between exons 3 and 4 (Figure 1) of RAC1 and contains 57 additional nucleotides that result in an inframe insertion of 19 amino acid residues between codons 75 and 76, in the vicinity of an important regulatory region of the GTPase, the switch II domain [25]. In colorectal cancer cells, it was shown that RAC1b alternative splicing occurs through the regulation of two antagonistic splicing factors (SR proteins), ASF/SF2 and SRp20 [34]. In other tissues, however, the signals involved may be different. In mouse mammary epithelial cells, for instance, the factor hnRNP A1 was shown to be involved in actively repressing the formation of the RAC1b during Rac1 splicing [35].
RAC1b is considered a highly activated variant of RAC1: despite the lower levels of expression compared to RAC1, RAC1b exists predominantly in the active GTP-bound state. This is essentially due to RAC1b disability to interact with Rho-GDI, which keeps this GTPase constitutively membrane-bound, a location that favors the interaction with activators, and consequently promotes the active GTP-bound state [24]. Moreover, RAC1b shows impaired intrinsic activity and increased GDP to GTP exchange rates, although this variant can still be down regulated by activated GAPs and it is influenced by GEFs action [14,24,36,37]. Also, RAC1b's additional amino acids seem to confer to this variant a selective downstream signaling, since several pathways activated by RAC1, are not activated by RAC1b [24].
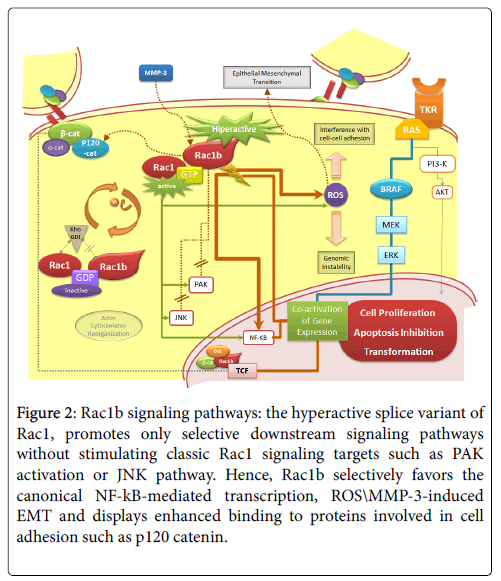
In fact, compared to RAC1, RAC1b shows a selective downstream signaling (Figure 2). Unlike RAC1, GTP-bound RAC1b ability to induce actin cytoskeleton reorganization is impaired-RAC1b is unable to induce lamellipodia formation. RAC1b is also unable to interact with two other well-established RAC1 signaling pathways: RAC1b is incapable to activate PAK1 effector and stimulate the JNK cascade [24,36,37]. On the other hand, RAC1b was shown to bind more effectively than RAC1 to p120 catenin, SmgGDS, and RACK1, proteins that can promote loss of epithelial cell structure and increased cell proliferation [38]. Also, RAC1b favors specific pathways conducting to the production of reactive oxygen species (ROS) and NF-κB activation [24,33,39,40].
Figure 2: Rac1b signaling pathways: the hyperactive splice variant of Rac1, promotes only selective downstream signaling pathways without stimulating classic Rac1 signaling targets such as PAK activation or JNK pathway. Hence, Rac1b selectively favors the canonical NF-kB-mediated transcription, ROS\MMP-3-induced EMT and displays enhanced binding to proteins involved in cell adhesion such as p120 catenin.
