Research Article Open Access
Quantitative Fit Analysis of Orthopedic Bone Plates: Methods, Criteria and Approach
Hazreen Harith, Javad Malekani, Beat Schmutz, Michael Schuetz and Prasad KDV Yarlagadda*Science and Engineering Faculty, Queensland University of Technology (QUT), Australia
- Corresponding Author:
- Prasad KDV Yarlagadda
Science and Engineering Faculty
Queensland University of Technology (QUT)
2 George Street, Brisbane, QLD 4001, Australia
Tel: +61 7 31385167
E-mail: y.prasad@qut.edu.au
Received date September 03, 2013; Accepted date October 25, 2013; Published date November 01, 2013
Citation: Harith H, Malekani J, Schmutz B, Schuetz M, Yarlagadda PKDV (2013) Quantitative Fit Analysis of Orthopedic Bone Plates: Methods, Criteria and Approach. J Biomim Biomater Tissue Eng 18:116. doi:10.4172/1662-100X.1000116
Copyright: © 2013 Harith H, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Biomimetics Biomaterials and Tissue Engineering
Abstract
Studies on quantitative fit analysis of precontoured fracture fixation plates emerged within the last few years and therefore, there is a wide research gap in this area. Quantitative fit assessment facilitates the measure of the gap between a fracture fixation plate and the underlying bone, and specifies the required plate fit criteria. For clinicallymeaningful fit assessment outcome, it is necessary to establish the appropriate criteria and parameter. The present paper studies this subject and recommends using multiple fit criteria and the maximum distance between the plate and underlying bone as fit parameter for clinically relevant outcome. We also propose the development of a software tool for automatic plate positioning and fit assessment for the purpose of implant design validation and optimization in an effort to provide better fitting implant that can assist proper fracture healing. The fundamental specifications of the software are discussed.
Keywords
Fracture fixation plate; 3D bone model; Fit assessment; Anatomical fit; Fit criteria; Automation
Introduction
Since the introduction of orthopedic bone plates in 1896, they have evolved substantially in design, implementation technique and material. Compression Plates (CP), Dynamic Compression Plate (DCP), Limited Contact Dynamic Compression Plate (LC-DCP), Point Contact Fixator (PC-Fix), Less Invasive Stabilization System (LISS) plate, Locking Compression Plate (LCP) and Precontoured LCP have been introduced so far. Plates have changed from a solid fixator to a bioactive healing facilitator [1]. However, they are not optimal yet. From structural point of view, while precontoured LCPs are sitespecific, they cannot fit to the underlying bone in all surgical cases [2-7]. This is mainly due to variation in bone morphology, which is contributed by various factors including gender, age, race and nutrition. Nevertheless, providing multiple plate shapes for any anatomical site results in an immense increase of the already large plate inventory and leads to significant logistical problems for hospitals and manufacturers. Therefore, in most cases, plates are designed based on the average bone shapes for the target population as it is impossible to have one shape that fits the entire population.
Clinically, an anatomically well-fitting plate can greatly facilitate the process of reduction in terms of axial and rotational alignments of the main fragments, and minimizes the soft-tissue impingement/ irritations in areas where the soft tissue covering is minimal. The locked internal fixator technique achieves stable fracture fixation without the need to compress the plate on the bone. Therefore, biomechanically, a perfect fit between plate and bone is not required. However, basic mechanics of fixed-angle locking plates dictate that load transfer is most optimal when the plate screw interface is closest to the near cortex [8]. The greater the distance between plate and bone, the higher the bending moment on the screw and the less efficient the construct becomes for transfer of loads [2]. On the one hand, a large gap between plate and bone increases stress at the fracture site and inhibits healing process. On the other hand, the absence of gap between plate and bone not only increases the stress at the fracture site [9], but also may lead to bone re-fracture especially for osteoporotic bone due to insufficient blood supply during fracture healing. Therefore, a balance is required in managing the amount of gap between the plate and underlying bone to promote optimal fracture healing. For this purpose, an anatomical plate that fits nicely to the underlying bone will help to achieve this balance.
