Quantifying Selective Solvent Transport Under an Electric Field in MixedSolvent Electrolytes
Received: 02-May-2023 / Manuscript No. ico-23-100911 / Editor assigned: 04-May-2023 / PreQC No. ico-23-100911 (PQ) / Reviewed: 18-May-2023 / QC No. ico-23-100911 / Revised: 24-May-2023 / Manuscript No. ico-23-100911 (R) / Published Date: 30-May-2023 DOI: 10.4172/2469-9764.1000221
Abstract
Electrolytes in lithium-ion batteries comprise solvent mixtures, but analysis of ion transport is always based on treating the solvents as a single-entity. We combine electrophoretic NMR (eNMR) measurements and molecular dynamics (MD) simulations to quantify electric-field-induced transport in a concentrated solution containing LiPF6 salt dissolved in an ethylene carbonate/ethyl methyl carbonate (EC/EMC) mixture. The selective transport of EC relative to EMC is reflected in the difference between two transference numbers, defined as the fraction of current carried by cations relative to the velocity of each solvent species. This difference arises from the preferential solvation of cations by EC and its dynamic consequences. The simulations reveal the presence of a large variety of transient solventcontaining clusters which migrate at different velocities. Rigorous averaging over different solvation environments is essential for comparing simulated and measured transference numbers. Our study emphasizes the necessity of acknowledging the presence of four species in mixed-solvent electrolytes.
Introduction
Many electrolytes of commercial importance consist of solvent mixtures. In conventional lithium-ion batteries, the solvent is a mixture of high permittivity cyclic carbonates such as ethylene carbonate (EC) and low permittivity linear carbonates such as ethyl methyl carbonate (EMC). A high permittivity component is crucial for ion dissociation but it is also characterized by high viscosities, which are detrimental for ion transport. In fact, EC is a crystalline solid at room temperature. A low permittivity component is essential for obtaining electrolyte solutions with low viscosities and enabling fast ion transport. Recent work also showed that manipulation of solvent–solvent interactions helps regulate the solvation structure and stabilize the electrolyte. In an important publication, Doyle and Newman modeled ion transport in lithium-ion batteries based on concentrated solution theory [1]. It is assumed that a mixture of low and high permittivity components can be approximately treated as a single species. Nearly every publication on modeling lithium-ion batteries is based on this assumption.
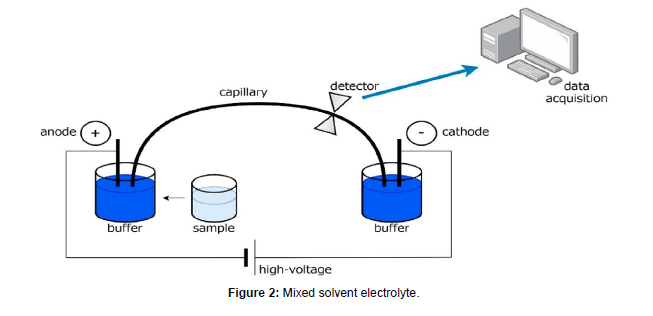
In a recent experimental publication, Wang et al. show that under an applied electric field, the high permittivity solvent accumulates near the negative electrode while low permittivity solvent accumulates near the positive electrode. Selective solvent transport has important implications on the overall functioning of the battery because electrochemical reaction kinetics depend on the local composition of the electrolyte solution at the electrode–electrolyte interface. Modeling solvent partitioning under an applied electric field requires going beyond the single-solvent approximation. In this work, we present the first steps toward the development of such models. The structure of the solvation shells surrounding dissociated lithium ions in mixed-solvent electrolytes has been studied extensively by computer simulations and experiments. One may expect a larger number of cyclic carbonate molecules in the vicinity of lithium ions due to their high permittivity. However, other factors such as denticity of the solvent molecules and steric hindrance also play important roles. Nevertheless, it is generally accepted that solvation shells are enriched in cyclic carbonates relative to the bulk composition. This conclusion is consistent with direct spectroscopic experiments. It should be evident that even the static structure of the solvation shell in mixed-solvent electrolytes is not entirely settled. While it is reasonable to expect the local solvation shell around lithium ions to have compositions that differ from that of the bulk mixture, the effect of this local heterogeneity on continuum transport has not been established (Figure 1 and 2).