Increased cellular ROS cause oxidative damage and induce genomic instability, stimulating carcinogenesis. At least in the colorectal biological system, RAC1b retains the capacity to stimulate the NF-κB pathway, although its action is limited to the classical RelA-dependent NF-κB pathway, contrary to RAC1 that also stimulates the noncanonical pathway (RelB-dependent). The RAC1b stimulation of NF- κB classical pathway increases the cell cycle progression and cell survival, reducing the apoptotic rates [33,39]. Consistently, Rac1b has been shown to sustain tumor cell survival in colorectal cancer [13]. RAC1b was also shown to have the ability to mediate epithelial - mesenchymal transition (EMT) in breast [40]. This was shown to occur, via a mechanism dependent on both Rac1b-induced ROS and MMP3 (matrix metalloproteinase-3), in mouse mammary epithelial cells [40,41]. Uncontrolled proliferation and EMT are ultimately involved in the development of tumor formation, invasion, and metastasis [16] In addition, a role for nuclear Rac1b in the Wnt pathway was also pointed out: nuclear expression of Rac1b was shown to be able to recruit Dishevelled and β-catenin to the promoters of Wnt target genes in the absence of Wnt3A stimulation, suggesting a role for Rac1b as a transcriptional co-activator in β-catenin/TCF-mediated transcription [42].
RAC1b over Expression and Malignant Progression
Alternative spliced Rac1b was first identified in breast, skin and epithelial tissue of the intestinal tract [14,25] and was found to be overexpressed in colorectal and lung cancer, as compared with the respective normal tissue [13,15,38,43].
Matos et al documented, in a subset of colorectal tumors, that cell survival was dependent on functional cooperation between the overexpression of Rac1b and mutant BRAFV600E [13]. Also, RAC1b overexpression was reported to have a key role in the malignant progression of breast and lung tumors [15,44,45]. Moreover, RAC1b overexpression was shown to constitute a marker of poor prognosis in KRAS/BRAFWT colorectal cancer patients treated with first - line FOLFOX/XELOX chemotherapy [46].
The Rac1b tumorigenic proprieties, its cooperation with BRAFV600E in the maintenance of colorectal tumor cell survival and the high prevalence of BRAFV600E oncogenic mutation amongst PTCs, led us to investigate whether Rac1b expression had also a relevant role in thyroid malignancies. We have found that RAC1b is overexpressed in PTCs compared to normal thyroid tissue. Moreover, we have shown that RAC1b overexpression is significantly associated with BRAFV600E mutation and poor clinical outcome [17].
The potential role of Rac1b overexpression in thyroid tumorigenesis
The canonical MAPK oncogenic pathway is considered to have key role in thyroid tumorigenesis. In fact, activating mutations of several genes in the MAPK pathway have been identified in more than 70% of PTCs, the BRAF V600E mutation was found to be the most frequent genetic alteration, occurring in about 45% of cases [8]. The V600E mutation constitutively activates BRAF kinase, leading to prolonged stimulation of the MAPK pathway, which ultimately leads to uncontrolled cell proliferation and faulty apoptosis [47,48].
Despite several studies suggesting that BRAFV600E may condition the development of tumors with aggressive behavior, the prognostic value of this mutation in PTC patients remains incompletely established [49], thus justifying the search for additional molecular markers. In fact, without neglecting the role of the BRAF mutation in PTCs with poor prognosis, additional genetic alterations are likely to be associated with the progression of PTC to more aggressive phenotypes. Our recent findings point to an important role of RAC1b in PTC and provide first evidence for a potential interplay between BRAFV600E and Rac1b modulating thyroid cancer progression, similarly to what happens in colorectal cancer cells [17]. In fact, we have accessed Rac1b expression by RT-qPCR in a total of 61 PTC samples and correlated it with BRAFV600E mutational status and clinical outcome based on the analysis of patient longitudinal evolution. Rac1b overexpression was present in 46% of PTCs and was significantly associated with both V600E mutation (68% of Rac1b overexpressing PTCs were also BRAFV600E positive) and poor clinical outcome (up to 73% of PTCs subgroup representing the poorer outcomes overexpressed Rac1b) [17].
