Research Article Open Access
Quantitative Determination of Topiramate in Human Breast Milk
Cristina Cifuentes, Sigrid Mennickent* and Marta De DiegoUniversity of Concepción, Concepción, Chile
- *Corresponding Author:
- Sigrid Mennickent
Department of Pharmacy Faculty of Pharmacy
University of Concepción PO Box 237, Concepción, Chile
Tel: 0412204208
Fax: 56412207086
E-mail: smennick@udec.cl
Received date: August 03, 2016; Accepted date: August 19, 2016; Published date: August 24, 2016
Citation: Cifuentes C, Mennickent S, Diego MD (2016) Quantitative Determination of Topiramate in Human Breast Milk. J Anal Bioanal Tech 7:334. doi: 10.4172/2155-9872.1000334
Copyright: © 2016 Cifuentes C, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Analytical & Bioanalytical Techniques
Abstract
A thin-layer chromatographic (HPTLC) method for quantification of topiramate in human breast milk was developed using liquid –liquid extraction with n-hexane and methanol as extraction solvents, fluorescence activation with ninhidrine (1% ethanolic solution) and chlorpromazine as internal standard.
Thin-layer chromatographic separation was performed on precoated silica gel F 254 HPTLC plates using a mixture of toluene: ethanol (25:10, v/v), as mobile phase. Densitometric detection was done at 326 nm. The method was validated for linearity, precision, selectivity, LOD and LOQ, and accuracy. Linear calibration curves in the range of 0.30 to 50.00 µg/mL showed correlation coefficient of 0.991. The intra-assay and inter-assay precision, expressed as the relative standard deviation (RSD), were in the range of 3.04% - 3.14% (n=3) and 1.81%-4.10% (n=9), respectively. The limit of detection was 0.24 µg/mL, and the limit of quantification was 0.30 µg/mL Accuracy, calculated as percentage recovery, was between 101.65% and 109.51%, with a RSD not higher than 0.41%. Topiramate is well resolved from others antiepileptic drugs and from the internal standard (Rs=5.20). In conclusion, the method is precise, accurate, reproducible and selective for the analysis of topiramate in human breast milk.
Keywords
Topiramate; Breast milk; Antiepileptics; Thin-layer chromatography
Introduction
Topiramate (TPM) is a monosaccharide drug with a molecule of fructopyranose in its chemical structure [1] (Figure 1). TPM is an anticonvulsant drug indicated for the treatment and control of partial seizures and severe tonic-clonic (grand mal) seizures in adults [2], including Lennox-Gastant Syndrome in children [3,4]. It is also used for prophylaxis of migraine [5,6], mood instability disorder [7], post-traumatic stress disorder, bulimia nervosa and binge-eating disorders [8]. It is also investigated as promising agent for obesity treatment [9], release of neuropathic pain [10], treatment of sleep related eating disorder [11], alcohol and drug addiction therapy [12,13] and smoking cessation [14-16].
These effects of TPM results from its multiple pharmacological actions, as the enhancement of the activity of γ-aminobutyric acid type A, inhibition of some voltage-gated sodium and calcium channels, inhibition of some glutamate receptors, as well a little inhibition of carbonic anhydrase [17-19].
Food and Drug Administration (FDA) suggest avoid the use of TPM in pregnant woman and in her babies, because the reports of risk oral birth defects (cleft lip and clef palate) in children born to mothers taking TPM (FDA New Release, March 2011). However, sometimes is more difficult to change the antiepileptic drug to some patients, including pregnant women, increasing the risks for their babies.
Some studies shown when the mother was taking 200 mg of topiramate daily had average milk levels of 0.6 μg/mL to 1.2 μg/mL, and these works estimated that the infants received doses between 0.1 and 0.7 mg/kg/day which was between 3 and 23% of the mother's weight-adjusted dose. Serum levels in a 24-day-old infant whose mother was taking 150 mg daily were about 0.5 μg/mL. Another infant whose mother was taking 200 mg daily had an average serum level of 0.6 μg/mL at 20 days of age and 0.7 μg/mL at 97 days of age. Overall, their plasma levels were about 10 to 20% of maternal plasma levels. Baby blood topiramate concentration is higher as baby age is increased, because the breast –feeding increased too, therefore, the blood concentration of topiramate. Moreover, this drug is a lipophilic compound, increasing its body accumulation [19,20].