Quantifying transport properties in mixed-solvent electrolytes is another challenge. Continuum transport in a ternary electrolyte comprising two ionic species and two solvent species (A and B) is characterized by six Stefan–Maxwell diffusion coefficients.
Materials
Mixed-solvent electrolyte: Prepare a mixed-solvent electrolyte solution by mixing two or more solvents with suitable electrolytes. The selection of solvents and electrolytes depends on the specific experimental requirements and target application.
Electrodes: Prepare two electrodes, typically made of conductive materials such as platinum or graphite. The choice of electrode material depends on the compatibility with the electrolyte and the experimental setup.
Container: Use a container or cell to hold the electrolyte solution and electrodes during the experiment. The container should be chemically inert and provide a suitable environment for the solvent transport measurements.
Experimental setup
Place the electrodes in the container, ensuring proper spacing between them. The electrode configuration can vary depending on the experimental requirements, such as parallel plate electrodes or concentric electrodes [2]. Connect the electrodes to a power supply or voltage source, allowing for the application of an electric field across the electrolyte solution. The voltage and duration of the applied electric field can be adjusted based on the desired experimental conditions. Ensure proper electrical connections and precautions for safe and controlled application of the electric field.
Solvent transport measurements
Choose a suitable technique to measure the solvent transport under the applied electric field. Some commonly used techniques include:
Electrochemical impedance spectroscopy (EIS): Measure the impedance response of the electrolyte solution to characterize the transport properties.
Differential scanning calorimetry (DSC): Monitor the changes in heat flow during the experiment to evaluate the transport behavior.
Optical microscopy: Observe the movement of solvent molecules or changes in the electrolyte solution's appearance under the electric field.
Other spectroscopic or imaging techniques: Use techniques such as nuclear magnetic resonance (NMR), infrared spectroscopy (IR), or confocal microscopy to study solvent transport.
Data analysis
• Analyze the obtained data using appropriate methods to extract meaningful insights into solvent transport behavior.
• Perform statistical analysis, curve fitting, or modeling techniques to interpret the experimental results.
• Compare the results with control experiments or theoretical predictions to validate the findings.
• Present the data and analysis in a clear and concise manner, using appropriate figures, tables, and graphs to support the conclusions.
Safety considerations
• Handle the electrolyte solutions and electrodes with care to avoid any spills or accidents.
• Follow proper safety protocols and use appropriate personal protective equipment (PPE) during the experiment.
• Adhere to local regulations and guidelines for the handling and disposal of chemicals.
Note: The specific details of the materials and methods can vary depending on the experimental setup, measurement techniques, and research objectives [3]. It is important to tailor the procedures to the specific requirements of the study and consider any additional factors or considerations unique to the research.
The study of solvent transport under an electric field in mixedsolvent electrolytes has gained significant interest due to its potential applications in various fields, including energy storage, electrochemical devices, and separation processes. In this discussion, we will explore the key findings and implications of the research related to solvent transport in mixed-solvent electrolytes under the influence of an electric field. One of the important observations in this study is the influence of solvent composition on the transport behavior. The choice of solvents and their ratio in the mixed-solvent electrolyte significantly affects the solvent transport properties. Different solvents possess varying dielectric constants, viscosities, and molecular structures, which can impact the overall mobility and diffusion of solvent molecules under an electric field [4]. It is crucial to understand the interactions between solvents and electrolytes to optimize the mixed-solvent electrolyte composition for specific applications. The applied electric field plays a crucial role in controlling and manipulating the solvent transport. The electric field creates a driving force that influences the movement of solvent molecules, leading to phenomena such as electro-osmosis and electrophoresis. Electro-osmosis involves the bulk movement of the solvent under the electric field, while electrophoresis refers to the migration of charged species, such as ions or colloidal particles, leading to solvent flow. The magnitude and direction of the electric field, as well as the applied voltage, can be adjusted to control the solvent transport rate and directionality.
