Quantitative EEG Analysis in COVID-19 Encephalopathy: Promising Findings from a Case Report
Received: 21-Nov-2021 / Accepted Date: 06-Dec-2021 / Published Date: 13-Dec-2021
Abstract
Objective: Tracking cognitive impairment in patients with coronavirus disease 2019 (COVID-19) requires objective markers to monitor therapy responses and prognosticate neurological recovery. We sought to assess if quantitative EEG (QEEG) analysis could help identifying peculiar features suggesting COVID-19 related encephalopathy regardless of brain damage related to pulmonary failure.
Methods: A patient, who survived to a severe respiratory failure due to COVID-19 pneumonia, developed a severe neurocognitive syndrome. The patient underwent QEEG monitoring (global coherence, GC) before after one month of intensive cardiopulmonary and motor rehabilitation.
Results: We found a high-frequency strong central-temporal-parietal connectivity at baseline. This was replaced by low-frequency frontal-parietal connectivity. EEG signals and their modifications were unrelated to the former acute respiratory failure.
Conclusion: A decrease of front parietal GC in the upper alpha and beta band in resting state may be a key feature of COVID-19-related encephalopathy. This may depend on a virus-induced brain damage causing loss of connections that are essential to orchestrate the interactions between brain regions at a global level concerning cognitive functions. Significance: Our data suggest that assessing GC seems promising to evidence a frontoparietal connectivity impairment sustaining the COVID-19 neurocognitive syndrome. Once confirmed in larger samples, these QEEG findings may support clinicians in the management and prognosis of COVID-19 patients.
Keywords: Quantitative EEG; Global coherence; COVID-19 neurocognitive syndrome; SARS-CoV-2; Functional connectivity
Introduction
Central Nervous System (CNS) involvement has been frequently reported in both the acute and the after-recovery phases of Coronavirus Disease 2019 (COVID-19) [1,2,3]. It includes stroke, headache, seizures, encephalopathy (an acute or subacute major neuropsychiatric disorder characterized by impaired cognition, altered mental status, confusional state, consciousness impairment, psychosis, dementialike syndrome, or affective disorder), and encephalitis (acute or subacute infectious toxic, metabolic, systemic toxemic, or hypoxic encephalopathy [4,5,6].
CNS involvement can be secondary to the respiratory failure due to COVID-19 pneumonia (and related issues, including sepsis, sedating and anesthetic drugs, and mechanical ventilation), up to an acute respiratory distress syndrome (ARDS) and concurrent cytokine storm. On the other hand, the SARS-CoV-2 can directly cause an encephalitis/ encephalopathy owing to:
1) A direct hematogenous attack by the virus by biding to the Angiotensin Converting Enzyme-2 receptor expressed in endothelial cells at the blood–brain barrier interface;
2) A retrograde ascent via peripheral nerve fibers of the upper respiratory tract or via the olfactory bulb); 6 and
3) A cytokine-mediated inflammation of the nervous system and vasculature (which can thus be para-infectious or post-infectious). It is therefore reasonable that a direct CNS damage may occur regardless of a systemic involvement, i.e., due to pulmonary failure. However, the knowledge about the neuropathological substrate of the cognitive dysfunction in the aftermath of COVID-19 infection is still partial [7,8]
Noteworthy, this pathophysiological knowledge may be useful concerning the diagnosis, prognosis, and pharmacological and rehabilitative management of COVID-19 patients. Actually, the COVID-19 cognitive dysfunction can cause lifelong disability, which implies long-term cares and potentially large health, social, and economic costs [4, 9-12]
In this regard, EEG analysis can provide us with useful information concerning brain network functioning during normal and pathological conditions. A bulk of studies focused on EEG profiles of patients with COVID-19, reporting either mostly normal EEGs to diffuse slowing [9,10] Some studies reported a preponderance of frontal patterns and continuous, slightly asymmetric, monomorphic, biphasic and slow delta waves, putatively linked to the mode of entry of the virus into the brain [13]. Conversely, few specific quantitative EEG studies have been reported to date, to the best of our knowledge [9,10]. Herein, we report on a COVID-19 patient who survived to a severe respiratory failure due to COVID-19 pneumonia, and developed a severe neurocognitive syndrome that was unrelated to respiratory failure. This neurocognitive syndrome was characterized by peculiar pattern at the quantitative EEG analysis that could be used to diagnose and monitor COVID-19 related encephalopathy.