The review of literature revealed that TPM has no ultraviolet, visible or fluorescence absorption [21,22]. Analysis of topiramate in pharmaceutical formulation and in biological fluids has been reported by HPLC with precolumn or post column derivatization, and by capillary electrophoresis [23-30], and by LC-MS [31-34]. None method by HPTLC was founded by quantitative determination of TPM in breast milk.
High performance thin-layer chromatography (HPTLC) is a technique carried out within a short period of time, requires few mobile phase and allows for the analysis of a large number of samples simultaneously. A plate of 10 × 20 cm. allows applying up to 33 spots (27 samples plus standards). HPTLC allows to detect quantities in the order of micrograms and of nanograms (in UV absorbance mode) and smaller than picograms (in fluorescence mode). Therefore, HPTLC allows a fast analysis, with minor cost than other techniques, and with a high selectivity, accuracy and reproducibility.
The developed method can be used to quantitative determination of TPM in human breast milk, suitable for therapeutic drug monitoring of this drug in this matrix, considering as the woman in lactation period as her baby. From these values, could be estimate the baby blood topiramate concentration without the risks to take blood sample from his (her).
Experimental
Instrumentation and reagents
USP standards of topiramate and chlorpromazine were purchased from Sigma- Aldrich, St. Louis, MO. Methanol, ethanol, toluene and ninhidrine, were obtained from Merck, Darmstadt, Germany. All of the reagents were pro-analysis quality.
Preparation of standard solutions
Stock solutions containing 200 μg/mL of TPM were prepared in methanol. Separate solutions were prepared for the calibration standards and quality control samples. These solutions were diluted immediately before use with methanol, to obtain working solutions of 0.3 – 1.2 – 1.4 – 2.0 – 5.0 – 10.0 – 20.0 – 30.0 – 40.0 and 50.0 μg/mL All of solutions were stored at 4°C for about two days.
Spiking procedure for calibration and quality control (QC) samples
The calibration samples were prepared immediately before use by spiking 1 mL of pooled human breast milk with 0.1 mL of a convenient working solution in methanol. Quality control samples were used to determine the intra and inter-assay precision and accuracy of the method. Human breast milk used for the validation of the method was obtained from healthy volunteers.
Sample preparation
Human breast milk samples were stored at -20°C until required for analysis. Calibration and quality control samples were thawed at room temperature. Immediately after thawing, 2 mL of sample was processed by adding initially 150 μL of chlorpromazine (internal standard) (50 μg/mL) and 100 μL de NaOH 0.1 M. to the solution, which was subsequently vortexed and centrifuged for 5 minutes at 3000 rpm. Then, the supernatant was transferred to other recipient and 100 μL of methanol was added. Aqueous phase was evaporated under a gentle steam of dry nitrogen at 37°C, and the residue was dissolved in 300 μL of methanol. An aliquot of 1 μL of this solution was spotted for analysis, to obtain the required quantity/spot. All the procedure was accomplished under safety conditions.
Instrumentation and chromatographic conditions
The HPTLC system consisted of a TLC Scanner 3 (CAMAG, Muttenz, Switzerland), equipped with software winCATS 1.4.2 (CAMAG); band application device Linomat V (CAMAG); twin trough chromatographic chamber (CAMAG) 10 × 10 cm. and 20 × 10 cm; and HPTLC glass backed plates 10 × 10 cm. and 20 × 10 cm. Precoated with silica gel F 254, layer thickness 0.2 mm (Merck, Darmstadt, Germany), previously washed with methanol and activated at 120°C during 20 minutes.
Serum samples, calibration and quality control samples application volumes were 1 μL of each of them. Sample application was done on 4 mm bands. Number of tracks depended of each assay. Mobile phase consisted of a mixture of toluene: ethanol (25:10, v/v). The chamber was previously saturated. Migration distance was 8 cm. Derivatization of topiramate was done with ninhidrine (1% ethanolic solution), by immersion of the plates into Deeping Device (Camag). This procedure makes topiramate fluorescent and the background dark. Densitometry scanning was performed at 326 nm.
Stability study
To establishment the stability of TPM samples, in normal storage conditions, the study was performed as follows: six extractions solutions with derivative agent were used at four different concentrations: 0.3, 1.2 μg/mL, 10 μg/mL and 50 μg/mL. These solutions were storage at three different conditions: freezer temperature, room temperature with light protection, and room temperature without light protection. Concentration determination was evaluated at 0, 1, 4, 7, 11 and 15 days of storage. Each sample was determined by duplicated.