The understanding of the transport mechanisms in mixedsolvent electrolytes is essential for the development of advanced electrochemical devices. For instance, in energy storage applications such as lithium-ion batteries, the movement of solvent molecules influences the transport of lithium ions, which directly impacts the battery's performance. By studying the solvent transport behavior, researchers can gain insights into the electrolyte's performance, stability, and overall electrochemical behavior. This knowledge can guide the design and optimization of electrolytes for enhanced battery performance and safety [5]. Moreover, the investigation of solvent transport in mixed-solvent electrolytes is valuable for separation processes. The ability to control the solvent transport under an electric field can be utilized in techniques such as electrophoretic deposition, electrodialysis, and electrokinetic chromatography. These techniques rely on the manipulation of solvent flow and the migration of charged species for effective separation and purification of target substances. Understanding the underlying transport mechanisms in mixedsolvent electrolytes enables the development of more efficient and selective separation processes. Despite the progress made in studying solvent transport in mixed-solvent electrolytes, several challenges and opportunities for future research exist. Further investigations are needed to understand the specific interactions between solvents and electrolytes, as well as their impact on transport behavior. The development of advanced characterization techniques, such as in situ spectroscopy or microscopy, can provide real-time insights into the solvent transport phenomena [6-8]. Additionally, computational modeling and simulation studies can complement experimental findings, aiding in the prediction and optimization of solvent transport properties.
Conclusion
The study of solvent transport under an electric field in mixedsolvent electrolytes offers valuable insights into the behavior of solvents and their impact on electrochemical processes. The understanding of transport mechanisms, solvent composition effects, and the role of the electric field provides a foundation for the design and optimization of electrolytes for various applications. Continued research in this field has the potential to drive advancements in energy storage, separation processes, and electrochemical devices, enabling more efficient and sustainable technologies.
References
- Pacios M, Valle MD, Bartroli J, Esplandiu MJ (2008)Electrochemical behavior of rigid carbon nanotube composite electrodes. J Electroanal Chem 619: 117-124.
- Salinas-Torres D, Huerta F, Montilla F, Morallon E (2011)Study on electroactive and elctrocatalytic surfaces of single walled carbon nanotube-modified electrodes. Electrochim Acta 56: 2464-2470.
- Arvand M, Dehsaraei M (2013)A simple and efficient electrochemical sensor for folic acid determination in human blood plasma based on gold nanoparticles–modified carbon paste electrode. Mater Sci Eng C 33: 3474-3480.
- Chandra U, Kumara Swamy BE, Gilbert O, Sherigara BS (2010)Voltammetric resolution of dopamine in the presence of ascorbic acid and uric acid at poly (calmagite) film coated carbon paste electrode . Electrochim Acta 55: 7166-7174.
- Adam RN (1996)Electrochemistry at Solid Electrodes. Marcel Dekker, New York 73: 1098.
- Chandra U, Kumara Swamy BE, Gilbert O, Pandurangachar M, Sherigara BS (2009)Voltammetric Resolution of Dopamine in presence of Ascorbic Acid at Polyvinyl Alcohol Modified Carbon Paste Electrode. Int J Electrochem Sci 4:1479-1488.
- Kutluay A, Aslanoglu M (2013)Modification of electrodes using conductive porous layers to confer selectivity for the voltammetric detection of paracetamol in the presence of ascorbic acid, dopamine and uric acid. Sensors and Actuators B 185: 398-404.
- Chandra P, Son NX, Noh HB, Goyal RN, Shim YB (2013)Investigation on the down regulation of dopamine by acetaminophen administration based on their simultaneous determination in urine. Biosens Bioelectron 39: 139-144
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Citation: Brown T (2023) Quantifying Selective Solvent Transport Under an Electric Field in Mixed-Solvent Electrolytes. Ind Chem, 9: 221. DOI: 10.4172/2469-9764.1000221
Copyright: © 2023 Brown T. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 1868
- [From(publication date): 0-2023 - Nov 16, 2025]
- Breakdown by view type
- HTML page views: 1539
- PDF downloads: 329