Besides MAPK pathway, NF-KB activation has been also reported to play an important role in thyroid malignancies [50-52]. While BRAFV600E has been reported to be responsible for both tumorigenesis initiation and progression, NF-KB activation has been associated with resistance to apoptosis and maintenance of the transformed phenotype [51,52]. Yet, the mechanism leading to NF-KB activation in thyroid tumorigenesis is still poorly defined [52]. Rac1b might contribute for this process. Due to its high activity and selective downstream signaling, RAC1b was shown to be a potent activator of the NF-KB pathway [39]. Moreover, Rac1b plays a role in other tumorassociated processes such as signaling pathways controlling cell adhesion, migration, and induction of epithelial-mesenchymal transition, which may also be involved in the development of thyroid malignant phenotype [37,40,41,43,52,53].
Conclusion
RAC1 and RAC1b have been implicated in several cellular processes associated with malignant transformation, namely cell survival, by stimulating cell cycle progression and by increasing responses for apoptosis evasion. RAC1b in particular, given its hyper-activatable properties and selective overexpression in cancerous tissue, has been recently highlighted as one promising therapeutic target.
For thyroid malignancies in particular, RAC1b overexpression in a subset of papillary thyroid carcinomas associated with unfavorable outcome suggests a role for RAC1b in the modulation of PTCs’ malignant progression, contributing to poorer clinical outcomes. Further studies are needed to validate the use of RAC1b as prognostic marker. In this context, the assessment of RAC1b overexpression by immunohistochemistry in paraffin-embedded tissues might be relevant for diagnosis and prognosis purposes and should be further explored since a RAC1b specific antibody is commercially available. Furthermore, the role of RAC1b might as well be explored in the context of other thyroid malignancies. Gaining mechanistic insights into how RAC1b overexpression specifically reprograms the thyroid neoplastic cells could be further explored to define a broader panel of molecular markers associated with disease prognosis or to characterize new pathways for therapeutic intervention.
References
- Hanahan D, Weinberg RA (2011) Hallmarks of cancer: the next generation. Cell 144: 646-674.
- Serpa J, Dias S (2011) Metabolic cues from the microenvironment act as a major selective factor for cancer progression and metastases formation. Cell Cycle Georget Tex 10:180-181.
- Meacham CE, Morrison SJ (2013) Tumour heterogeneity and cancer cell plasticity. Nature 501: 328-337.
- Liotta L, Petricoin E (2000) Molecular profiling of human cancer. Nat Rev Genet 1: 48-56.
- Siegel R, Naishadham D, Jemal A (2013) Cancer statistics, 2013. CA Cancer J Clin 63:11-30.
- Muro-Cacho CA, Ku NN (2000) Tumors of the thyroid gland: histologic and cytologic features: part 1. Cancer Control J Moffitt Cancer Cent 7: 276-287
- Nikiforov YE, Nikiforova MN (2011) Molecular genetics and diagnosis of thyroid cancer. Nat Rev Endocrinol 7:569-580.
- Bhaijee F, Nikiforov YE (2011) Molecular analysis of thyroid tumors. Endocr Pathol 22:126-133.
- Durante C, Haddy N, Baudin E (2006) Long-term outcome of 444 patients with distant metastases from papillary and follicular thyroid carcinoma: benefits and limits of radioiodine therapy. J Clin EndocrinolMetab 91: 2892-2899.
- Sipos JA, Mazzaferri EL (2010) Thyroid cancer epidemiology and prognostic variables. Clin Oncol R Coll Radiol GB 22: 395-404.
- Romitti M, Ceolin L, Siqueira DR (2013) Signaling pathways in follicular cell-derived thyroid carcinomas (review). Int J Oncol 42: 19-28.
- Boureux A, Vignal E, Faure S, Fort P (2007) Evolution of the Rho family of ras-like GTPases in eukaryotes. Mol Biol Evol 24: 203-216.
- Matos P, Oliveira C, Velho S (2008) B-Raf(V600E) cooperates with alternative spliced Rac1b to sustain colorectal cancer cell survival. Gastroenterology 135: 899-906.
- Schnelzer A, Prechtel D, Knaus U (2000) Rac1 in human breast cancer: overexpression, mutation analysis, and characterization of a new isoform, Rac1b. Oncogene 19:3013-3020.