Method validation
Validation of developed LC method was carried out as per the International Conference on Harmonization (ICH) guidelines, and as per FDA [33,34].
Results and Discussion
During method development different conditions for sample extraction and chromatographic conditions was tried to achieve optimal results.
The selection of the mobile phase was carried out on the basis of polarity i.e., choice a solvent system that would give dense and compact spots with appropriate Rf value, as well as a satisfactory separation of TPM and internal standard and good peak symmetry. Several trials for optimization of mobile phase were taken and finalized as toluene: ethanol (25:10, v/v). This solvent system gave compact spots for topiramate.
Some wavelengths were tried, chosen 326 as working wavelength. Complete resolution of the peaks with clear baseline separation was obtained of this way.
Sample extraction was optimized to eliminate the laborious extraction steps, with minimal losses of carbamazepine and very good recoveries from spiked serum samples.
Calibration curves
Calibration curves were constructed over the concentration range of 0.3 to 50.0 μg/mL. Each solution was spotted three times. This range of solution concentrations include the TPM concentrations expected in human breast milk [19,20].
The mean equation (curve coefficients ± standard deviation) for the calibration curve (n=5), obtained from five points, was y=0.3577x(± 0.1) x+0.7893(± 0.2) with a correlation coefficient, r=0.99. ANOVA assay showed a relationship between the ratio of peak area of the analyte to the internal standard as a function of the concentration added, with p<0.005.
Precision and accuracy
The intra assay precision and accuracy was calculated at low (L), medium (M), and high (H) quality control levels for three replicates each of the same analytical run (each replicate was spotted three times), and inter assay precision and accuracy was calculated after repeated analysis in three different analytical runs.
Each experiment included the sample extraction step. The precision and the accuracy of the assay were measured by the relative standard deviation (RSD) over the concentration range. TPM solutions were of 0.3, 10.0 and 50.0 μg/mL. RSD for intra–assay study was between 3.04% and 3.14%, and for inter-assay was between 1.81% and 4.10%.
Accuracy was calculated from the test results as the percentage of analyte recovered by the assay, determined by linear regression equation of peak area vs. drug concentration. Accuracy was between 101.65% and 109.51%, expressed as analyte recovery percentage. The results are presented in Tables 1 and 2 (precision) and (accuracy) respectively.
| Concentrationµg/mL | RSD Intra-assay | RSD Inter-assay |
|---|---|---|
| 0.3 | 3.04 | 3.46 |
| 10.0 | 3.10 | 1.81 |
| 50.0 | 3.14 | 4.10 |
Table 1: Precision of Topiramate in human breast milk.
| Intra-assay | |||
| Initialconcentration (µg/mL) | Founded concentration (µg/mL) | Recovery (%) | RSD |
| 0.3 | 1.34 ± 0.07 | 109.51 | 0.41 |
| 10.0 | 10.40 ± 0.01 | 101.65 | 0.01 |
| 50.0 | 49.96 ± 0.03 | 101.97 | 0.04 |
| Inter-assay | |||
| Initialconcentration (µg/mL) | Founded concentration (µg/m) | Recovery (%) | RSD |
| 0.3 | 1.35 ± 0.50 | 105.14 | 0.30 |
| 10.0 | 11.21 ± 0.09 | 108.47 | 0.08 |
| 50.0 | 50.93 ± 0.05 | 101.90 | 0.04 |
Table 2: Accuracy of Topiramate in human breast milk.
Detection and quantification limits
The limit of detection (LOD) and limit of quantification (LOQ) were calculated preparing solutions at three concentrations (0.1- 0.2-0.3 μg/mL) in the lower range of linear regression curve for both biological matrices.
LOD was 0.24 μg/mL and LOQ was 0.30 μg/mL, determined using the equations [35,36]: LOD=3.3 σ/b; LOQ=10 σ/b, where σ is the standard deviation of the values, and “b” corresponds to the slope obtained from the curve peak area versus concentration of the analyte. These values were experimentally verified.