Since both bones and plates have irregular shapes, each plate shape has varying requirements in terms of what constitutes a clinically acceptable fit. Therefore, it is not possible to specify a single value that defines an optimal gap [3]. Quantitative fit assessment will help to establish appropriate measurement for the gap between plate and the bone, and define the plate fitting criteria. The present paper studies the current quantitative fit assessment methods, the fit criteria for a distal tibial plate as a case study and recommends the best approach.
Fit Assessment Methods
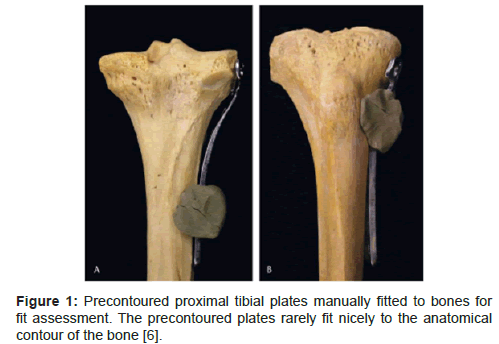
Studies on quantitative fit analysis of precontoured plates emerged within the last few years. Prior to that, studies on orthopedic implants focused on the structural evolution of implants [1]. Fit analysis of plate implant is commonly performed by visually inspecting a plate that is fitted to a set of cadaver or prototype bones (Figure 1). One source of cadaver bones is from museum collection, which includes older bones. Therefore, using them for fit assessment may disregard the morphological changes through generations. Furthermore, visual inspection of plate fit results in qualitative fit outcome. To address these limitations, more recent studies have focused on computer methods (hereafter referred as virtual methods) for quantitative fit assessment in an effort to establish a standardized and objective fit assessment method and outcome.
The first report on quantitative fit analysis assessed the fit for proximal tibia plates [5]. The quantification of plate fit was performed by first digitizing and mapping the plate and bone using a special MicroScribe digitizer, and then capturing the points manually to create 3D images of the bone and plate. Nevertheless, the authors used cadaver bones for fit assessment. Therefore, whether or not the outcome is applicable for the current populations is uncertain. In term of method, the main limitations of this approach are: i) usage of cadaver bones from museum collections, and ii) usage of physical bone models as it does not permit simultaneous evaluation of multiple shapes at the same anatomical site.
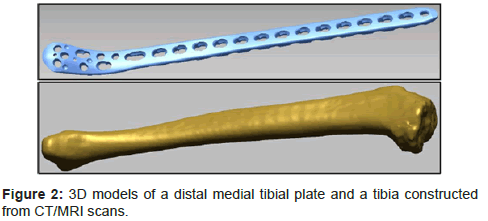
Advancement in medical imaging technology allows wider access to bone data from current populations. This advantage drives the development of the semi-automated and automated methods. Both are entirely virtual process and utilize 3D bone models reconstructed from CT/MRI scans and 3D plate models for fit assessment (Figure 2).
The general process of the semi-automated method involves importing the models into 3D modelling software, with which the plate positioning and fit assessment are then performed using the tools available within the software [2,3,10]. Schmutz et al. [2] first proposed this method to evaluate the fitting of a distal medial tibial plate on 21 tibiae, and used the same approach successfully on tibial nails [11]. The same approach was also adopted to assess the fit of plates from different anatomical sites [10,12]. Although this method eliminates the limitations of using cadaver or prototype bones, plate positioning is still performed manually using the software tool. Therefore, processing large bone data still requires long processing time and results in operatordependent outcome.
These limitations can be addressed to some extent using an automated method. However, as with automating any process, detailed and careful planning of the process is imperative to ensure correct outcome. Specifically for this problem, it is essential that the outcome is correct as well as clinically meaningful. The first automated method was proposed by Kozic et al. [6] for fit assessment of a proximal tibia plate on statistical bone models derived from 92 tibiae. Although the process was automated, there are critical limitations related to the selection of fit assessment parameter and criteria which may impact the validity of the outcome. These issues will be discussed in the following sections.
