Research Article Open Access
Quantitation of Insulin Analogue Glargine and Its Two Metabolites M1 and M2 on Triple Quad 6500 and Triple TOF 5600 LC-MS/MS Systems in a Dog Toxicokinetics Study
Yong-Xi Li1*, Yan Ke1, Junyu Li1, Yu Li2, Run Li2, Xiaofeng Chen2, Sahana Mollah3 and Xu Wang31Medpace, Bioanalytical Laboratories, Cincinnati, Ohio, USA
2HEC Pharma Co. Ltd, 5th Industrial Area Shangsha, China
3AB SCIEX, Framingham, Massachusetts, USA
- *Corresponding Author:
- Yong-Xi Li
Medpace, Bioanalytical Laboratories, 5365 Medpace Way
Cincinnati, Ohio, 45227, USA
Tel: +1-513-579-9911 ext 2070
Fax: +1-513-579-0444
E-mail: y.li@medpace.com
Received date: November 15, 2013; Accepted date: December 18, 2013; Published date: December 20, 2013
Citation: Li YX, Ke Y, Li J, Li Y, Li R, et al. (2013) Quantitation of Insulin Analogue Glargine and Its Two Metabolites M1 and M2 on Triple Quad 6500 and Triple TOF 5600 LC-MS/MS Systems in a Dog Toxicokinetics Study. J Anal Bioanal Tech S5: 004. doi: 10.4172/2155-9872.S5-004
Copyright: © 2013 Li YX, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Analytical & Bioanalytical Techniques
Abstract
Glargine is a long lasting bioengineered insulin analogue commonly used in the medical treatment of insulindependent diabetes mellitus. After subcutaneous injection, glargine undergoes enzymatic process generating two metabolites, M1 and M2. Quantitative evidences of their presence and concentration after doping multiple concentrations are important for clinical study. Such information can also help to understand the pharmacokinetics, pharmacodynamics, and toxicology during the insulin drug development. We developed and validated a high throughput analytical method coupled with solid-phase extraction (SPE) and LC-MS/MS for the quantitation of intact insulin in plasma samples for use in pre-clinical and clinical studies. The multiple-reaction-monitoring (MRM) experiments performed on a triple-quadruple mass spectrometry instrument were used for quantitation. A 3.5 min UHPLC method was developed to achieve high throughput. An eight-point standard calibration curve with concentration from 0.2 ng/mL to 20 ng/mL was constructed corresponding to the normal non-fasting insulin levels in plasma. The acceptance criteria for values of accuracy, precision, and linearity of the quantitation curve were developed in accordance to the bioanalytical method validation guidance published by Food and Drug Administration (FDA) and European Medicines Agency (EMA). This validated MRM method was applied for toxicity study of insulin analogues in dog. The toxicokinetic results were reported. Moreover, we compared the MRM results to the MS quantitation results acquired on a hybrid quadruple-quadruple time-of-flight (QqTOF) and showed the high resolution instrument can be an option for peptide and biotherapeutic protein quantitation.
Keywords
Insulin; Glargine; Glargine metabolite 1; Glargine metabolite 2; SPE; LC-MS/MS; MRM; QqTOF; Biotherapeutic protein quantitation; Toxicokinetics in dog
Abbreviations
LLOQ: Lower Limit of Quantification; QC: Quality Control; LQC: Low QC; MQC: Middle QC; HQC: High QC; SPE: Solid Phase Extraction; MRM: Multiple Reaction Monitoring; CV: Coefficient of Variation; RE: Relative Error; QqTOF: Quadrupole-Quadrupole- Time-Of-Flight; FDA: Food and Drug Administration; EMA: European Medicines Agency; XIC: Extracted-Ion Chromatogram; AUC: Area under the Curve; Cmax: Maximum Concentration
Introduction
Human insulin is a peptide hormone composed of 51 amino acids, and has a molecular weight of 5808 Da. It is a heterodimer of two peptide chains connected by disulfide bonds that is secreted by beta cells of pancreas, and is central to regulating carbohydrate and fat metabolism in the body [1]. Insulin disturbance can cause diabetes mellitus, a condition in which the pancreas no longer produces enough insulin or cells stop responding to the insulin that is produced, so that glucose in the blood cannot be absorbed into the cells.
Animal form insulin, including Porcine and Bovine insulin, has been used clinically for the treatment of diabetes. However, biosynthetic human insulin is preferred because side reactions are generally less common. Biotechnology started introduction of reengineered human insulin in the late 1980s [2], and this insulin can achieve somewhat different absorption or duration of action characteristics. For example, NovoRapid® and Apidra® are rapid-acting analogues, and Lantus® and Levemir® are the types of long-last insulin analogues. During the drug development and clinical study, the fundamental information including insulin presence, concentration, metabolism of insulin and its related compounds collected needs to be answered to understand medical treatments for patients suffering from different types of diabetes under individual conditions [3,4].