Case Description
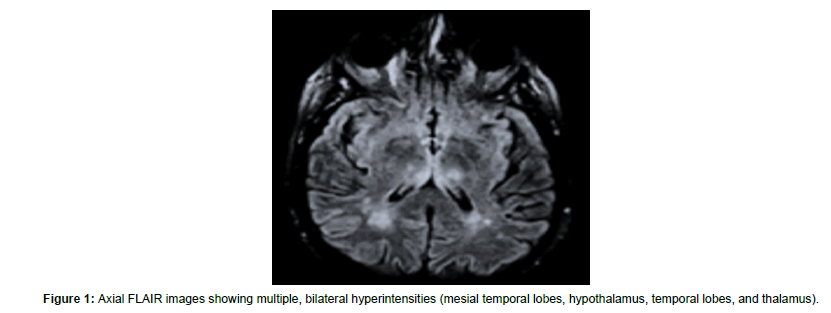
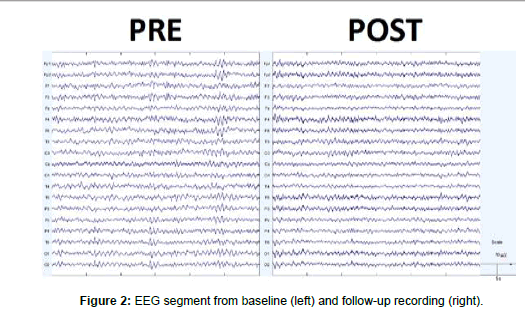
On January 2021, an otherwise healthy 59-year-old male started to complain of fever, cough, and generalized malaise since five days. Ha accessed the ER where he was provided with a COVID-19 nasopharyngeal swab sample that was positive. He was thus hospitalized at the infectious diseases unit for competence. Owing to the onset of acute hypoxemic respiratory failure two days after the recovery, he was intubated and transferred to the COVID intensive care unit (ICU). During recovery, he was provided with chest CT that showed round glass changes and peripheral opacities in upper and mid zones of both lungs. One week after the recovery in ICU, he was weaned from pharmacological sedation, extubated, and provided with non-invasive ventilation. A nasopharyngeal tube was used for feeding. The patient opened eyes spontaneously, localized to painful stimuli, and complained of dysphagia, global muscle weakness, and frequent, transient episodes of psychomotor agitation with aggressive behavior and irritability, and sexual disinhibition. Furthermore, disorientation, distractibility, confabulation, and paranoid affect were also appreciable. He was thus diagnosed with adjustment disorder, prescribed with antipsychotic and mood-stabilizer drugs, and transferred to the infectious diseases unit. After 25 days, he was admitted to our cardiopulmonary rehabilitation unit, once the nasopharyngeal molecular swab for SARS-CoV-2 was negative. His neurological condition were stable, the patient required noninvasive ventilation only nightlong and intermittent O2 administration during the day. He then was provided with a brain MRI scan that showed areas of demyelination suggestive of para-/postinfectious encephalitis (Figure 1). And EEG recording (diffuse slowing, some bi-hemispheric synchronous spikes) (Figure 2). He was managed with conventional respiratory and motor rehabilitation for one month. At the discharge, his respiratory and neurological condition were improved, as he had only a residual impairment in attention, immediate and delayed memory for verbal and visual information, processing speed, verbal fluency, and visuospatial construction and skills. He was thus prescribed with low-dose olanzapine and citalopram. Another EEG recording was conducted (reduction of diffuse slowing), and outpatient followed-up. To date, his neuropsychiatric conditions are largely improved concerning attention, processing speed, memory, verbal fluency, and impulsivity, whereas a residual deficit in executive and visuospatial functions is yet appreciable. The institutional review board approved this study and caregiver’s patient gave written informed consent for data analysis and study publication.