- Zhou C, Licciulli S, Avila JL (2012) The Rac1 splice form Rac1b promotes K-ras-induced lung tumorigenesis. Oncogene.
- Mehner C, Miller E, Khauv D (2014) Tumor Cell-Derived MMP3 Orchestrates Rac1b and Tissue Alterations That Promote Pancreatic Adenocarcinoma. Mol Cancer Res 12: 1430-1439.
- Silva AL, Carmo F, Bugalho MJ (2013) RAC1b overexpression in papillary thyroid carcinoma: a role to unravel. Eur J EndocrinolEur Fed Endocr Soc 168: 795-804.
- Hankins WD, Scolnick EM (1981) Harvey and Kirsten sarcoma viruses promote the growth and differentiation of erythroid precursor cells in vitro. Cell 26: 91-97.
- Rojas AM, Fuentes G, Rausell A, Valencia A (2012) The Ras protein superfamily: evolutionary tree and role of conserved amino acids. J Cell Biol 196:189-201.
- Madaule P, Axel R, Myers AM (1987) Characterization of two members of the rho gene family from the yeast Saccharomyces cerevisiae. Proc Natl Acad Sci USA 84: 779-783.
- Wennerberg K, Rossman KL, Der CJ (2005) The Ras superfamily at a glance. J Cell Sci 118: 843-846.
- Heasman SJ, Ridley AJ (2008) Mammalian Rho GTPases: new insights into their functions from in vivo studies. Nat Rev Mol Cell Biol 9: 690-701.
- Fukuda T, Kiuchi K, Takahashi M (2002) Novel mechanism of regulation of Rac activity and lamellipodia formation by RET tyrosine kinase. J Biol Chem 277: 19114-19121.
- Matos P, Collard JG, Jordan P (2003) Tumor-related alternatively spliced Rac1b is not regulated by Rho-GDP dissociation inhibitors and exhibits selective downstream signaling. J Biol Chem 278:50442-50448.
- Jordan P, Brazao R, Boavida MG (1999) Cloning of a novel human Rac1b splice variant with increased expression in colorectal tumors. Oncogene 18:6835-6839.
- Matos P, Skaug J, Marques B (2000) Small GTPase Rac1: structure, localization, and expression of the human gene. Biochem Biophys Res Commun 277: 741-751.
- Wennerberg K, Der CJ (2004) Rho-family GTPases: it’s not only Rac and Rho (and I like it). J Cell Sci 117: 1301-1312.
- Jaffe AB, Hall A (2005) Rho GTPases: biochemistry and biology. Annu Rev Cell Dev Biol 21: 247-269.
- Symons M, Settleman J (2000) Rho family GTPases: more than simple switches. Trends Cell Biol 10: 415-419.
- Bosco EE, Mulloy JC, Zheng Y (2009) Rac1 GTPase: a “Rac” of all trades. Cell Mol Life Sci CMLS 66: 370-374.
- Nagase M, Fujita T (2013) Role of Rac1-mineralocorticoid-receptor signalling in renal and cardiac disease. Nat Rev Nephrol 9: 86-98.
- Hinz M, Krappmann D, Eichten A (1999) NF-kappaB function in growth control: regulation of cyclin D1 expression and G0/G1-to-S-phase transition. Mol Cell Biol 19: 2690-2698.
- Matos P, Jordan P (2006) Rac1, but not Rac1B, stimulates RelB-mediated gene transcription in colorectal cancer cells. J Biol Chem 281: 13724-13732.
- Gonçalves V, Matos P, Jordan P (2009) Antagonistic SR proteins regulate alternative splicing of tumor-related Rac1b downstream of the PI3-kinase and Wnt pathways. Hum Mol Genet 18: 3696-3707.
- Pelisch F, Khauv D, Risso G (2012) Involvement of hnRNP A1 in the matrix metalloprotease-3-dependent regulation of Rac1 pre-mRNA splicing. J Cell Biochem 113: 2319-2329.