In our work, we focused on glargine, a long acting insulin analogue. Design of insulin glargine (6063 Da) followed the physiology of human insulin formation in β–cells in which 31B-Arg-32B-Arg-human insulin is a final intermediate of the processing from proinsulin to human insulin [5]. After multiple dosing in subjects with type I and type II diabetes, glargine shows nearly flat action profile and duration beyond 24 h [6,7]. In in vivo, after subcutaneous injection, glargine undergoes an enzymatic removal of the basic arginine pair at positions 30B and 31B to yield 21A-Gly-human insulin (metabolite 1 [M1] (5751 Da)), analogous to prohormone activation [8], with some further loss of threonine to 21A-Gly-des-30B-Thr-human insulin (metabolite 2 [M2] (5650 Da)) [9,10]. Generation of M1 and M2 at the subcutaneous injection site and in plasma carries the notion that the proteolytic degradation products of insulin glargine contribute to the long lasting systemic metabolic activity, and possible side effects, such as growthpromoting effects [11,12].
LC-MS/MS based targeted MRM assays have been used very successfully to quantify small molecules (e.g., hormones, drugs and their metabolites) in pharmaceutical and clinical research [13]. More recently, several laboratories demonstrated the feasibility of using similar concept for quantitation of peptides derived from proteins in multiple bio-matrix resources [14-20], thus making LC-MS/MS quantitation methods increasingly applicable in clinical research [21]. Modern mass spectrometry offers excellent sensitivity, specificity, and dynamic range for both small molecule and peptide quantitation [14,22]. In our work, we developed a rapid, sensitive, and validated analytical method to quantitatively measure glargine and its metabolites for toxicokinetics in dog plasma.
Experimental
Materials
All solvents, methanol, ethanol, acetonitrile, 2-propanol, and water were purchased from Fisher Scientific (Pittsburgh, PA) at HPLC grade. Solid phase extraction (SPE) plate was obtained from Phenomenex (Torrance, CA). Acetic acid and bovine insulin were purchased from Sigma-Aldrich (St. Louis, MO). Dog plasma was purchased from BioChemed (Winchester, VA). Glargine, M1, and M2 were provided by HEC Pharma.
Sample Preparation
All calibration standards were prepared by using 0.3 mL dog plasma fortified with reference compounds glargine, M1, and M2 at concentration of 0.2, 0.5, 1.0, 2.0, 5.0, 10.0, 15.0, and 20.0 ng/mL according to the normal non-fasting insulin plasma levels. Quality control (QC) samples (LLOQ, LQC, MQC, HQC, and dilution QCs) were prepared with respective concentration of 0.2, 0.6, 4.0, 16.0, and 150.0 ng/mL. Glargine is a long-acting insulin analogue, which shows smooth absorption with no obvious peak. Moreover, Glargine will be rapidly metabolized to M1 and M2, but mainly M1. The LLOQ of 0.2 ng/mL was chosen for current study but lower concentration standards will be included in human study. Bovine insulin (molecular weight of 5734 Da) used as internal standard (IS) was spiked at concentration of 3 ng/mL from stock solution of concentration 400 ng/mL prepared in dilution solution (0.1% acetic acid in Methanol/H2O 20/80). Plasma samples were then treated with cold ethanol at ratio of 1.5:1 (solvent:sample) to precipitate large proteins. The precipitant was removed by centrifugation at 13,000 rpm for 5 minutes. The supernatant was then transferred to a new sample tube and mixed with 1.5 mL of water containing 0.1% acetic acid to reduce ethanol concentration for following SPE purification.
SPE and Chromatography
The SPE purification had been demonstrated as a most efficient method to clean up and concentrate sample prior to LC-MS analysis [3,23,24]. The operation was performed on a positive pressure manifold from SPEware (Baldwin Park, CA). A C18 reversed phase 96 well SPE plate was used to purify insulin. First, the plate was sequentially conditioned with 2 column volumes of wash solution (0.1% acetic acid in H2O), 1 mL of methanol, and 1 mL of water. Then, 2.25 mL of diluted crashed plasma samples were loaded onto the plate and washed with 1 mL of wash solution (10/90 methanol/H2O v/v) twice to remove salts and other small molecules. Finally, insulin was eluted with 0.1% acetic acid in methanol. This step was repeated twice for a total volume of 500 μL eluent. Eluent fractions were lyophilized and reconstituted in 60 μL of solvent (0.1% acetic acid in 20/80 methanol/H2O v/v) for subsequent analysis.
Chromatography separation of intact insulin was performed on a Shimadzu Nexera UHPLC LC-30 system using Kinetex C18 column (2.1×50 mm, particle size 2.6 μm, pore size 100 Ǻ). The column oven was operated at 50°C. Mobile phase A consisted of H2O and 0.1% acetic acid, and mobile phase B consisted of 2-propanol/acetonitrile (50/50 v/v) and 0.1% acetic acid. An optimized rapid gradient: 20% B for 0.5 min, from 20% B to 30% B in 1.0 min, from 30% B to 90% B in 0.3 min, 90% B for 0.7 min, from 90% B to 20 % B in 0.1 min, 20% B for 0.9 min at a flow rate of 0.7 mL/min was used to maximally separate analyte from matrix interferences. The total run time of each injection was 3.5 min. Sample injection volume was 10 μL.