EEG Analysis and Findings
Both EEG were recorded in resting condition, with the eyes closed, using a Nihon-Kohden device (Shinjuku; Tokyo, Japan) equipped with a standard 21-electrode headset. EEG was sampled at 512 Hz, band-pass filtered at 0.3-45 Hz, 50 Hz-notched, and referenced to FCz. Impedance was kept below 5 kΩ. After artifact removal (visual inspection and ICA), the EEG was segmented into 10-sec epochs (5120 samples) and submitted to a second ICA. Then, global coherence (GC) was computed separately in four frequency bands (delta, 1–4 Hz, theta, 4–7 Hz, alpha, 7–13 Hz, and beta, 13–30 Hz) by dividing the square of the magnitude of the cross spectrum by the product of power spectra of two time-series [14].
In this regard, we used 30 overlapping (750 ms) 1-sec epochs (512 samples, frequency resolution of 1 Hz. Coherence was computed for all possible combinations of the 21 EEG channels, and then averaged for GC. In addition, we calculated the global correlation dimension (D2) using spatial embedding and the algorithm of Grassberger and Procaccia [15].
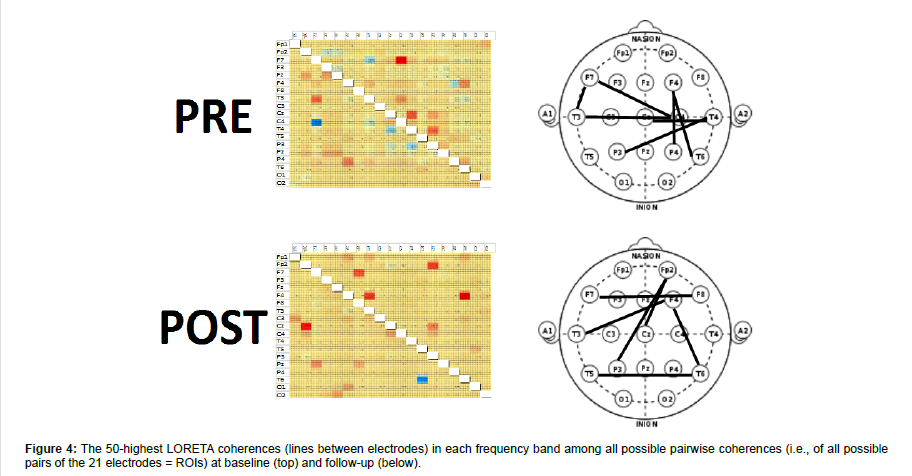
Generally, the higher the D2 value, the greater the degrees of freedom, the lesser the signal coupling, and the greater the global dynamical complexity were. Lastly, we estimated the spatial distribution of the 50 strongest pair-wise coherences by using standardized low resolution electromagnetic tomography (sLORETA). The significance of post-pre changes was assessed using the reliable change index (RCI).
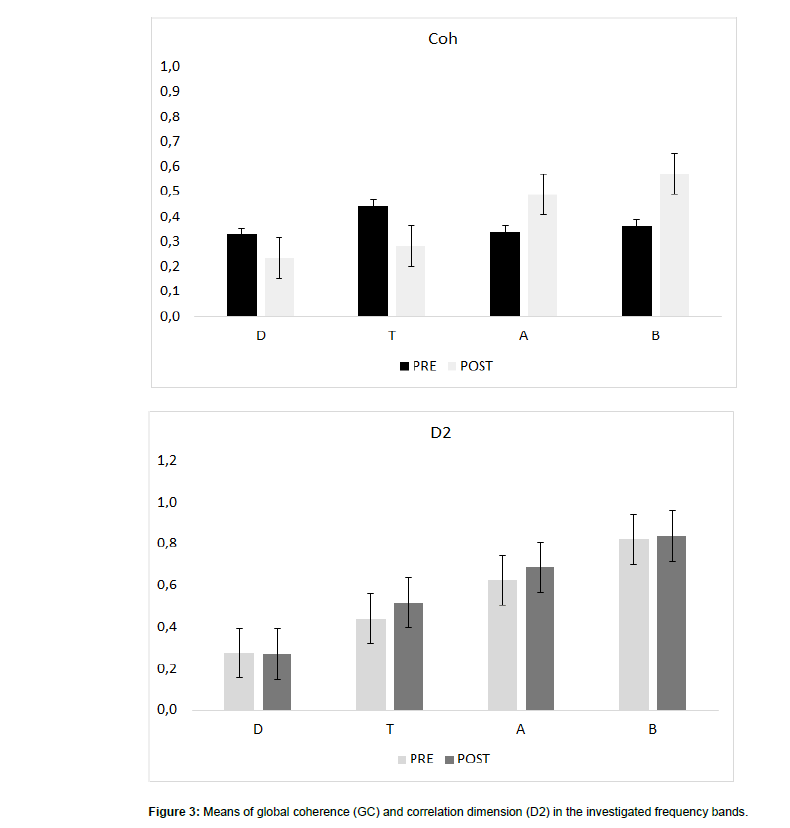
The first EEG showed a global slowing of the background activity, some bi-hemispheric synchronous spikes (Figure 3). And slightly higher GC values in delta and theta band (Figure 3). LORETA coherence showed a coherence increase limited to central-temporalparietal areas (Figure 4).