To this end, we propose that the virtual method is a better option for fit assessment of plate implant as it lends flexibility in using bone data that are representative of the current populations to develop better fitting implant, as well as performing quantitative fit assessment for objective and standardized fit assessment outcome. Furthermore, an automated method is a better option than a semi-automated method as it reduces processing time and minimizes operator-dependent outcome. However, it is imperative that such method produces correct and clinically meaningful outcome.
Fit Assessment Parameters
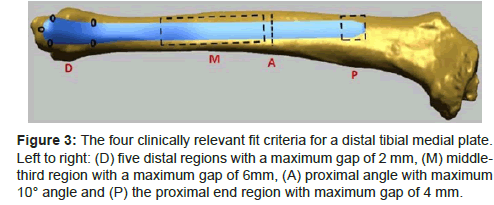
Fit assessment parameter and criteria are two main factors that determine the clinical relevance of any fit assessment outcome. Regardless of the fitting method, the outcome will render useless if the parameter and criteria selection are clinically irrelevant. In term of fit assessment parameter, previous studies have used the average distance between the plate surface and the underlying bone surface [6,10,13], the maximum distance between the plate and the underlying bone surfaces [2,3,12] and also the volume between the plate and underlying bone surface [5]. Indeed, measuring the gap between bone and plate in general is practical because the plate should be able to fit nicely to the bone. However, using the average distance over the entire plate surface may not necessarily provide a correct indication on how well the plate fits to the bone. This is important when the anatomical site involves regions with varying amount of soft tissue covering such as the site for the distal tibial medial plate [2,3]. For this plate, the distal medial end of the plate is located at the ankle where the soft tissue covering is thin, and therefore plate protrusion due to poor fit may cause discomfort to patients. In contrast, the amount of soft tissue in the tibia shaft is greater; therefore, greater gap can be tolerated in that area. As such, the authors have specified that the tolerated gap in the distal region is 2mm, and the tolerated gap in the shaft region (middle-third region) is 6mm (Figure 3). In this case, the maximum distance between the plate and underlying bone in the distal medial area will accurately indicate plate fit, compared to the average distance between the plates and underlying bone.
Figure 3: The four clinically relevant fit criteria for a distal tibial medial plate. Left to right: (D) five distal regions with a maximum gap of 2 mm, (M) middlethird region with a maximum gap of 6mm, (A) proximal angle with maximum 10° angle and (P) the proximal end region with maximum gap of 4 mm.
Similarly, the empty volume between the plate under surface and the bone provides a weaker indication in term of plate position and plate fit with respect to the bone surface. Although in many cases 3D measurements provide a more meaningful data, the variation in bone shape makes it impossible to establish a default value that can delineate well-fitting and poor-fitting cases.
Therefore, we propose that the maximum distance between the plate and underlying bone is a better option for fit parameter as it provides more accurate representation of plate fit.
Selection of Fit Criteria
Another important aspect in quantitative fit analysis is the selection of fit criteria to determine plate fit. Due to complex bone shape, it is only natural that the criteria are plate-dependent. However, more importantly, the criteria should be clinically relevant and meaningful. Previous studies have used from one [6,13] to four [2,5] fit criteria for different plate shapes. Since the fit criteria are plate-dependent though, the number of fit criteria does not indicate its clinical relevance. On the other hand, it is also quite impossible that one criterion is sufficient to represent a clinically relevant fit outcome, especially due to complex bone shape. In fact, this is one of the limitations of the recently proposed automated method in which one fit criterion was selected, and the criterion as based on the average distance of between the plate and underlying bone surface [6].