Mass spectrometry
The MRM experiment was performed on an AB SCIEX Triple Quad™ 6500 system (AB SCIEX, Framingham, MA) using an IonDriveTM Source in the positive ion mode at an ion spray voltage of 5500 V. The conditions used for LC-MS/MS quantitation of intact insulin were: Curtain gas, 30 psi; ion source gas 1, 70 psi; ion source gas 2, 70 psi; temperature, 600°C; declustering potential, 80 V; Entrance potential, 10 V. After optimizing sensitivity and specificity, the MRM transitions of 867->984, 960->652, 942->930, and 957->315 were selected for glargine, M1, M2, and Bovine insulin for quantification. Detailed compound parameters are listed in the Table 1.
The intact insulin analogues were also analyzed with a TripleTOF®™ 5600 system (AB SCIEX, Framingham, MA) using a DuoSpray™ ion Source in the positive ion mode at an ion spray voltage of 5500 V. An automated Calibrant Delivery System (AB SCIEX, Framingham, MA) was used to deliver the APCI positive calibration solution (AB SCIEX, Framingham, MA) through the APCI probe for external mass calibration. The source condition used for quantitation of intact insulin were: curtain gas, 30 psi; ion source gas 1, 70 psi; ion source gas 2, 70 psi; temperature, 600°C; declustering potential, 80 V; collision energy, 10 eV. TOF MS accumulation time was 250 msec, and TOF MS mass range was m/z 400-1800.
Toxicokinetic study in dog
Beagle dogs (6-12 kg) were used in this study. The dogs had access to water and libitum, and been fed with standardized canine diet. Three dose groups (0.5, 1, 2 U/kg) of 6 dogs at each dose group (3 males and 3 females) were administered insulin glargine subcutaneously. The recommended starting dose for human is 0.2 U/kg for glargine injection. Therefore, the low dosage of 0.5 U/kg in the dog study was chosen which was a bit above the equivalent dosage with no toxicity. The high dosage was 2 U/kg under that the dogs show some toxicity. The middle dosage was 1 U/kg that was added between the low and high dosage so that it can better evaluate the dose-response relationships. To determine systemic exposure, plasma concentrations of glargine, M1, and M2 in plasma were determined at each dosage level. Blood samples were collected from all dogs at approximately 0 (before dosing), 1, 2, 4, 8, and 24 hours postdose. Samples were analyzed on an AB SCIEX Triple Quad™ 6500 system with MRM method described above.
Data analysis
The chromatographic peak area integration was carried out by MultiQuant™ 2.1.1 (AB SCIEX, Framingham, MA) using MQ4 algorithm. The quantitation precision was measured by calculated percent coefficient of variation (% CV) from 18 replicates of QC samples (3 replicates of dilution QC) during the course of validation. The quantitation accuracy was defined by the percentage of variation calculated by peak area ratio between analyte and IS versus calculated concentration of standard samples to their respective nominal values (Relative Error, RE%). The linearity of standard calibration curve was determined by the weighted least square regression from the plot of eight-point standard curve. Toxicokinetics were completed by using Phoenix WinNonlin 6.3 program from Certara (St. Louis, MO).
Results
Insulin quantitation with MRM approach on a triple quad 6500 LC-MS/MS system
The scope of this insulin study was to develop and validate a rapid and reliable mass spectrometry assay allowing the determination of synthetic insulin analogues in dog plasma doped at different concentrations. The precursors of [M+7H]7+ at m/z 867 (glargine), [M+6H]6+ at m/z 960 (M1), and [M+6H]6+ at m/z 942 (M2) were isolated for MS/MS. Although [M+6H]6+ is the second abundant charge state of M1 and M2 precursors, their MRM transitions showed higher signal intensity than the ones from [M+5H]5+, the most abundant charge state. Due to the intra- and intermolecular disulfide bonds, fragmenting insulin analogues only generated limited product ions that can be selected for MRM transitions (Figure 1). The MRM assay configurations including transitions and compound specific parameters (Table 1) were developed on an AB SCIEX Triple QuadTM 6500 instrument. The specimens were prepared and analyzed as described in the method.
| Name | Q1 | Q3 | Dwell time | CE | CXP |
|---|---|---|---|---|---|
| Bovine Insulin, IS | 956.7 | 315.0 | 50 ms | 52 | 19 |
| Glargine | 867.0 | 984.0 | 50 ms | 27 | 45 |
| M1 | 959.8 | 652.0 | 50 ms | 32 | 15 |
| M2 | 942.0 | 930.0 | 50 ms | 23 | 8 |
Table 1: Transition and compound parameters of glargine, M1, and M2 for MRM experiment.