After one month, our patient got a significant improvement in the background EEG activity (Fig.2), a GC decrease in delta and theta ranges (both RCI >1.96) and an increase in alpha and beta frequency band (both RCI >1.96), and an increase of D2 in the beta range (RCI=-6) (Figure 3). LORETA coherence showed a shift of high-value coherences towards frontal-parietal electrodes (Figure 4).
Discussion
CNS symptoms directly related to COVID-19 infection are numerous and often difficult to differentiate from COVID-19 systemic disease. Our QEEG data suggest the possibility to identify specific EEG patterns that link CNS symptoms directly to COVID-19 infection compared to other non-specific encephalopathy patterns. [2,16,17]. Actually, we found QEEG patterns largely distinguishable from the other most common encephalopathy, i.e., severe hypoxic condition [9,10,18-21]. Therefore, there is suggestion for EEG testing to be used for COVID-19 diagnosing and monitoring [9,10,18-21].
On one hand, QEEG analysis seems capable to differentiate differentiate neurotrophic related specific changes from those possibly resulting from COVID-19 pulmonary or systemic deterioration, including brain hypoxia, microemboli, or prolonged ICU treatment with long term sedation, which may all result in encephalopathy. On the other hand, one could concern that neuroimaging may offer more robust findings on differentiating COVID-19 related vs. systemic features of encephalopathy. In addition, patients with COVID-19 can show different, patterned or not, EEG abnormalities, epileptiform changes and seizures/status epilepticus, and a diffuse slowing of EEG activity, which altogether give evidence for a diffuse encephalopathy, correlate with both the clinical status of the patient and the pre-existing neurological diseases, but are non-specific of COVID-19 related encephalopathy [22, 23].
Nonetheless, other peculiar EEG abnormalities within frontal region, including frontal epileptiform discharges, have been proposed as biomarkers for such condition. In addition, neuroimaging performing is not always straightforward or diriment, including the fresh or disseminated white matter hyper-intensity. Furthermore, the data we show are consistent with clinical (anosmia and ageusia) and anatomical-electrophysiological correlations [20]. In particular, greater delta-theta band spectral power, higher temporal variance, diversity in frequency band and spatial extent have been reported as quantitative EEG findings suggesting the involvement of the frontal lobe, the piriform cortex, and limbic structures as markers of COVID-19 related encephalopathy. [9,10,13,18-21].
Despite these issues, one could criticize that EEG assessment is challenged in the acute and post-acute phases by many confounding factors, including pulmonary involvement/hypoxia, cardiac arrest, other metabolic changes, sedation, systemic inflammatory response syndrome, hypercoagulability, vasculitis, strokes, the older age of patients, a larger proportion of pre-existing neurological conditions, and the possibility of sicker patients. However, despite these limitations, several authors commented that a wider use of EEG would help characterize more patients with COVID-19 related encephalopathy. In this regard, global connectivity markers, including global coherence and correlation dimension, are proposed to provide some useful information for COVID-19 related encephalopathy assessment, as they contain information about the correlation between channels when measured in a multichannel way, which betrays the frontoparietal connectivity impairment [18]. Actually, GC reflects the amount of large-scale functional interactions, which may account for a neocortical disconnection syndrome [24, 25] the D2 is a reflection of the ‘degrees of freedom’ necessary to describe the dynamics of the system under study, thus being a measure of brain complexity [26].