Selectivity
The selectivity of the assay was checked by analyzing three independent blank human breast milk samples. The chromatograms of these blanks samples were compared with chromatograms obtained by analyzing the biological fluid samples spiked with TPM and chlorpromazine, the internal standard, and with other antiepileptic drugs: carbamazepine and phenytoin.
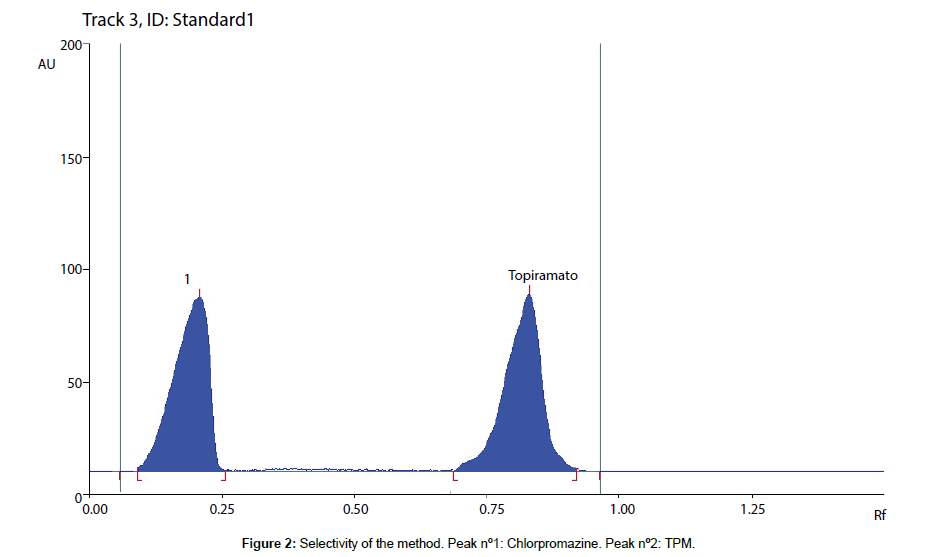
The solutions were prepared at a concentration of 20 μg/mL of each compound. TPM and chlorpromazine were well separated, with a resolution (R) value between both peaks of 5.20 (Figure 2). Carbamazepine and phenytoin run with the solvent line. Moreover, no interference was observed in drug free samples, indicating the high selectivity of the developed method.
Extraction recovery
The extraction recovery of TPM from human breast milk and that of the internal standard were quantified using the concentrations of 0.3, 10.0 and 50 μg/mL for the drug, and 10 μg/mL of the internal standard. The extraction recoveries were calculated by comparing the observed concentrations obtained from the processed standard samples to the concentrations obtained from the standards solutions added to the human breast milk after the extraction, which represented 100% recovery. The extraction recovery of TPM from biological fluid ranged from 92.3% to 95.7%. The internal standard extraction recoveries were found between 91.6% and 94.8%.
Stability
Stability of TPM human breast milk was assessed with concentration solutions of 0.3, 1.2, 10.0 and 50.0 μg/mL, stored at -20°C for up to 12 weeks. Reference solutions for corresponding calibration curves were prepared freshly on each day of measurement (days 0, 10, 20, 40, 60 and 84). Observed concentrations of TPM during this time ranged between 96.5%-97.7%.
Freeze-thaw stability of TPM in biological fluid was studied using solutions exposed to three cycles of freezing-thawing versus regularly treated quality control samples. The measured concentrations of TPM ranged between 95.4% and 97.8%.
Application of the method
The developed method was linear between the concentrations range expected, precise, accurate, sensible, and selective for the quantitative determination of TPM in studied matrix: human breast milk. It is very important because TPM can produce severe damage to the fetus, and not always is possible that the epileptic woman change this drug when she is pregnant, because sometimes no other antiepileptic drug is effective in some people.
Using human breast milk we can estimate the drug quantity in baby blood, without the ethic aspects to withdrawal blood of them. It is possible to establish a relationship between the drug quantity in breast milk of epileptic woman with topiramate as her medication for the disease, and dug quantity in baby blood. These steps will be a continuation of this work, including another biological fluid studied before: human serum and umbilical cord blood. Of this way, we can compare the TPM quantity in these three biological fluids, establishing a correlation between drug quantity in serum of the mother, the drug quantity in umbilical cord blood, in breast milk, and the drug quantity in baby blood, using the values founded in serum´s mother, umbilical cord and in breast milk.