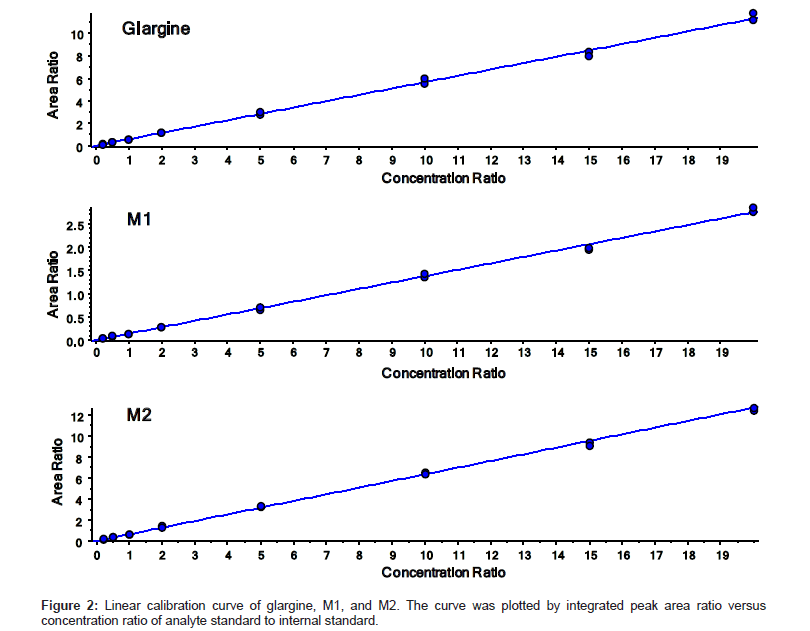
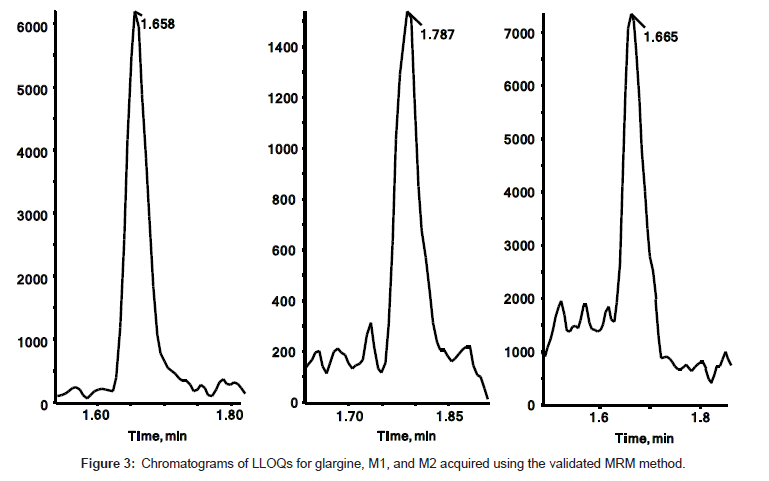
The quantitation precision and accuracy of standards from six singlet eight-point standard curves for glargine, M1, and M2 in dog plasma ranged from 95% to 112% (Table 2). The precision and accuracy for all QCs from 18 replicates (3 replicates of dilution QC) were presented in Table 3. All observed CV% and RE% of three insulin analogues from their respective concentrations were lower than 15%, which is considered acceptable as published in the validated bioanalytical method according to United States FDA [25] and European EMA guidelines [26]. The assay demonstrated a linear relationship within the concentration range from 0.2 ng/mL to 20 ng/mL. The linearity of calibration curves were calculated based on the equation of y=0.5642x+0.0057, (r2=0.998), y=0.1375x+0.0018 (r2=0.996), and y=0.6365x-0.0040 (r2=0.999) for glargine, M1, and M2 respectively (Figure 2). Freeze/thaw (3 cycles) stability, bench top stability, autosampler stability, extract refrigerator stability, and one month storage stabilities at -20°C and -80°C were all successfully validated (data not shown). Therefore, the method for glargine, M1, and M2 in dog plasma on a triple quad 6500 system was fully validated. Chromatograms of LLOQs for glargine, M1, and M2 in the validated MRM method are presented in Figure 3. The spectrum quality at 0.2 ng/mL indicated that the instrument can quantitate insulin analogues at even lower concentration when needed with a little method modification.
| Analyte | Standard & Concentration (ng/mL) |
Mean (ng/mL) |
Precision %CV |
Accuracy %RE |
|---|---|---|---|---|
| Glargine | 0.2 (N=6) | 0.20 | 6.50 | -1.00 |
| 0.5 (N=6) | 0.52 | 2.80 | 3.00 | |
| 1.0 (N=6) | 1.00 | 6.00 | -0.20 | |
| 2.0 (N=6) | 2.12 | 1.60 | 6.00 | |
| 5.0 (N=6) | 4.97 | 4.30 | -0.60 | |
| 10.0 (N=6) | 10.00 | 1.90 | -0.60 | |
| 15.0 (N=6) | 14.40 | 3.40 | -4.00 | |
| 20.0 (N=6) | 19.70 | 2.20 | -1.50 | |
| M1 | 0.2 (N=6) | 0.20 | 5.00 | 1.00 |
| 0.5 (N=6) | 0.49 | 11.70 | -2.60 | |
| 1.0 (N=6) | 0.98 | 6.60 | -0.23 | |
| 2.0 (N=6) | 2.00 | 7.60 | 0.00 | |
| 5.0 (N=6) | 5.19 | 6.30 | 3.80 | |
| 10.0 (N=6) | 10.20 | 3.40 | 2.00 | |
| 15.0 (N=6) | 14.70 | 6.00 | -2.00 | |
| 20.0 (N=6) | 19.90 | 2.50 | -0.50 | |
| M2 | 0.2 (N=6) | 0.20 | 13.80 | 0.00 |
| 0.5 (N=6) | 0.49 | 8.50 | -2.00 | |
| 1.0 (N=6) | 1.02 | 8.30 | 2.00 | |
| 2.0 (N=6) | 2.01 | 5.00 | 0.50 | |
| 5.0 (N=6) | 5.03 | 3.80 | 0.60 | |
| 10.0 (N=6) | 10.30 | 1.00 | 3.00 | |
| 15.0 (N=6) | 14.70 | 5.90 | -2.00 | |
| 20.0 (N=6) | 19.40 | 2.60 | -0.30 |
Table 2: Accuracy and precision of calibration standards for glargine, M1 and M2.