Strengths of QEEG Assessment
Our data support the usefulness of investigating COVID-19 EEG with these nonlinear time series analysis, which has been conducted for the first ever time, to the best of our knowledge. Actually, our patient showed a global EEG slowing similarly to that commonly found in non-COVID-19 pulmonary failure-related encephalopathy. Furthermore, our patient did not show an increase in GC values in delta and theta band, which is instead commonly reported in non-COVID-19 pulmonary failure-related encephalopathy [27]. Therefore, the current results suggest a decrease of coupling between EEG channels in the higher frequency ranges with particular regard to the frontal-parietal connections to the advantage of the centraltemporal- parietal ones; in other words, they reflect impairment in large-scale functional interactions. This finding, together with the shift of frequency ranges from the low- to the high-range (as indicated by the D2 data), suggests a decrease in brain complexity as reflected by the prominent frontoparietal connectivity failure (in low-frequency range) paralleled by a probably compensatory (or maladaptive) high-frequency central-temporal-parietal connectivity increase. This model may be consistent with the postulated virus-induced damage of frontal bran structures [20, 13,19] which has relevant consequences on different neurocognitive processes as already reported in other diseases based on such a mechanism, including Alzheimer’s disease and schizophrenia [28]. The importance of these quantitative EEG changes in COVID-19 encephalopathy is also suggested by the specific EEG connectivity changes occurred after the cardiopulmonary and motor rehabilitation period. Actually, the patient disclosed a partial enhancing of background EEG activity, the disappearance of the synchronous epileptiform discharges, a decrease of low frequency GC, and an increase of GC in alpha and beta frequencies and of D2 in beta range. Furthermore, we appreciated a shift of high-value coherences towards frontal-parietal electrodes. On the contrary, patients with non-COVID19 pulmonary failure-related encephalopathy do not usually show any significant change in GC at a one-month follow-up [27]. Noteworthy, these EEG changes in our patient were correlated with neither the neurological nor the respiratory picture. Overall, the findings of specific EEG connectivity changes (from a detrimental high-frequency central-temporal-parietal to a more functional lowfrequency frontal-parietal connectivity) suggest that a decrease of functional connectivity in the upper alpha and beta band in resting state may be a key feature of COVID19-related encephalopathy. This is also confirmed by the restoration of connectivity patterns in resting state from the high-frequency central-temporal-parietal to the lowfrequency frontal-parietal paths as per LORETA data. This baseline frontoparietal connectivity decrease may depend on the virus-induced loss of connections, which are essential to orchestrate the interactions between brain regions at a global level as well as the cognitive functions that resulted impaired in our patient [29]. Given that these EEG changes were not correlated with the clinical picture modification, differently from other markers reported in the literature, global connectivity measures may represent an independent diagnostic indicator, whereas the prognostic role needs further assessment [23].
Furthermore, consistently with the baseline EEG differences between non-COVID-19 pulmonary failure-related encephalopathy and our patient and the evolution of the clinical picture and of the EEG pattern, we may hypothesize that our patient suffered from a COVID-19 related encephalopathy resulting from direct brain involvement, rather than from a systemic involvement secondary to COVID-19. This hypothesis is supported by some evidences beyond the EEG picture, including the brain MRI scan that did not showed any significant sign of hypoxic brain injury (as the pulmonary failure was promptly and well managed, without significant complication), CNS vasculopathy, and stroke/hemorrhage. Moreover, the possible biasing effect of pharmacological therapies on the neuropsychiatric profile of our patient (including steroids, sedating and anesthetic drugs) can be excluded consistently with small cumulative doses and the relatively short-lasting ICU stay. Lastly, blood and urine sample tests were negative.