Generally, based on previous studies, a set of clinically meaningful criteria had higher number of criteria and lower number of fit cases. For example, one study had four fit criteria and only four fit cases from 101 dataset [5], and another had four fit criteria and six fit cases from 45 dataset [3]. In both studies, the number of fit cases was low, which highlighted the need for implant design optimization. In addition to providing clinically meaningful fit assessment outcome, clinically relevant fit criteria assists in highlighting specific areas on the plate for shape optimization purposes. For example, using four clinically relevant fit criteria for the distal tibial medial plate (Figure 3), the authors were able to determine that the current shape required further optimization in the distal and middle third regions [2,3].
Discussion and Conclusion
Virtual methods for fit analysis enable the usage of bone datasets representative of current populations for implant design and optimization. Additionally, with the advancement of medical imaging technology, larger bone datasets are available. In this regard, manual or semi-automated fit assessment method is less suitable as they are both operator-dependent process and require long processing time. An automated fit assessment method that integrates plate positioning and fit assessment processes is desirable as it enables batch processing, which will increase the efficiency of task execution and produce operator-independent outcome.
Nevertheless, the only automated method proposed in the literature used one fit criterion based on the average distance between the investigated plate and underlying bone surface [6]. Although this reduces the complexity of the problem, the fit criterion may not be clinically meaningful. Furthermore, the plate was fitted on statistical bone models. Therefore, it is uncertain whether the overall fit assessment outcome would be similar when using actual bone models. Finally, although the positioning criteria were discussed, no validation was offered yet for the final positioning result to show whether it is surgically acceptable or otherwise.
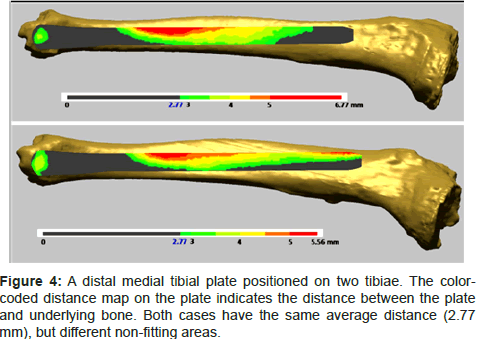
An example to illustrate the unsuitability of using the average distance as a fit criterion is the distal tibial medial plate. We present in Figure 4 two cases for which the plate was positioned at a clinically acceptable position. These cases were part of previous studies by one of the authors, in which they proposed four fit criteria and assessed the plate fit for 45 tibiae [2,3]. Both cases had the same average distance between the plate and underlying bone. However, one case was not fitting in the middle third region (Figure 4, top), while another was not fitting in the distal and proximal end regions (Figure 4, bottom). As discussed earlier, clinically, non-fitting in the distal region is lesstolerated than the middle third region because the soft tissue covering in the region is nominal. Such details are not readily available when using one fit criterion or using the average distance as fit parameter. Therefore, automatic fit analysis utilizing a set of relevant and clinically meaningful criteria is imperative for correct fit assessment outcome.
The fit assessment process involves two main steps, the positioning or alignment of the plate on the bone and the calculation for fit assessment. To date, there is no commercial software available to perform simultaneous automatic plate positioning and fit assessment of clinically relevant fit criteria and results in surgically correct plate position. Currently, these processes are performed iteratively by manipulating various tools in the software, such as the tools to move the plate, colour-coded distance map to keep track of the distance between the bone and plate and the collision detection tool to ensure that the bone and plate do not intersect each other [2]. Each of these tools has to be manipulated individually which increases the processing time for positioning the plate on larger bone dataset.
To conclude, this paper has reviewed the literature on quantitative fit assessment of anatomically precontoured fracture fixation plate and proposed that:
i. Automating the fit assessment process will enable proper handling of larger bone dataset and provide more standardized and objective fit assessment outcome.
ii. Clinically relevant fit criteria are necessary for correct fit assessment outcome. As highlighted earlier, it is highly unlikely that one unique criterion can highlight the clinical relevance of the outcome. Furthermore, it is best that a specific criterion is set for different areas on the plate based on their clinical requirements as proposed in previous studies [2,5].
iii. Specifying acceptable tolerance for the distance between the plate and underlying bone surface is more accurate to provide a correct indication of plate fit as opposed to using overall average distance.
iv. The development of a software tool for positioning and fit assessment of fracture fixation plates will greatly assist implant design validation and optimization efforts. Ideally, this software should be able to handle various plate shapes for various anatomical sites. Additionally, it should be able to interact efficiently with currently available medical imaging equipment such as MRI and CT to retrieve bone images, as well as 3D modelling and reverse-engineering software to build the 3D bone model. Finally, it also should be able to interact with CAD software tools to be able to apply the CAD models of the plates.