| Analyte | QC & Concentration (ng/mL) |
Mean (ng/mL) |
Precision %CV |
Accuracy %RE |
|---|---|---|---|---|
| Glargine | LLOQ 0.2 (N=18) | 0.2 | 11.20 | -5.00 |
| LQC 0.6 (N=18) | 0.6 | 10.70 | 0.80 | |
| MQC 4.0 (N=18) | 3.8 | 4.10 | 4.30 | |
| HQC 16.0 (N=18) | 15.5 | 7.80 | -3.10 | |
| Dilution QC 150.0 (N=3) | 152.0 | 4.10 | 1.30 | |
| M1 | LLOQ 0.2 (N=18) | 0.2 | 14.60 | -5.50 |
| LQC 0.6 (N=18) | 0.6 | 11.20 | 0.70 | |
| MQC 4.0 (N=18) | 3.8 | 9.60 | -5.30 | |
| HQC 16.0 (N=18) | 15.9 | 7.40 | -0.06 | |
| Dilution QC 150.0 (N=3) | 153.0 | 7.90 | 1.80 | |
| M2 | LLOQ 0.2 (N=18) | 0.2 | 10.90 | 1.50 |
| LQC 0.6 (N=18) | 0.6 | 9.30 | -2.30 | |
| MQC 4.0 (N=18) | 3.9 | 5.40 | -3.50 | |
| HQC 16.0 (N=18) | 15.6 | 7.00 | -2.50 | |
| Dilution QC 150.0 (N=3) | 154.0 | 5.10 | 2.70 |
Table 3: Accuracy and precision of QC samples for glargine, M1 and M2.
Toxicokinetics
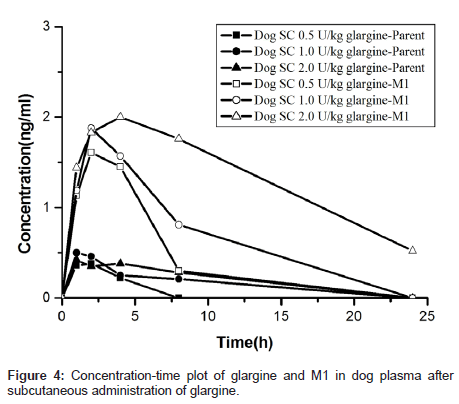
To facilitate toxicology study, the concentration-time profiles of glargine and M1 were obtained using the validated method described above. The toxicokinetics parameters were calculated by established non-compartmental methods in PhoenixWinNonlin. The results are summarized in Table 4. The area under the plasma concentration versus time curve from 0 to t (AUC0-t), where t is last time point with concentration above the lower limit of quantitation, was calculated using linear trapezoidal interpolation in the ascending slope and logarithmic trapezoidal interpolation in the descending slope. Figure 4 showed the concentration versus time profiles of glargine and its major metabolite M1 in dog plasma after a single subcutaneous injection of glargine. The toxicokinetic model of M2 was not carried out because it has low concentration during glargine metabolism [5]. The results represented that most, if not all, of the glargine injected subcutaneously in dogs (as well as in humans in our other work) was rapidly transformed to M1 resulting in minimal, if any, plasma exposure to parent glargine.
| Analyte | Dose(U/kg) | Tmax(h) | Cmax(ng/ml) | AUC0-t(h*ng/ml) |
|---|---|---|---|---|
| Glargine | 0.5 | 1.33 | 0.38 | 0.55 |
| 1.0 | 1.33 | 0.53 | 1.50 | |
| 2.0 | 3.20 | 0.49 | 2.35 | |
| M1 | 0.5 | 2.67 | 1.64 | 5.45 |
| 1.0 | 2.83 | 1.91 | 10.35 | |
| 2.0 | 4.50 | 2.14 | 18.48 |
Table 4: Summary of toxicokinetics parameters in dog toxicology study after subcutaneous injection of glargine (n=6).