Conclusion
The most significant advantage of the present HPTLC method is this allows the quantitation of TPM in human breast milk with the aim of predict the drug concentration in the baby blood using a relationship between these levels. Of this way, it is not necessary to obtain blood from the baby to quantify the drug levels.
The chromatographic conditions are simple, the analysis requires a short period of time (HPTLC separation was obtained within twelve minutes), and the method allows for the analysis of many samples simultaneously with a very good accuracy, sensitivity and precision.
None method was founded by quantitative determination of TPM in breast milk.
Conflict of Interest Statement
The authors declare that they have no financial/commercial conflicts of interest.
Acknowledgements
The authors would like to thank the Research Council at the University of Concepción (Project UCO 12.01). This work is part of the Thesis of Master in Pharmaceutical Sciences (University of Concepción, Chile) of Miss Cristina Cifuentes.
References
- Summary of Product Characteristics-Topamax® (2009) 15 mg 25 mg 50 mg tablets (topiramate). Ortho-McNeil-Janssen Pharmaceuticals Inc.
- Sachdeo RC (1998) Topiramate Clinical profile in epilepsy. Clinical Pharmacokinetics 34: 335-346.
- Glauser TA, Levisohn PM, Ritter F, Sachdeo RC(2000) Topiramate in Lennox-Gastaut syndrome: open-label treatment of patients completing a randomized controlled trial. Epilepsia41: S86-S90.
- Kugler SL, Sachdeo RC (1998) Topiramate efficacy in infancy. Pediatric Neurology 19: 320-322.
- Huang WY, Lo MC, Wang SJ, Tsai JJ, Wu HM (2010) Topiramate in prevention of cluster headache in the Taiwanese. Neurology India 58: 284-287.
- Kanemura H, Sano F, Tando T, Sugita K, Aihara M (2015) Effects of topiramate on headache in children with epilepsy.Brain & development47: 18-22.
- Sahraian A, Bigdeli M, Ghanizadeh A, Akhondzadeh S (2014) Topiramate as an adjuvant treatment for obsessive compulsive symptoms in patients with bipolar disorder: a randomized double blind placebo controlled clinical trial. Journal of affective disorders166: 201-205.
- Correll CU, Sheridan EM, Del Bello MP (2010) Antipsychotic and mood stabilizer efficacy and tolerability in pediatric and adult patients with bipolar I mania: a comparative analysis of acute, randomized, placebo-controlled trials. Bipolar disorders 12: 116-141.
- Alfaris N, Minnick AM, Hopkins CM, Berkowitz RI, Wadden TA (2015) Combination phentermine and topiramate extended release in the management of obesity. Expert opinion on pharmacotherapy16; 1263-1274.
- Wiffen PJ, Derry S, Lunn MP, Moore RA (2013)Topiramate for neuropathic pain and fibromyalgia in adults. CDSR 8: CD008314.
- Chiaro G, Caletti MT,Provini F(2015) Treatment of sleep-related eating disorder. Current Treatment Options in Neurology17: 361.
- Guglielmo R, Martinotti G, Quatrale M, Ioime L, Kadilli I, et al. (2015) Topiramate in Alcohol Use Disorders: Review and Update. CNS drugs 29:383-395.
- Hammond CJ, Niciu MJ, Drew S, Arias AJ (2015) Anticonvulsants for the treatment of alcohol withdrawal syndrome and alcohol use disorders. CNS drugs 29: 293-311.
- Khazaal Y, Zullino DF (2009) Topiramate for smoking cessation and the importance to distinguish withdrawal-motivated consumption and cue-triggered automatisms. Journal of Clinical Psychopharmacology29: 192-193.
- Oncken C, Arias AJ, Feinn R, Litt M, Covault J, et al. (2014) Topiramate for smoking cessation: a randomized, placebo-controlled pilot study. Nicotine & tobacco research 16: 288-296.
- Reid MS, Palamar J, Raghavan S, Flammino F (2007) Effects of topiramate on cue-induced cigarette craving and the response to a smoked cigarette in briefly abstinent smokers. Psychopharmacology192: 147-158.
- Hardman J, Limbird L (2006) Las Bases Farmacológicas de la Terapéutica. McGraw-Hill, Mexico, pp. 550.
- McEvoy G (2012) AHFS Drug Information American Society of Health- System Pharmacists. Bethesda, pp: 2219-2223.