Acknowledgement
This work is supported by an Australian Research Council (ARC) Linkage Grant (ARC LP: LP0990250) in conjunction with faculty of Science and Engineering, and Institute of Health and Biomedical Innovation (IHBI), Queensland University of Technology (QUT).
References
- Javad M, Beat S, YuanTong G, Michael S, Prasad KY (2011) Orthopedic bone plates: Evolution in Structure Implementation technique and biomaterial. GSTF J Eng Technol 1: 135-140.
- Schmutz B, Wullschleger ME, Kim H, Noser H, Sch├?┬╝tz MA (2008) Fit assessment of anatomic plates for the distal medial tibia. J Orthop Trauma 22: 258-263.
- Schmutz B, Wullschleger ME, Noser H, Barry M, Meek J, et al. (2011) Fit optimisation of a distal medial tibia plate. Comput Methods Biomech Biomed Engin 14: 359-364.
- Hazreen H, Beat S, Michael S, YuanTong G, Prasad KY (2011) Quantitative Fit Assessment of a Precontoured Fracture Fixation Plate: Its Automation and an Investigation on the Borderline Cases. Adv Mater Res 339: 685-689.
- Goyal KS, Skalak AS, Marcus RE, Vallier HA, Cooperman DR (2007) Analysis of anatomic periarticular tibial plate fit on normal adults. Clin Orthop Relat Res 461: 245-257.
- Kozic N, Weber S, B├?┬╝chler P, Lutz C, Reimers N, et al. (2010) Optimisation of orthopaedic implant design using statistical shape space analysis based on level sets. Med Image Anal 14: 265-275.
- Bou-Sleiman H, Ritacco LE, Nolte LP, Reyes M (2011) Minimization of intra-operative shaping of orthopaedic fixation plates: a population-based design. Med Image Comput Comput Assist Interv 14: 409-416.
- Ahmad M, Nanda R, Bajwa AS, Candal-Couto J, Green S, et al. (2007) Biomechanical Testing of the Locking Compression Plate: When Does the Distance Between Bone and Implant Significantly Reduce Construct Stability? Injury 38: 358-364.
- Fouad H (2010) Effects of the bone-plate material and the presence of a gap between the fractured bone and plate on the predicted stresses at the fractured bone. Med Eng Phys 32: 783-789.
- Park AY, DiStefano JG, Nguyen TQ, Buckley JM, Montgomery WH 3rd, et al. (2012) Congruency of scapula locking plates: implications for implant design. Am J Orthop (Belle Mead NJ) 41: E53-56.
- Schmutz B, Rathnayaka K, Wullschleger ME, Meek J, Schuetz MA (2010) Quantitative fit assessment of tibial nail designs using 3D computer modelling. Injury 41: 216-219.
- Huang JI, Toogood P, Chen MR, Wilber JH, Cooperman DR (2007) Clavicular anatomy and the applicability of precontoured plates. J Bone Joint Surg Am 89: 2260-2265.
- Schulz AP, Reimers N, Wipf F, Vallotton M, Bonaretti S, et al. (2012) Evidence Based Development of a Novel Lateral Fibula Plate (VariAx Fibula) Using a Real CT Bone Data Based Optimization Process During Device Development. Open Orthop J 6: 1-7.
Relevant Topics
Recommended Journals
Article Tools
Article Usage
- Total views: 25159
- [From(publication date):
November-2013 - Apr 19, 2025] - Breakdown by view type
- HTML page views : 20375
- PDF downloads : 4784