Insulin quantitation on a QqTOF hybrid mass spectrometry instrument (TripleTOF)
Intact glargine, M1, and M2 were also analyzed on a TripleTOF®™ 5600 system. Figure 5a displayed the charge distributions of three intact insulin analogues. The mass accuracy and isotopic distribution of the three most abundant precursor ions are shown in Figure 5b. The concentrations of standard calibration curve were same as the ones analyzed on the triple quadrupole instrument. The quantitation was achieved by extracted ion chromatogram (XIC) peak from the high resolution precursor spectrum acquired in TOF MS full scan. The extraction width is a mass range centered at the m/z of the ion of interest. A fixed value with absolute units, such as Daltons (Da) or mili- Daltons (mDa) was used for extraction of all ions of interest.
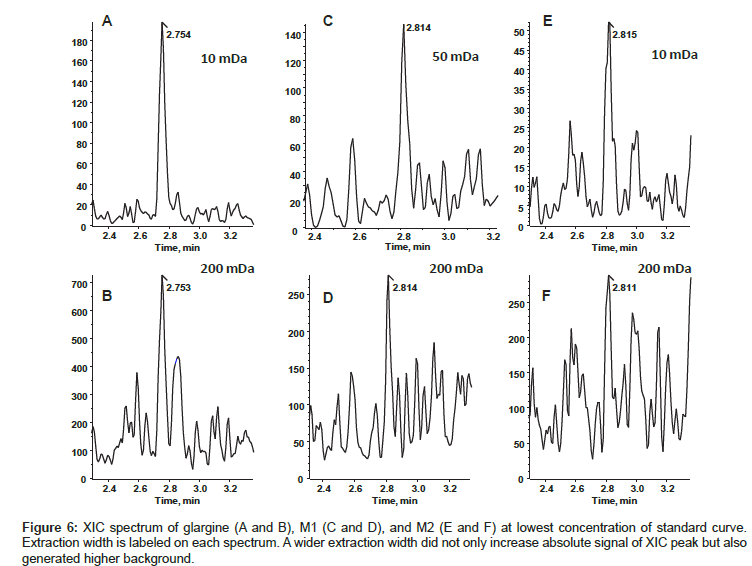
By following the rule of thumb, the most abundant respective precursor ions of glargine, M1, and M2 were selected for quantitation. During analysis, we summed the top four most abundant isotopic peaks of each charge state, but didn’t observe the same good quality of quantitation that was observed by summing multiple charge states. Extraction width of 5, 10, 50, 100, and 200 mDa were compared for the quality of quantitation. The CV, accuracy, and s/n (signal to noise ratio) of lowest concentration from standard curve with different extraction width were shown in Table 5. Based on the best s/n, CV, and accuracy, we chose 10 mDa for glargine and M2, 50 mDa for M1 data analysis. Figure 6 shows spectra of LLOQ generated with selected extraction widths. Both glargine and M1 showed linear relationship within the concentration range from 0.2 ng/mL to 20 ng/mL, whereas the lowest limit quantitation was 0.5 ng/mL for M2. The quantitation accuracy of the standard calibration curve for glargine ranged from 82% to 119%; M1 ranged from 80% to 119%; and M2 ranged 84% to 108%. The values of % CV were less than 15% for all concentrations.
| Glargine | M1 | M2 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Extraction Width |
CV (%) |
Accuracy (%) |
S/N | CV (%) |
Accuracy (%) |
S/N | CV (%) |
Accuracy (%) |
S/N |
| 5 mDa | 11 | 87 | 8.5 | 8 | 122 | 6.2 | 10 | 106 | 9.0 |
| 10 mDa | 11 | 87 | 11.0 | 15 | 119 | 5.4 | 11 | 114 | 6.0 |
| 50 mDa | 15 | 87 | 9.6 | 20 | 111 | 7.7 | 25 | 98 | 4.2 |
| 100 mDa | 13 | 86 | 3.3 | 29 | 118 | 2.1 | 9 | 108 | 2.8 |
| 200 mDa | 14 | 85 | 2.0 | 17 | 112 | 2.0 | 13 | 105 | 2.2 |
*Data was from LLOQ of each compound.
Table 5: CV, accuracy, and s/n for three insulin analogues at different extraction width.
Discussion
Multiple reaction monitoring (MRM) is still the most sensitive and selective scan function on a triple-quadrupole mass spectrometry instrument, which can be operated in a high throughput fashion for targeted quantitative bioanalysis [13,14,27]. We developed a validated method with 3.5 min per sample turnover rate, while reaching the level of sensitivity and linearity to cover the concentration range of normal non-fasting insulin in plasma. This method was applied for toxicokinetics study in dog plasma and in human plasma (in our other ongoing projects). The results showed: 1) after subcutaneous injection, insulin glargine is rapidly metabolized to its major metabolite M1 (>87%) in dogs, with little or no parent or M2 detectable in plasma. The kinetic profile suggests that M1, rather than glargine, is the major driver in pharmacokinetics of glargine, and responsible for glargine’s long-acting profile. 2) A dose dependent increase of M1 plasma levels was observed, which was confirmed by AUC. Cmax of M1 was 2.7 h in low dose treatment, and 4.5 h after high dose treatment. 3) The AUC or Cmax of M1 (Parent was a little) was lower than the declared data in the application materials of Lantus in CDER [28] and Japan [29]. The methods of sample analysis for concentrations of glargine, M1, and M2 in these two applications were radioimmunoassay (RIA), which is different from the method in this paper. Meanwhile, the analysis and validation results in this paper proved that the MRM method on LCMS/ MS system is of high specificity and avoided any other analogues interference.