Limitations Of QEEG Assessment
The main methodological limitation in our study is the lack of a direct control. Furthermore, other non-COVID-19 encephalopathy is age-related entities. In addition, a partial effect of even transient brain hypoxia may still have a pathophysiological role, which requires further and longer assessments. The main clinical limitation is the challenging differential diagnosis with the post-intensive care syndrome (PICS), which also includes mobility problems of neuromuscular origin, altered cognition, and psychotic manifestations [30]. Despite PICS is not characterized by an encephalopathy, we can neither firmly exclude this possibility as a cause of COVID-19 encephalopathy, nor consider it as the first option [31].
Conclusion
We suggest that QEEG analysis may be useful in the differential diagnosis of COVID-19 related encephalopathy (that include background EEG slowing and high GC values in the low-frequency ranges and low GC values in the high-frequency ranges) towards other COVID-19-related encephalopathy (that include globally low GC values indicating the presence of nonspecific encephalopathy and cortical irritability). EEG abnormalities affecting the frontoparietal connectivity seems to be common in COVID-19 encephalopathy and may be proposed as a potential biomarker of COVID-19 neurocognitive syndrome if recorded consistently. Our data suggest that assessing global coherence and related measures seems promising to evidence a frontoparietal connectivity impairment sustaining the COVID-19 neurocognitive syndrome. Therefore, once confirmed in larger samples, these EEG findings may support clinicians in the management and prognosis of COVID-19 patients.
None of the authors have potential conflicts of interest to be disclosed.
References
- Rogers JP, Chesney E, Oliver D, Pollak TA, McGuire P, et al. (2020) Psychiatric and neuropsychiatric presentations associated with severe coronavirus infections: a systematic review and meta-analysis with comparison to the COVID-19 pandemic. Lancet Psychiatry 7: 611-27.
- Asadi-Pooya AA, Simani L (2020) J Central nervous system manifestations of COVID- 19: a systematic review. Neurol Sci 413: n116832.
- Ahmed MU, Hanif M, Ali MJ, Haider MA, Kherani D, et al. (2020) Neurological Manifestations of COVID-19 (SARS-CoV-2): A Review. Front Neurol 11: 518.
- Ellul MA, Benjamin L, Singh B, Lant S, Michael BD, et al. (2020) Neurological associations of COVID-19. Lancet Neurol 19: 767-83.
- Parra A, Juanes A, Losada CP, Ãlvarez-Sesmero S, Santana VD, et al. (2020) Psychotic symptoms in COVID-19 patients. A retrospective descriptive study. Psychiatry Res 291: 113254.
- Zubair AS, McAlpine LS, Gardin T, Farhadian S, Kuruvilla DE, et al. (2020) Neuropathogenesis and Neurologic Manifestations of the Coronaviruses in the Age of Coronavirus Disease 2019: A Review. JAMA Neurol 77: 1018-27.
- Desforges M, Le Coupanec A, Brison E, Meessen-Pinard M, Talbot PJ (2020) Human Coronaviruses and Other Respiratory Viruses: Underestimated Opportunistic Pathogens of the Central Nervous System? Viruses 12: 14.
- Ritchie K, Chan D, Watermeyer T (2020) The cognitive consequences of the COVID-19 epidemic: collateral damage? Brain Commun 2: fcaa069.
- Kopanska M, Banas-Zabczyk A, Lagowska A, Kuduk B, Szczygielski (2021) J Changes in EEG Recordings in COVID-19 Patients as a Basis for More Accurate QEEG Diagnostics and EEG Neurofeedback Therapy: A Systematic Review. J Clin Med 10: 1300.
- Antony AR, Haneef Z (2020) Systematic review of EEG findings in 617 patients diagnosed with COVID-19. Seizure.
- Chia KX, Polakhare S, Bruno SD (2020) Case report Possible affective cognitive cerebellar syndrome in a young patient with COVID-19 CNS vasculopathy and stroke. BMJ Case Rep 13: e237926.
- Lleó A, Alcolea D (2020) The cognitive aftermath of COVID-19.Brain Commun.
- Petrescu AM, Taussig D, Bouilleret V (2020) Electroencephalogram (EEG) in COVID-19: A systematic retrospective study. Neurophysiol Clin 50: 155-65.