- Ohman I, Vitols S, Luef G (2002) Topiramate kinetics during delivery, lactation, and in the neonate: preliminary observations. Epilepsia 43:1157-1160.
- Froscher W, Jurges U (2006) Topiramate used during breast feeding. AktuelNeurol33:215-217.
- Sweetman S (2006) Martindale, Guía Completa de Consulta Farmacoterapéutica. PharmaEditores SL, Barcelona,pp: 500-501.
- Guerrini R, Parmeggiani L (2006) Topiramate and its clinical applications in epilepsy. Expert Opinion on Pharmacotherapy 7: 811-823.
- Bahrami G, Mirzaeei S, Mohammadi B, Kiani A (2005) High performance liquid chromatographic determination of topiramate in human serum using UV detection. Journal of Chromatography B: Biomedical Sciences and Applications822: 322-325.
- Walker RB, Mohammadi A, Rezanour N, Ansari M (2010) Development of a Stability-Indicating High Performance Liquid Chromatographic Method for the Analysis of Topiramate and Dissolution Rate Testing in Topiramate Tablets. Asian Journal of Chemistry22: 3856-3866.
- Styslo-Zalasik M, Li W (2005) Determination of topiramate and its degradation product in liquid oral solutions by high performance liquid chromatography with a chemiluminescent nitrogen detector. Journal of Pharmaceutical and Biomedical Analysis 37: 529-534.
- Jalili R, Majnooni MB, Mohammadi B, Fakhri S, Mirzaei S, et al. (2014) Development and validation of a new method for determination of topiramate in bulk and pharmaceutical formulation using high performance liquid chromatography UV detection after precolumnderivatization. Journal of Reports in Pharmaceutical Sciences 3: 179-183.
- Mandrioli R, Musenga A, Kenndler E, De Donno M, Amore M, et al. (2010) Determination of topiramate in human plasma by capillary electrophoresis with indirect UV detection.Journal of Pharmaceutical and Biomedical Analysis 53: 1319-1323.
- Roskar R, Milosheska D, Vovk T, Grabnar I (2015) Simple and Sensitive High Performance Liquid Chromatography Method with Fluorescence Detection for Therapeutic Drug Monitoring of Topiramate. ActaChimica.Slovenica62: 411-419.
- Bahrami G, Mohammadi B (2007) A novel high sensitivity HPLC assay for topiramate, using 4-chloro-7-nitrobenzofurazan as pre-column fluorescence derivatizing agent. Journal of Chromatography B: Biomedical Sciences and Applications 850: 400-404.
- Bahrami G,Mirzaeei S, Kiani A (2004) Sensitive analytical method for Topiramate in human serum by HPLC with pre-column fluorescent derivatization and its application in human pharmacokinetic studies. Journal of Chromatography B: Biomedical Sciences and Applications 813: 175-180.
- Contin M, Riva R, Albani F, Baruzzi A (2001) Simple and rapid liquid chromatographic-turbo ion spray mass spectrometric determination of topiramate in human plasma. Journal of Chromatography B: Biomedical Sciences and Applications 761:133-137.
- Mercolini L, Mandrioli R, Amore M, Raggi MA (2010) Simultaneous HPLC-F analysis of three recent antiepileptic drugs in human plasma. Journal of Pharmaceutical and Biomedical Analysis53: 62-67.
- Popov TV, Maricic LC, Prosen H, Voncina DB (2013) Determination of topiramate in human plasma using liquid chromatography tandem mass spectrometry. ActaChimicaSlovenica60:144-150.
- Kuchekar SR, Zaware BH, Kundlik ML (2010) Rapid and Specific Approach for Direct Measurement of Topiramate in Human Plasma by LC-MS/MS: Application for Bioequivalence Study. Journal of Bioanalysis&Biomedecine2: 107-112.
- The Sixth ICH International Conference on Armonization of Technical Requirements for Registration of Pharmaceuticals for Human Use (2003) Osaka, Japan.
- The United States Pharmacopeia(USP30) (2007)United States Pharmacopeial Convection, Inc., Rocville, USA.
Relevant Topics
Recommended Journals
Article Tools
Article Usage
- Total views: 11653
- [From(publication date):
October-2016 - Apr 11, 2025] - Breakdown by view type
- HTML page views : 10724
- PDF downloads : 929