- Fiegen D, Haeusler LC, Blumenstein L (2004) Alternative splicing of Rac1 generates Rac1b, a self-activating GTPase. J Biol Chem 279: 4743-4749.
- Singh A, Karnoub AE, Palmby TR (2004) Rac1b, a tumor associated, constitutively active Rac1 splice variant, promotes cellular transformation. Oncogene 23: 9369-9380.
- Orlichenko L, Geyer R, Yanagisawa M (2010) The 19-amino acid insertion in the tumor-associated splice isoform Rac1b confers specific binding to p120 catenin. J Biol Chem 285: 19153-19161.
- Matos P, Jordan P (2005) Expression of Rac1b stimulates NF-kappaB-mediated cell survival and G1/S progression. Exp Cell Res 305: 292-299.
- Radisky DC, Levy DD, Littlepage LE (2005) Rac1b and reactive oxygen species mediate MMP-3-induced EMT and genomic instability. Nature 436: 123-127.
- Stallings-Mann M, Radisky D (2007) Matrix Metalloproteinase-Induced Malignancy in Mammary Epithelial Cells. Cells Tissues Organs 185: 104-110.
- Bapat B (2011) Rac1b recruits Dishevelled and β-catenin to Wnt target gene promoters independent of Wnt3A stimulation
- Matos P, Jordan P (2008) Increased Rac1b expression sustains colorectal tumor cell survival. Mol Cancer Res MCR 6: 1178-1184.
- Nelson CM, Khauv D, Bissell MJ, Radisky DC (2008) Change in cell shape is required for matrix metalloproteinase-induced epithelial-mesenchymal transition of mammary epithelial cells. J Cell Biochem 105: 25-33.
- Stallings-Mann ML, Waldmann J, Zhang Y (2012) Matrix metalloproteinase induction of Rac1b, a key effector of lung cancer progression. Sci Transl Med 4: 142-195.
- Alonso-Espinaco V, Cuatrecasas M, Alonso V (2014) RAC1b overexpression correlates with poor prognosis in KRAS/BRAF WT metastatic colorectal cancer patients treated with first-line FOLFOX/XELOX chemotherapy. Eur J Cancer 50: 1973-1981.
- Kimura ET, Nikiforova MN, Zhu Z (2003) High prevalence of BRAF mutations in thyroid cancer: genetic evidence for constitutive activation of the RET/PTC-RAS-BRAF signaling pathway in papillary thyroid carcinoma. Cancer Res 63: 1454-1457.
- Wan PTC, Garnett MJ, Roe SM (2004) Mechanism of activation of the RAF-ERK signaling pathway by oncogenic mutations of B-RAF. Cell 116: 855-867.
- Puxeddu E, Moretti S (2007) Clinical prognosis in BRAF-mutated PTC. Arq Bras EndocrinolMetabol 51: 736-747.
- Pacifico F, Leonardi A (2010) Role of NF-kappaB in thyroid cancer. Mol Cell Endocrinol 321: 29-35.
- Bommarito A, Richiusa P, Carissimi E (2011) BRAFV600E mutation, TIMP-1 upregulation, and NF-κB activation: closing the loop on the papillary thyroid cancer trilogy. EndocrRelat Cancer 18: 669-685.
- Palona I, Namba H, Mitsutake N (2006) BRAFV600E promotes invasiveness of thyroid cancer cells through nuclear factor kappaB activation. Endocrinology 147: 5699-5707.
- Esufali S, Charames GS, Pethe VV (2007) Activation of tumor-specific splice variant Rac1b by dishevelled promotes canonical Wnt signaling and decreased adhesion of colorectal cancer cells. Cancer Res 67: 2469-2479.
Relevant Topics
Recommended Journals
Article Tools
Article Usage
- Total views: 11480
- [From(publication date):
April-2016 - Apr 01, 2025] - Breakdown by view type
- HTML page views : 10585
- PDF downloads : 895