- Lopes da Silva FH, Vos JE, Mooibroek J, Van Rotterdam A (1980) Partial coherence analysis of thalamic and cortical alpha rhythms in dog a contribution towards a general model of the cortical organisation of rhythmic activity. In: Pfurtscheller G, et al. (Eds.), Rhythmic EEG activities and cortical functioning, Elsevier, Amsterdam Pp: 33-59.
- Di C, Wang T, Yang X, Li S (2018) An improved Grassberger-Procaccia algorithm for analysis of climate system complexity. Hydrol Earth Syst. Sci Discuss 22: 5069-79.
- Baig AM (2020) Neurological manifestations in COVID-19 caused by SARS-CoV-2. CNS Neurosci. Ther 26: 499-501.
- Li Z, Liu T, Yang N, Han D, Mi X, et al. (2020) Neurological manifestations of patients with COVID-19: Potential routes of SARS-CoV-2 neuroinvasion from the periphery to the brain. Front Med 14: 533-41.
- Pasini E, Bisulli F, Volpi L, Minardi I, Tappatà M, et al. (2020) EEG findings in COVID-19 related encephalopathy. Clin Neurophysiol 131: 2265-67.
- Vellieux G, Rouvel-Tallec A, Jaquet P, Grinea A, Sonneville R, et al. (2020) COVID-19 associated encephalopathy: Is there a specific EEG pattern?. Clin Neurophysiol 131: 1928-30.
- Pastor J, Vega-Zelaya L, MartÃn Abad E (2020) Specific EEG Encephalopathy Pattern in SARS-CoV-2 Patients. J Clin Med 9: 1545.
- Flamand M, Perron A, Buron Y, Szurhaj W (2021) Pay more attention to EEG in COVID-19 pandemic. Clin. Neurophysiol 131: 2062-64.
- Pilato MS, Urban A, Alkawadri R, Barot NV, Castellano JF, et al. (2020) EEG Findings in Coronavirus Disease. J Clin Neurophysiol.
- Pati S, Toth E, Chaitanya G (2020) Quantitative EEG markers to prognosticate critically ill patients with COVID-19: A retrospective cohort study. Clin Neurophysiol 131: 1824-26.
- Belisle M (2005) Measuring landscape connectivity: the challenge of behavioural landscape ecology. Ecology 86: 1988-95.
- Sakkalis V (2011) Review of advanced techniques for the estimation of brain connectivity measured with EEG/MEG. Comput. Biol. Med 41: 1110-17.
- Lane D (2011) Online Statistics Education. In: Lovric M. (Ed)International Encyclopedia of Statistical Science, Springer.
- Yu L, De Mazancourt M, Hess A, et al. (2016) Functional connectivity and information flow of the respiratory neural network in chronic obstructive pulmonary disease. Hum. Brain Mapp 37: 2736-54.
- Mutlu J, Landeau B, Gaubert M, de La Sayette V, Desgranges B, et al. (2017) Distinct influence of specific versus global connectivity on the different Alzheimer's disease biomarkers. Brain 140: 3317-3328.
- Dixon ML, De La Vega A, Mills C, Andrews-Hanna J, Spreng RN, et al. (2018) Heterogeneity within the frontoparietal control network and its relationship to the default and dorsal attention networks. Proc Natl Acad Sci 115: E1598-E1607.
- Bryant SE, McNabb K (2019) Postintensive Care Syndrome Crit Care. Nurs Clin North Am 31: 507–516.
- Lee M, Kang J, Jeong YJ (2019) Risk factors for post–intensive care syndrome: A systematic review and meta-analysis. Aust Crit Care 12
Citation: Luana B, Antonino N, Flavio C, Marco F, Fabio C, et al. (2021) Quantitative EEG Analysis in COVID-19 Encephalopathy: Promising Findings from a Case Report. J Neuroinfect Dis 12: 364.
Copyright: © 2021 Luana B, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Usage
- Total views: 2193
- [From(publication date): 0-2021 - Feb 22, 2025]
- Breakdown by view type
- HTML page views: 1705
- PDF downloads: 488