Although in our study we did not observe non-specific interference and very high background noise causing higher lower limit of quantification (LLOQ), there are instances where low resolution nominal mass spectrometry instruments may not be specific enough and encounter these issues. In such cases, high resolution mass spectrometry instrument can be used to separate interferences from analyte and improve detection and quantitation limit [30-32]. In the past, high resolution mass spectrometry instruments could not fulfill the need for reliable quantification in bioanalysis due to the limitations in sensitivity, linear dynamic range, and speed. In our work, we demonstrated the TripleTOF instrument is able to overcome these limitations and become an option for large molecule quantitation. Depending on the problems associated with a bioassay, such as high background noise, matrix interference or sensitivity requirement, the quantification can be done with either XIC of precursor ion(s) in MS scan or XIC of product ion(s) in the MS/MS spectrum (MRM like approach). While the method of product ion extraction provides better selectivity to avoid interferences and generates chromatographic peaks with lower background noise; however, signal loss during fragmentation can reduce overall sensitivity. For such cases precursor mass extraction can provide better sensitivity, and require very little to no compound optimization for large therapeutic peptides and proteins quantification during method development. It also allows for both targeted and non-targeted quantitation in a single method. In this study, we used an MS based method for quantitation of insulin analogues on the TripleTOF®™ 5600 system.
In terms of data analysis, both high resolution and MRM data use integrated chromatographic peak area to represent signal intensity of the target analyte. However, the cycle time for high resolution method is longer than a MRM method. When processing high resolution data, XIC extraction width, number of isotopic ions and number of the charges to sum for each species should be evaluated. Adjusting these parameters leverages between analyte signal intensity and background noise for optimum sensitivity and specificity. Based on the results shown above, although the quantitation quality of XIC of precursor ions is comparable to MRM approach, MRM results from triple quad 6500 system still shows relatively better sensitivity, reliability, and reproducibility.
Conclusion
Our results demonstrated that we have developed a rapid and reliable LC-MS/MS method for insulin quantification in dog plasma over a concentration range of 0.2 ng/mL to 20 ng/mL. We also showed feasibility of using a TripleTOF for high throughput intact large therapeutic peptide quantification without tryptic digestion. The MRM method provided best sensitivity, accuracy, precision and linear dynamic range, whereas the modern QqTOF instrument could be used in the experiments needing higher specificity. This validated MRM method had been successfully applied to toxicokinetics study of glargine and M1. The results from dog toxicokinetics study showed that majority of glargine after subcutaneous injection was rapidly transformed to M1 resulting in minimal, if any, plasma exposure to parent glargine.
Acknowledgements
The authors thank Nicole Roenker and Elise Snider for experimental help and Dr. Xinfa Tang for the support of the study.
References
- Burtis CA, Ashwood ER (1997) Tietz textbook of clinical chemistry. 2nd edition, W.B. Saunders, Philadephia, PA, USA.
- Brange J, Ribel U, Hansen JF, Dodson G, Hansen MT, et al. (1988) Monomeric insulins obtained by protein engineering and their medical implications. Nature 333: 679-682.
- Thevis M, Thomas A, Delahaut P, Bosseloir A, Schänzer W (2005) Qualitative determination of synthetic analogues of insulin in human plasma by immunoaffinity purification and liquid chromatography-tandem mass spectrometry for doping control purposes. Anal Chem 77: 3579-3585.
- Polonsky KS, O'meara NM (2001) In Endocrinology. 4th edition, Philadephia, PA.
- Bolli GB, Hahn AD, Schmidt R, Eisenblaetter T, Dahmen R, et al. (2012) Plasma exposure to insulin glargine and its metabolites M1 and M2 after subcutaneous injection of therapeutic and supratherapeutic doses of glargine in subjects with type 1 diabetes. Diabetes Care 35: 2626-2630.
- Porcellati F, Rossetti P, Busciantella NR, Marzotti S, Lucidi P, et al. (2007) Comparison of pharmacokinetics and dynamics of the long-acting insulin analogs glargine and detemir at steady state in type 1 diabetes: a double-blind, randomized, crossover study. Diabetes Care 30: 2447-2452.
- Lucidi P, Porcellati F, Rossetti P, Candeloro P, Cioli P, et al. (2011) Pharmacokinetics and pharmacodynamics of therapeutic doses of basal insulins NPH, glargine, and detemir after 1 week of daily administration at bedtime in type 2 diabetic subjects: a randomized cross-over study. Diabetes Care 34: 1312-1314.
- Steiner DF (2011) Adventures with insulin in the islets of Langerhans. J Biol Chem 286: 17399-17421.
- Kohn WD, Micanovic R, Myers SL, Vick AM, Kahl SD, et al. (2007) pI-shifted insulin analogs with extended in vivo time action and favorable receptor selectivity. Peptides 28: 935-948.
- Kuerzel GU, Shukla U, Scholtz HE, Pretorius SG, Wessels DH, et al. (2003) Biotransformation of insulin glargine after subcutaneous injection in healthy subjects. Curr Med Res Opin 19: 34-40.
- Kurtzhals P, Schäffer L, Sørensen A, Kristensen C, Jonassen I, et al. (2000) Correlations of receptor binding and metabolic and mitogenic potencies of insulin analogs designed for clinical use. Diabetes 49: 999-1005.
- Le Roith D (2007) Insulin glargine and receptor-mediated signalling: clinical implications in treating type 2 diabetes. Diabetes Metab Res Rev 23: 593-599.
- Wilcken B, Wiley V, Hammond J, Carpenter K (2003) Screening newborns for inborn errors of metabolism by tandem mass spectrometry. N Engl J Med 348: 2304-2312.
- Anderson L, Hunter CL (2006) Quantitative mass spectrometric multiple reaction monitoring assays for major plasma proteins. Mol Cell Proteomics 5: 573-588.
- Barnidge DR, Dratz EA, Martin T, Bonilla LE, Moran LB, et al. (2003) Absolute quantification of the G protein-coupled receptor rhodopsin by LC/MS/MS using proteolysis product peptides and synthetic peptide standards. Anal Chem 75: 445-451.
- Barnidge DR, Goodmanson MK, Klee GG, Muddiman DC (2004) Absolute quantification of the model biomarker prostate-specific antigen in serum by LC-Ms/MS using protein cleavage and isotope dilution mass spectrometry. J Proteome Res 3: 644-652.
- Gerber SA, Rush J, Stemman O, Kirschner MW, Gygi SP (2003) Absolute quantification of proteins and phosphoproteins from cell lysates by tandem MS. Proc Natl Acad Sci U S A 100: 6940-6945.
- Keshishian H, Addona T, Burgess M, Kuhn E, Carr SA (2007) Quantitative, multiplexed assays for low abundance proteins in plasma by targeted mass spectrometry and stable isotope dilution. Mol Cell Proteomics 6: 2212-2229.
- Kuhn E, Wu J, Karl J, Liao H, Zolg W, et al. (2004) Quantification of C-reactive protein in the serum of patients with rheumatoid arthritis using multiple reaction monitoring mass spectrometry and 13C-labeled peptide standards. Proteomics 4: 1175-1186.
- Wang X, Stewart PA, Cao Q, Sang QX, Chung LW, et al. (2011) Characterization of the phosphoproteome in androgen-repressed human prostate cancer cells by Fourier transform ion cyclotron resonance mass spectrometry. J Proteome Res 10: 3920-3928.
- Rauh M (2012) LC-MS/MS for protein and peptide quantification in clinical chemistry. J Chromatogr B Analyt Technol Biomed Life Sci 883-884: 59-67.
- Hoofnagle AN, Wener MH (2009) The fundamental flaws of immunoassays and potential solutions using tandem mass spectrometry. J Immunol Methods 347: 3-11.
- Lame ME, Chambers EE, Blatnik M (2011) Quantitation of amyloid beta peptides Aß(1-38), Aß(1-40), and Aß(1-42) in human cerebrospinal fluid by ultra-performance liquid chromatography-tandem mass spectrometry. Anal Biochem 419: 133-139.
- Wang X, Emmett MR, Marshall AG (2010) Liquid chromatography electrospray ionization Fourier transform ion cyclotron resonance mass spectrometric characterization of N-linked glycans and glycopeptides. Anal Chem 82: 6542-6548.
- USFDA (2001) Guidance for Industry: Bioanalytical Method Validation. Food and Drug Administration.
- EMA (2011) Guideline on bioanalytical method validation. Committee for Medicinal Products for Human Use.
- Ramagiri S, Garofolo F (2012) Large molecule bioanalysis using Q-TOF without predigestion and its data processing challenges. Bioanalysis 4: 529-540.
- Matthews EJ, Benz RD, Contrera JF (2000) Use of toxicological information in drug design. J Mol Graph Model 18: 605-615.
- Absorb, distribute Metabolize and Excrete (ADME) Application materials of Lantus. Japan.
- Hewel JA, Phanse S, Liu J, Bousette N, Gramolini A, et al. (2013) Targeted protein identification, quantification and reporting for high-resolution nanoflow targeted peptide monitoring. J Proteomics 81: 159-172.
- Wang X, Tipton JD, Emmett MR, Marshall AG (2010) Sites and extent of selenomethionine incorporation into recombinant Cas6 protein by top-down and bottom-up proteomics with 14.5 T Fourier transform ion cyclotron resonance mass spectrometry. Rapid Commun Mass Spectrom 24: 2386-2392.
- Chen J, Cui W, Giblin D, Gross ML (2012) New protein footprinting: fast photochemical iodination combined with top-down and bottom-up mass spectrometry. J Am Soc Mass Spectrom 23: 1306-1318.
Relevant Topics
Recommended Journals
Article Tools
Article Usage
- Total views: 17580
- [From(publication date):
specialissue-2014 - Mar 31, 2025] - Breakdown by view type
- HTML page views : 12493
- PDF downloads : 5087